Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4341
Peer-review started: February 2, 2017
First decision: February 15, 2017
Revised: March 27, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 28, 2017
Processing time: 150 Days and 13.1 Hours
To investigate the mechanism of the antiproliferative effect of synthetic indole phytoalexin derivatives on human colorectal cancer cell lines.
Changes in cell proliferation and the cytotoxic effect of the tested compounds on human colorectal cancer cell lines and human fibroblasts were evaluated using MTS and BrdU assay, allowing us to choose the most potent substance. Cell cycle alterations were analyzed using flow cytometric analysis. The apoptosis-inducing effect of compound K-453 on the HCT116 cell line was examined with annexin V/PI double staining using flow cytometry, as well as acridine orange/propidium iodide (AO/PI) staining. The flow cytometry method also allowed us to measure changes in levels or activation states of other factors associated with apoptosis, such as poly (ADP-ribose) polymerase (PARP), caspase-3 and -9, cytochrome c, Bcl-2 family proteins, and also the integrity of the mitochondrial membrane. To evaluate activity of the transcription factors and proteins involved in signaling pathways we used Western blot analysis together with flow cytometry.
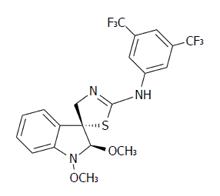
Among the ten tested compounds, compound K-453 {(±)-trans-1,2-dimethoxy-2’-(3,5-bis-trifluoromethylphenylamino)spiro{indoline-3,5’[4’,5’]dihydrothiazol} exhibited the most potent activity with IC50 = 32.22 ± 1.14 μmol/L in human colorectal HCT116 cells and was thus selected for further studies. Flow cytometric analysis revealed a K-453-induced increase in the population of cells with sub-G1 DNA content, which is considered as a marker of apoptotic cell death. The apoptosis-inducing effect of compound K453 was also confirmed by annexin V/PI double staining and AO/PI staining. The apoptosis was associated with the loss of mitochondrial membrane potential, PARP cleavage, caspase-3 and caspase-9 activation, release of cytochrome c, as well as changes in the levels of Bcl-2 family members. Moreover, flow cytometry showed that compound K-453 stimulates phosphorylation of p38 MAPK but decreases phosphorylation of Akt and Erk 1/2. Activation of p38 MAPK was also confirmed using Western blot analysis. This analysis also revealed down-regulation of NF-κB1 (p50) and RelA (p65) proteins and the loss of their anti-apoptotic activity.
In our study compound K-453 exhibited an antiproliferative effect by induction of intrinsic apoptosis as well as modulation of several signaling pathways.
Core tip: In this study we inform about our first results obtained from cell proliferation assays, flow cytometry analysis and Western blot analysis of HCT116 cells after treatment with compound K-453, which belongs to a group of synthetic derivatives of indole phytoalexins originally isolated from cruciferous vegetables. This study demonstrates that our compound induces the mitochondrial pathway of apoptosis and that the antiproliferative effect of our substance may be linked to inhibition of several signaling pathways. Our results provide a rationale for further in vivo anticancer efficacy studies.
- Citation: Tischlerova V, Kello M, Budovska M, Mojzis J. Indole phytoalexin derivatives induce mitochondrial-mediated apoptosis in human colorectal carcinoma cells. World J Gastroenterol 2017; 23(24): 4341-4353
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4341
Cancer is a leading cause of death in more as well as less economically developed countries. Based on GLOBOCAN estimates, about 8.2 million cancer deaths occurred in 2012 worldwide. From all cancers, colorectal cancer (CRC) is the third most common cancer in men and the second in women, with higher incidence in developed countries[1]. Despite improvements in cancer diagnosis and treatment, the mortality rate of CRC is still rising and is expected to increase from 693900 in 2012 to more than 1.1 million deaths by 2030[2]. Chemotherapy is known to be an effective strategy for colon cancer patients. On the other hand, due to the relative nonselectivity of current anticancer drugs (malignant vs non-malignant cells), severe chemotherapy-related adverse reactions often limit the therapeutic effectiveness of these agents[3]. Therefore, novel therapeutic agents for treatment of colorectal cancer are needed.
Natural compounds have attracted attention for use as agents for cancer chemoprevention and treatment. It is generally accepted that consumption of cruciferous vegetables is inversely associated with the risk for variety of cancers, including CRC[4-8]. It is believed that glucosinolates, sulfur-containing phytochemicals, and their metabolic derivatives (e.g., isothiocyanates) play an important function in the cancer-chemopreventive role of cruciferous plants[9]. They act through multiple mechanisms in the prevention of cancer. These include inhibition of metabolic activation or induction of metabolic deactivation of procarcinogens[10,11], inhibition of key steps in angiogenesis[12], induction of apoptosis and cell cycle arrest[13], inhibition of metastasis[14] or suppression of different signaling pathways associated with cell proliferation and growth[15,16].
Moreover, in the last ten years another group of cruciferous-derived compounds has attracted the interest of scientists: the indole phytoalexins. These secondary metabolites are produced de novo by plants, and they are involved in protection against biotic and abiotic stresses[17]. Although these phytochemicals are important components of plant defenses against bacterial and fungal infection, it has been observed that indole phytoalexins may also have health-promoting effects in humans[18]. Beside other effects, some reports have documented an antiproliferative effect of cruciferous phytoalexins.
Recently, we found that brassinin and its derivatives (e.g., 1-methoxybrassinin, homobrassinin) caused blocking of the cell cycle in the G2/M phase and dysregulation of tubulins expression in colorectal cancer cells. Moreover, both compounds increased the number of cells having sub-G1 DNA content, which is considered to be a marker of apoptotic cell death simultaneously with down-regulation of antiapoptotic as well as up-regulation of proapoptotic genes. Furthermore, we detected increased production of reactive oxygen species (ROS), decreased mitochondrial membrane potential (MMP) and modulation of selected signaling pathways in cancer cells treated with indole phytoalexins[19,20]. Another natural indole phytoalexin, camalexin, was found to inhibit proliferation of human leukemia cells[21]. It activated different caspases, decreased MMP and also increased the concentration of ROS. Later, we observed that the addition of benzene to the camalexin structure significantly increased its antiproliferative activity[22]. For more details about the mechanisms of the antiproliferative effect of indole phytoalexins, you may also read the review article by Chripkova and co-workers 2016[23].
Encouraged by our previous results we synthesized a series of new indole phytoalexins that underwent preliminary in vitro testing in our laboratory. Among the tested molecules, the compound (±)-trans-1,2-dimethoxy-2’-(3,5-bis-trifluoromethylphenylamino)spiro{indoline-3,5’[4’,5’]dihydrothiazol} (K-453) possessed the highest activity against HCT116 cells. Our results generate a rationale for in vivo efficacy studies with this compound in preclinical cancer models.
(±)-cis-1,2-dimethoxy-2’-(4-trifluoromethylphenylamino)spiro{indoline-3,5’[4’,5’]dihydrothiazol} (K-452), (±)-trans-1,2-dimethoxy-2’-(3,5-bis-trifluoromethylphenylamino)spiro{indoline-3,5’[4’,5’]dihydrothiazol} (K-453), 5-fluorobrassitin (K-455), 5-fluoro-1-methoxybrassitin (K-459), (±)-cis-5-fluoro-1-methoxyspirobrassinolmethylether (K-462), (±)-1-methoxy-2’-(4-bromophenylamino)spiro{indoline-3,5’-[4’,5’]-dihydrothiazol}-2-one (K-463), (±)-1-methoxy-2’-(4-trifluoromethylphenylamino)spiro{indoline-3,5’-[4’,5’]-dihydrothiazol}-2-one (K-465), (±)-1-methoxy-2’-(4-methylphenylamino)spiro{indoline-3,5’-[4’,5’]-dihydrothiazol}-2-one (K-466), trans-(±)-1-(terc-buthoxycarbonyl)-2-(1-piperidyl)-2’-(4-chlorophenylamino)spiro{indoline-3,5’-[4’,5’]dihydrothiazol} (K-467), trans-(±)-1-(terc-buthoxycarbonyl)-2-(1-piperidyl)-2’-(4-trifluoromethylphenylamino)spiro{indoline-3,5’-[4’,5’]dihydrothiazol} (K-471).
The compounds were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, Missouri, United States). The final concentration of DMSO in the culture medium was < 0.2% and exhibited no cytotoxicity.
The human cancer cell line HCT116 (human colorectal carcinoma) was cultured in RPMI 1640 medium (Biosera, Kansas City, MO, United States). Caco-2 (human colorectal adenocarcinoma) and 3T3 (murine fibroblasts) cell lines were maintained in a growth medium consisting of high glucose Dulbecco’s Modified Eagle Medium with sodium pyruvate (GE Healthcare, Piscataway, NJ, United States). The growth medium was supplemented with a 10% fetal bovine serum, penicillin (100 IU/mL) and streptomycin (100 μg/mL) (all Invitrogen, Carlsbad, CA, United States) in an atmosphere containing 5% CO2 in humidified air at 37 °C. Cell viability, estimated by trypan exclusion, was greater than 95% before each experiment.
Cell viability and proliferation were determined using colorimetric microculture assay with MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) dye (Promega, Madison, WI, United States). Cells were seeded at a density of 10 × 103 cells/well in 96-well polystyrene microplates. Twenty-four hours after cell seeding, different concentrations (100, 50, 10, 5, 1 and 0.1 μmol/L) of the compounds or Z-VAD-FM (50 µmol/L as pre-treatment; Enzo Life Sciences, Inc., Farmingdale, NY, United States) were tested. After 72 h of incubation, 10 μL of MTS were added to the each well. After an additional 2 h, cell proliferation was evaluated by measuring the absorbance at wavelength 490 nm using the automated Cytation™ 3 Cell Imaging Multi-Mode Reader (Biotek, Winooski, VT, United States). Absorbance of control wells was taken as 100%, and the results were expressed as a percentage of the untreated control.
Cell proliferation was confirmed using the 5-bromo-2’-deoxyuridine (BrdU) Cell Proliferation Assay Kit (Roche Diagnostics, Mannheim, Germany). Cells were seeded at a density of 10 × 103 cells/well in 96-well polystyrene microplates (SARSTEDT, Nümbrecht, Germany). Twenty-four hours after cell seeding, different concentrations (100, 50, 10 μmol/L) of compound K-453 were added. After 48 h of treatment, cells were incubated with BrdU labeling solution (10 mmol/L final concentration) for another 24 h at 37 °C followed by fixation and incubation with anti-BrdU peroxidase conjugate for an additional 1.5 h at room temperature. Finally, after substrate reaction, the “stop solution” was added (25 μL 1 mol/L H2SO4), and color intensity was measured with an automated Cytation™ 3 Cell Imaging Multi-Mode Reader (Biotek). Absorbance of control wells was taken as 100%, and the results were expressed as a percentage of the untreated control.
Floating and adherent HCT116 cells (1 × 106) were harvested 24, 48 and 72 h after treatment with the tested compound (K-453, c = 40 μmol/L), washed in PBS, fixed in cold 70% ethanol and kept at +4 °C overnight. Prior to analysis the cells were washed in PBS, resuspended in staining solution (0.2% Triton X-100, 0.5 mg/mL ribonuclease A and 0.025 mg/mL propidium iodide in 500 μL PBS; all Sigma Aldrich, St. Louis, MO, United States) and incubated for 30 min at room temperature in the dark. The DNA contents of the stained cells were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, United States). For each sample, a minimum of 1 × 104 cells were evaluated and the sample flow rate during analysis did not exceed 200-300 cells per second.
For apoptosis detection, floating and adherent HCT116 cells (1 × 106) were harvested 24, 48 and 72 h after treatment with the tested compound (K-453, c = 40 μmol/L), washed in PBS and stained using Annexin-V-FLUOS Staining Kit (Roche Diagnostics, Mannheim, Germany) for 15 min at room temperature in the dark. Prior to analysis the cells were stained with PI and were observed using a BD FACSCalibur flow cytometer.
HCT116 cells were seeded at a density of 5 × 104/well into 6-well polystyrene microplates (SARSTEDT). Twenty-four hours after seeding, cells were treated with K-453 at 40 μmol/L concentration. At 24, 48 and 72 h after treatment, the culture medium was removed; the cells were washed in PBS and fixed with 4% paraformaldehyde for 20 min. After fixation, the paraformaldehyde was removed, the cells were washed in PBS, and the staining solution (10 μg/mL Acridine Orange and 10 μg/mL propidium iodide; Sigma) was added to each well for 1 h incubation at room temperature. After incubation, the staining solution was removed, the cells were washed in PBS and apoptosis was observed using an automated Cytation™ 3 Cell Imaging Multi-Mode Reader (Biotek).
HCT116 cells (1 × 106) were treated with K-453 at 40 μmol/L concentration for 24, 48 and 72 h. Floating and adherent cells were harvested, washed in PBS, divided for particular analysis and stained prior to analysis (Table 1). Fluorescence was detected after 15 min incubation at room temperature in the dark using a BD FACSCalibur flow cytometer. A minimum of 1 × 104 events were analyzed per analysis.
| Analysis | Staining solution | Manufacturer |
| Caspase activation | Caspase-3-PE | BD Biosciences Pharmingen, San Diego, CA, United States |
| Cleaved Caspase-9-FITC | BIOSS Antibodies, Woburn, MA, United States | |
| Cytochrome c release | Cytochrome c Antibody (6H2) FITC Conjugate | Invitrogen, Carlsbad, CA, United States |
| Cleavage of PARP | Cleaved-PARP (Asp214) XP® Rabbit mAb (PE Conjugate) | Cell Signaling Technology, Danvers, MA, United States |
| Mitochondrial membrane potential | TMRE (tetramethylrhodamine ethyl ester perchlorate) Final concentration 0.1 µmol/L | Sigma-Aldrich, St. Louis, Missouri, United States |
| Protein analysis | Phospho-Bcl-2 (Ser70) Rabbit mAb Alexa Fluor® 488 Conjugate | Cell Signaling Technology®, Danvers, MA, United States |
| Phospho-Bad (Ser112) Rabbit mAb (PE Conjugate) |
Protein lysates from our cells were prepared using Laemli lysis buffer containing 1 mol/L Tris/HCl (pH 6.8), glycerol, 20% SDS (sodium dodecyl sulfate) and deionized H2O in the presence of PIC (protease inhibitor cocktail) and a sonication process. The concentration of proteins was evaluated using the Pierce® BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, United States) and measured using an automated Cytation™ 3 Cell Imaging Multi-Mode Reader (Biotek) at wavelength 570 nm. Proteins were separated on SDS-PAA gel (12%) at 100 V for 2 h. They were then transferred to a PVDF Blotting Membrane (GE Healthcare, Chicago, IL, United States) at 200 mA for 2 h using a BioRad Mini Trans-Blot cell (BioRad, Hercules, CA, United States). The membrane with the transferred proteins was blocked in 4% milk with TBS-Tween (pH 7.4) for 1 h at room temperature to minimize non-specific binding. The membrane was subsequently incubated with primary antibodies during the night at 4 °C. Immunoblotting was carried out with the antibodies stated below (Table 2). The next day, the membranes were washed in TBS-Tween (3 × 5 min) and incubated with a corresponding secondary antibody associated with horseradish peroxidase for 1 h at room temperature. After incubation, the membranes were again washed in TBS-Tween (3 x 5 min), and the expression of proteins was detected using a chemiluminescent ECL substrate (Thermo Scientific, Rockford, IL, United States) and MF-ChemiBIS 2.0 Imaging System (DNR Bio-Imaging Systems, Jerusalem, Israel).
| Name | Mr (kDA) | Origin | Manufacturer |
| Primary antibodies | |||
| PARP | 116 + 89 | Rabbit | Cell Signaling Technology®, Danvers, Massachusetts, United States |
| β-actin | 45 | Mouse | |
| p38 MAPK | 43 | Rabbit | |
| NF-κB p65 | 65 | Rabbit | |
| NF-κB p105/p50 | 105/50 | Rabbit | |
| Secondary antibodies | |||
| Anti-rabbit IgG HRP-Linked | - | Goat | Cell Signaling Technology®, Danvers, Massachusetts, United States |
| Anti-mouse IgG/HRP | - | Goat | Dako, Glostrup, Denmark |
Results are expressed as the mean ± SD. Statistical analysis of the data were performed using standard procedures, with one-way ANOVA followed by the Bonferroni multiple comparisons test. Differences were considered significant when P values were smaller than 0.05.
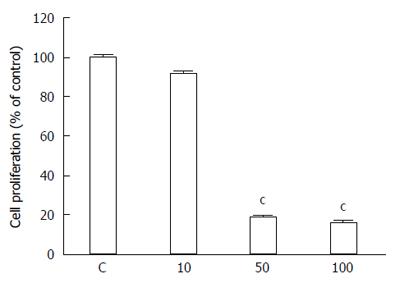
Using the colorimetric MTS assay, the antiproliferative effect of the studied substances was determined. The IC50 values of the newly synthesized derivatives of indole phytoalexins on human cancer and non-cancer (3T3) cell lines are presented in Table 3. Among the tested derivatives, the compound K-453 (Figure 1) exhibited the most significant inhibitory effects on the growth of HCT116 cells, with an IC50 value of 32.22 (± 1.14) μmol/L. Other tested derivatives of indole phytoalexins displayed weaker or no effect at all on cell proliferation. Based on these results, further experiments were performed with the most effective compound, K-453, on the most sensitive cancer cell line HCT116, using a concentration of 40 μmol/L.
| Compound | Cancer Cell Lines | ||
| CaCo-2 | HCT116 | 3T3 | |
| K-452 | > 100 | > 100 | > 100 |
| K-453 | 67.39 ± 0.78 | 32.22 ± 1.14 | 60.55 ± 3.35 |
| K-455 | > 100 | > 100 | > 100 |
| K-459 | > 100 | > 100 | > 100 |
| K-462 | > 100 | > 100 | > 100 |
| K-463 | 74.18 ± 0.001 | 33.04 ± 2.67 | 61.13 ± 2.25 |
| K-465 | 35.66 ± 6.49 | 33.74 ± 2.78 | 34.94 ± 2.01 |
| K-466 | > 100 | > 100 | > 100 |
| K-467 | > 100 | > 100 | > 100 |
| K-471 | > 100 | > 100 | > 100 |
5-bromo-2’-deoxyuridine (BrdU) is commonly used for labeling replicating DNA in proliferating cells of living tissues. BrdU is incorporated into newly synthesized DNA during the S phase of the cell cycle in place of thymidine. Specific antibodies for BrdU are then used to detect the incorporated nucleoside and to differentiate between living and dead cells. Our results showed significantly slower BrdU incorporation rate in the K-453-treated HCT116 cells compared with control (Figure 2), indicating cell proliferation inhibition.
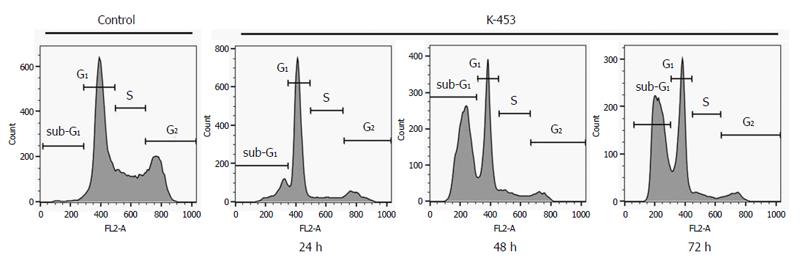
Cell cycle distribution was determined using flow cytometric analysis of HCT116 cells after treatment with K-453 for 24, 48 and 72 h. Results showed a significant increase of cells with sub-G1 DNA content after 24 h treatment which enhanced after 48 and 72 h. At the same time, a decrease in the population of cells in S and G2 phase was observed shortly after 24 h treatment with K-453 (Table 4, Figure 3). These findings suggest significant changes in cell cycle progression and induction of apoptosis.
| Treatment | Time (h) | sub-G1 | G0/G1 | S | G2/M |
| Control | 24 | 1.30 ± 0.07 | 48.38 ± 0.81 | 23.28 ± 0.53 | 27.05 ± 0.35 |
| 48 | 1.75 ± 0.22 | 52.1 ± 2.12 | 22.88 ± 0.08 | 23.30 ± 2.26 | |
| 72 | 1.64 ± 0.09 | 48.57 ± 0.52 | 24.85 ± 0.64 | 24.95 ± 0.21 | |
| K-453 | 24 | 19.7 ± 2.26b | 62.97 ± 0.37b | 7.05 ± 1.35b | 10.29 ± 1.29b |
| 48 | 48.9 ± 7.21c | 35.58 ± 3.22a | 9.31 ± 2.54b | 6.22 ± 1.45b | |
| 72 | 55.11 ± 14.13c | 34.11 ± 9.89a | 5.71 ± 1.73b | 5.07 ± 2.52b |
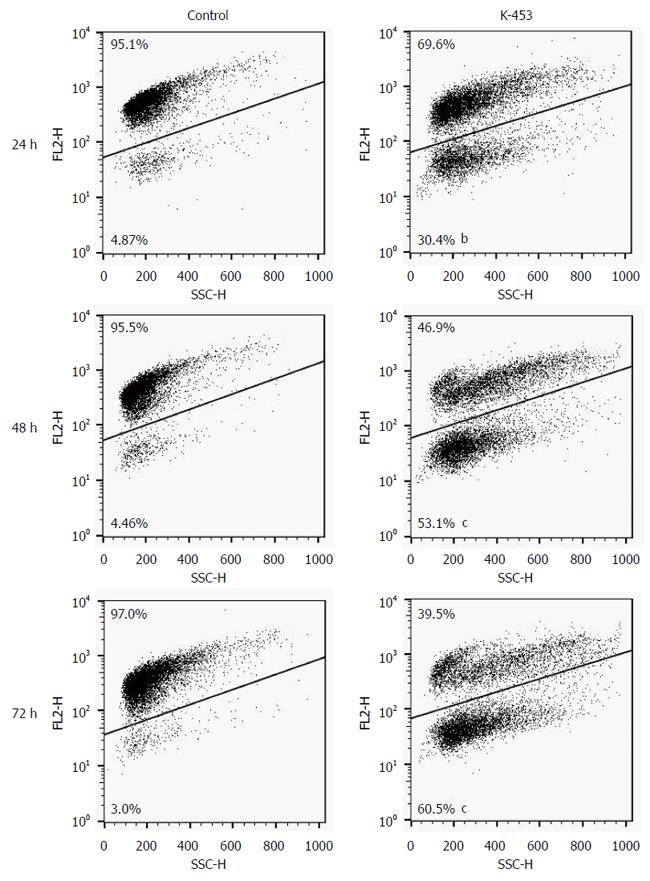
Phosphatidyl serine (PS) is normally localized on the internal surface of the lipid bilayer of the plasma membrane. When cells undergo apoptosis, the PS is externalized and available to detection by the annexin V-FITC conjugate. Annexin therefore acts as a marker of programmed cell death. Compound K-453 induced a significant increase in cellular apoptosis of HCT116 cells (in early stage) and PS externalization even after just 24 h treatment, with a persistent and slight increase after 48 h (Table 5). Moreover, we observed an increase in cells positively stained with both Annexin V and PI (late apoptotic events or cell death) after 48 h. Therefore, we suggest that apoptosis plays an important role in the death mechanisms of HCT116 cells treated with the K-453 derivative of indole phytoalexins.
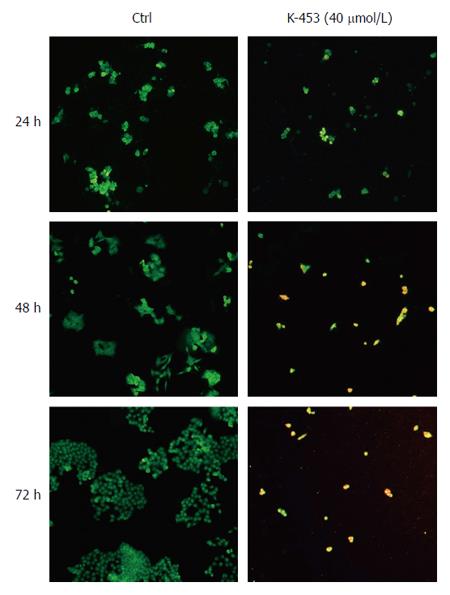
Apoptosis was also confirmed by staining with acridine orange and PI (AO/PI). Acridine orange is a vital dye that will stain both living and dead cells, whereas PI will stain only those cells that have lost their membrane integrity. Living cells stain uniformly green and can thus be distinguished from apoptotic cells. Microscopic detection showed a significant decrease in proliferation and viability of HCT116 cells after K-453 treatment (Figure 4). The number of cells stained red (necrotic) increased time dependently. This indicates that the most of the cells were not undergoing necrosis, and cell death occurred primarily through apoptosis.
Mitochondria are described as key factors in controlling apoptosis. The mitochondrial respiratory chain generates transmembrane potential (∆Ψm), and its decrease or even loss is one of the indicators of mitochondrial dysfunction. Integrity of the mitochondrial membrane and disruption of its potential can be detected using the cationic dye, TMRE (tetramethylrhodamine ethyl ester perchlorate). In our experiments, changes in the Mitochondrial membrane potential (MMP) were observed 24, 48 and 72 h after treatment with K-453, demonstrating significant impairment of mitochondrial function (Figure 5).
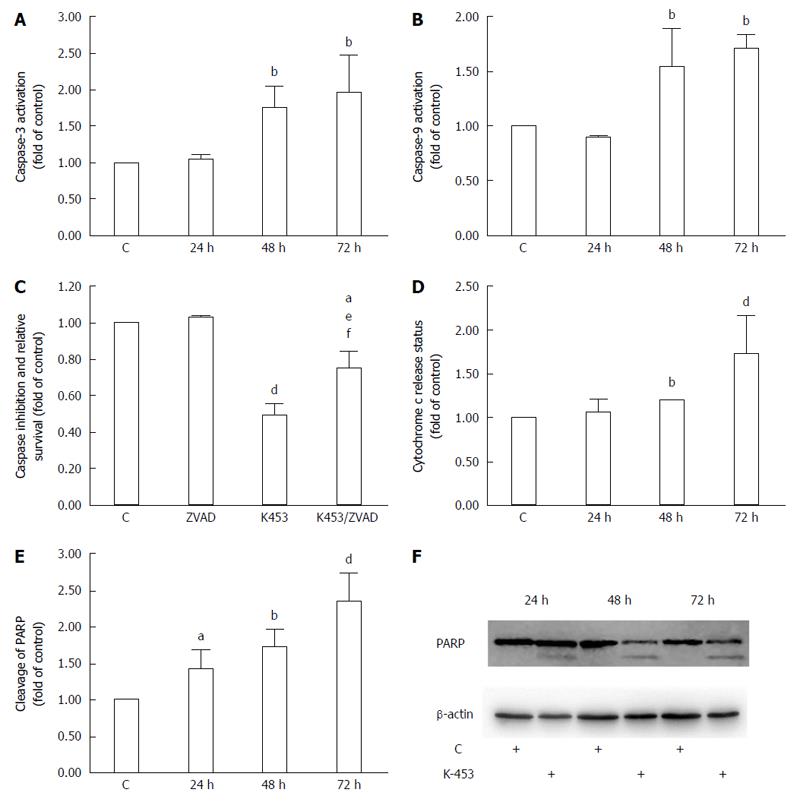
Poly (ADP-ribose) polymerase (PARP) is involved in several biological processes, such as cell cycle progression, DNA repair and regulation of transcription and programmed cell death. Proteolytic cleavage of PARP is nowadays used as a marker for apoptosis. Our flow cytometry (Figure 6E) and Western blot (Figure 6F) analysis showed cleavage of PARP after 24 h of incubation; however, the most significant increase was observed after 72 h of the treatment with K-453.
An important member of the apoptosis cascade as an intermediate is cytochrome c, a component of the electron transfer chain in mitochondria. During apoptosis, this protein is released from mitochondria to cytosol, where it binds to Apaf-1 protein creating the apoptosome complex and triggering activation of initiator caspase-9. Notable changes in the release of cytochrome c were observed using flow cytometry analysis, particularly 72 h after treatment with compound K-453 (Figure 6D).
Caspases are proteolytic enzymes playing an important role in controlling cell death. Initiator caspases (such as caspase-9) activate executioner caspases (including caspase-3) and subsequently coordinate their activity to impact main structural proteins and activate other enzymes, leading to apoptosis as well. Caspase-3 activity was in strong correlation with PARP cleavage, mainly 48 and 72 h after treatment with K-453 (Figure 6A). Similar effects are observed in caspase-9 activation, with significance after 48 h and 72 h of treatment (Figure 6B). Activity of both caspases was evaluated using flow cytometry analysis.
Caspase-dependent apoptotic pathways were also confirmed by using pan-caspase inhibitor Z-VAD-FM. Inhibition of caspases clearly demonstrated an increase of HCT116 cell viability after 72 h treatment with K-453 compound compared with the treated groups without inhibition (Figure 6C).
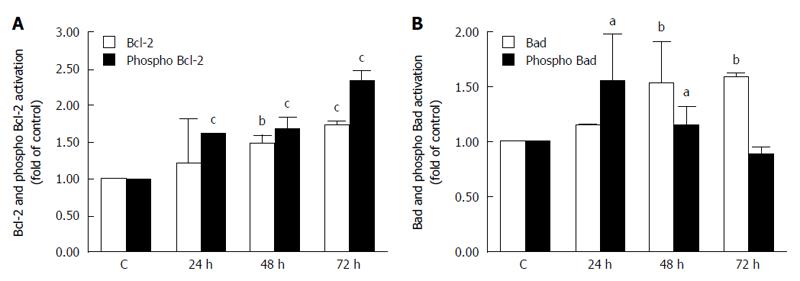
Activation of initiator caspases during apoptosis is mediated by two pathways: the mitochondrial (intrinsic) pathway and the death receptor (extrinsic) pathway. To determine activation of the intrinsic pathway of apoptosis after treatment with our compound, we evaluated changes in activation of Bcl-2 family members. Our results showed a significant increase in the levels of Bcl-2 (anti-apoptotic) (Figure 7A) and Bad (pro-apoptotic) (Figure 7B) proteins after 24, 48 and 72 h of treatment. After 24, 48 and mainly after 72 h, the amount of the phosphorylated (inactive) form of Bcl-2 significantly increased, as well (Figure 7A). On the other hand, phosphorylation of Bad increased shortly after 24 h and then decreased 48 and 72 h after treatment (Figure 7B).
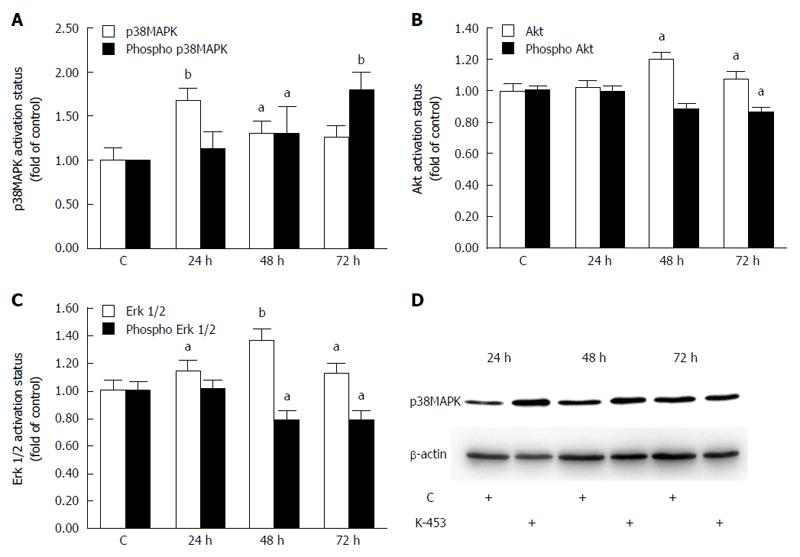
Several signaling pathways play an important role in cell death. To evaluate the mechanisms of apoptosis after K-453 treatment, activation of the p38 MAPK, ERK 1/2 and Akt signalling pathways was examined. Flow cytometry analysis revealed a significant increase in phosphorylation of p38 MAPK after 48 and 72 h of treatment with compound K-453 (40 μmol/L), thus stimulation of its tumor-suppressing activity (Figure 8A). On the other hand, phosphorylation of Akt (Figure 8B) and ERK 1/2 (Figure 8C) decreased 48 and 72 h after treatment. Alterations of the Akt and Erk signaling pathway plays a major role in proliferation, cell survival and apoptosis. Moreover, we observed an increase of p38 MAPK proteins in whole cell lysates (Figure 8D) from K-453-treated cells, which indicates stress promotion and activation of signal transduction mechanisms.
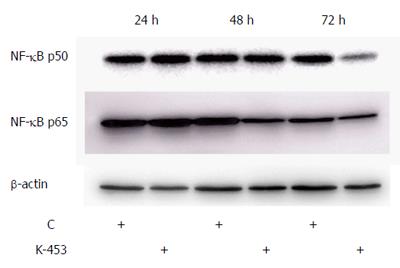
In our experiments we also analyzed the ability of the K-453 compound to modulate the activity of NF-κB/Rel proteins in cell death. The transcription factor NF-κB is involved in promoting proliferation and inhibition of apoptosis. Our results showed that treatment with K-453 caused down-regulation of NF-κB1 (p50) after 72 h and RelA (p65) protein mainly after 48 h (Figure 9). These results suggest that K-453 can suppress NF-κB activity, followed by cell growth suppression and apoptosis induction.
Apoptosis plays a key role in the elimination of needless cells, including malignant cells, and induction of apoptosis is crucial for the anticancer activity of many conventional antitumor agents. On the other hand, it is well known that most cancer cells acquire the ability to evade apoptosis[24].
The present study was designed to discover whether newly synthesized indole phytolalexins have an antiproliferative effect in HCT116 colorectal carcinoma cells and further to investigate the possible mechanisms of the pro-apoptotic effect of selected compounds. Using the MTS test we observed a broad range in antiproliferative activity of the studied compounds. Because of the highest antiproliferative effect in cancer cells as well as higher selectivity to cancer vs non-cancer cells, the compound (±)-trans-1,2-dimethoxy-2’-(3,5-bis-trifluoromethylphenylamino)spiro{indoline-3,5’ [4’,5’]dihydrothiazol} (K-453) was chosen for further study.
It has been observed that either naturally occurring indole phytoalexins or their synthetic derivatives induce cell cycle arrest and apoptosis in cancer cells[19,20,22,25]. Viability and proliferation assays showed that K-453 suppressed growth of HCT116 cells when compared with untreated cells. We demonstrated that the K-453-induced antiproliferative effect in human colorectal cells was associated with induction of apoptosis, as detected by several complementary assays.
We found that compound K-453 induced an increase in the G0/G1 phase of the cell cycle, with a simultaneous increase in cells having sub-G1 DNA content, which is considered to be a marker of programmed cell death. Compared with untreated cells, the number of cells with sub-G1 DNA content increased 15.1-, 27.9- and 33.6-fold after 24, 48 and 72 h of incubation, respectively. Moreover, in our study we observed a time-dependent significant increase in the number of apoptotic HCT116 cells, as evidenced by annexin V/PI, AO/PI staining and PARP cleavage.
Because the mechanisms of the apoptotic effect of K-453 in cancer cells has not yet been elucidated, in the present study we tried to analyze the machinery by which this compound induces apoptosis.
Mitochondria play a crucial role in regulating cell life and death[26]. Stress-induced mitochondrial dysfunction (e.g., DNA damage, chemotherapeutic agents) leads to apoptotic cell death[27]. Mitochondrial collapse can be connected with the loss of mitochondrial membrane potential (ΔΨm)[28] and the release of cytochrome c into the cytosol, where it triggers the formation of the apoptosome, which activates procaspase-9[29]. This activated caspase-9 in turn activates the executioner caspase-3, with subsequent execution of the apoptotic process[30]. In our experiments we observed a significant loss of MMP in the K-453-treated HCT116 cells after 24, 48 and 72 h of incubation, associated with an increase in cytosol cytochrome c content in cancer cells after 48 and 72 h of treatment. In the same treatment period we detected activation of both caspase-9 and caspase-3 in the K-453-treated colon cancer cells.
Furthermore, proteins of the Bcl-2 family, either anti-apoptotic (e.g., Bcl-2 and Bcl-xL) or pro-apoptotic (e.g., Bax and Bad), play an important role in the mitochondrial pathway of apoptosis via controlling of mitochondrial permeability[31]. Bcl-2 protein, a negative regulator of cell death, blocks apoptosis by preventing mitochondrial permeabilization via neutralizing the activity of pro-apoptotic proteins[32]. It is generally accepted that either phosphorylation or dephosphorylation may significantly influence the activity of the Bcl-2 family proteins. It has been suggested that phosphorylation of Bcl-2 can lead to its inactivation[33-36]. On the other hand, it was found that BAD dephosphorylation was associated with induction of apoptosis in prostate cancer cells[37]. Our results showed that K-453-induced apoptosis in HCT116 was associated with an increase in Bcl-2 phosphorylation after 24, 48 and 72 h of treatment as well as BAD dephosphorylation after 72 h of treatment.
Furthermore, modulation of the signaling pathways, including p38 MAPK, ERK1/2, Akt and NF-κB by K-453, was also examined. MAP kinases have been implicated in a variety of intracellular pathways associated with different cellular activities, such as cell survival, proliferation, transformation and apoptosis. Extracellular signal-regulated kinase (ERK), p38, and c-Jun NH2-terminal kinase (JNK) are members of the mammalian MAPK family[38].
The kinase p38, with an important function in regulating cellular response to a variety of stressors, is ubiquitous, and activation of the p38 MAPK signaling pathway resulted in apoptosis induction. Furthermore, activation of p38 requires its phosphorylation[39]. In the present study we observed that K-453-induced phosphorylation of p38 MAPK. As was mentioned above, phosphorylation of Bcl-2 protein can led to its inactivation. Activated p38 MAPK has been identified as a kinases able to mediate such phosphorylation, and it is suggested that p38 MAPK-induced Bcl-2 phosphorylation can be crucial for the induction of apoptosis under conditions of cellular stress[40].
In contrast to p38 MAPK, we found decreased phosphorylation of Akt and ERK 1/2 in K-453-treated colon cancer cells after 48 and 72 h treatment. Because activation (i.e., phosphorylation) of both kinases is mostly associated with cell survival, growth, proliferation, migration and apoptosis inhibition[41-43], we suggest that inactivation of both kinases may play a role in K-453-induced apoptosis.
Last but not least, treatment of HCT116 cells with K-453 resulted in induction of apoptosis, which correlated with lowering of NF-κB/p65 and p50 levels. NF-κB is generally regarded, although not absolutely, as an anti-apoptotic factor and its inactivation by anticancer compounds is possible method of eliminating malignant cells through apoptosis induction[44,45]. Since the NF-kB generally exists as a heterodimer of the p50 and p65 polypeptides[45], we suggest that lower expression of both subunits in K-453-treated cells may lead to dysregulation of the NF-kB pathway, with concomitant loss of the anti-apoptotic function of NF-κB.
Collectively, these findings demonstrated that K-453 induced caspase-dependent apoptosis in colon cancer cells. Loss of MMP, modulation of Bcl-2 and BAD phosphorylation and cytochrome c release indicate the mitochondrial pathway of apoptosis. In addition, K-453 induced apoptosis at least in part through activation of the p38 MAPK pathway as well as through inactivation of the ERK 1/2, Akt and NF-κB signaling pathways.
Indole phytoalexins represent a large group of natural plant metabolites involved in several cell functions, including antiproliferative functions. These substances are produced by plants several hours or days after a chemical, physical or biological stress attack. The structure of phytoalexins depends on the type of plant that synthesizes them and partially on the elicitor inducing their synthesis. Presently, the ability of indole phytoalexins to inhibit the growth of cells was tested in vitro in a number of cancer cell lines.
Besides the antimicrobial properties of indole phytoalexins, remarkable antiproliferative activity was discovered within these compounds that may be beneficial in chemotherapy of tumors in the human population. To improve the anti-cancer activity of natural phytoalexins, several synthetic derivates are now being introduced. However, further studies are necessary to determine mechanism of their activity.
This is the first study to demonstrate the complex antiproliferative mechanism of novel synthetic spiro derivates of indole phytoalexins in the HCT116 colon cancer cell line.
This study was designed to elucidate the apoptosis mechanism of antiproliferative action of the synthetic derivative K-453 in human colorectal cancer cell lines. They found activation of mitochondrial and stress pathway machinery after K-453 treatment. These results provide a rationale for further in vivo anticancer efficacy studies.
GLOBOCAN is the project aimed to provide actual estimates of the incidence, mortality and prevalence from major types of cancer for 184 countries around the world. Estimates are based on the most recent data available at International Agency for Research on Cancer.
Authors investigate the mechanism of indole phytoalexin on colorectal cancer cell lines. Research design was well established. Their results indicate interesting pathway of apoptosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Slovak Republic
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nagata J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21371] [Article Influence: 2137.1] [Reference Citation Analysis (3)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3306] [Article Influence: 413.3] [Reference Citation Analysis (3)] |
| 3. | Overbeek A, van den Berg MH, van Leeuwen FE, Kaspers GJ, Lambalk CB, van Dulmen-den Broeder E. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat Rev. 2017;53:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Azeem S, Gillani SW, Siddiqui A, Jandrajupalli SB, Poh V, Syed Sulaiman SA. Diet and Colorectal Cancer Risk in Asia--a Systematic Review. Asian Pac J Cancer Prev. 2015;16:5389-5396. [PubMed] |
| 5. | Hu J, Hu Y, Hu Y, Zheng S. Intake of cruciferous vegetables is associated with reduced risk of ovarian cancer: a meta-analysis. Asia Pac J Clin Nutr. 2015;24:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Thomson CA, Ho E, Strom MB. Chemopreventive properties of 3,3’-diindolylmethane in breast cancer: evidence from experimental and human studies. Nutr Rev. 2016;74:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Tse G, Eslick GD. Cruciferous vegetables and risk of colorectal neoplasms: a systematic review and meta-analysis. Nutr Cancer. 2014;66:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Veeranki OL, Bhattacharya A, Tang L, Marshall JR, Zhang Y. Cruciferous vegetables, isothiocyanates, and prevention of bladder cancer. Curr Pharmacol Rep. 2015;1:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Abdull Razis AF, Noor NM. Cruciferous vegetables: dietary phytochemicals for cancer prevention. Asian Pac J Cancer Prev. 2013;14:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Ioannides C, Konsue N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab Rev. 2015;47:356-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Konsue N, Ioannides C. Differential response of four human livers to modulation of phase II enzyme systems by the chemopreventive phytochemical phenethyl isothiocyanate. Mol Nutr Food Res. 2010;54:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6:e25799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Wu X, Zhu Y, Yan H, Liu B, Li Y, Zhou Q, Xu K. Isothiocyanates induce oxidative stress and suppress the metastasis potential of human non-small cell lung cancer cells. BMC Cancer. 2010;10:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 16. | Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: recent advances. Mol Nutr Food Res. 2014;58:1685-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 17. | Pedras MS, Yaya EE, Glawischnig E. The phytoalexins from cultivated and wild crucifers: chemistry and biology. Nat Prod Rep. 2011;28:1381-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Jeandet P, Hébrard C, Deville MA, Cordelier S, Dorey S, Aziz A, Crouzet J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules. 2014;19:18033-18056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Chripkova M, Drutovic D, Pilatova M, Mikes J, Budovska M, Vaskova J, Broggini M, Mirossay L, Mojzis J. Brassinin and its derivatives as potential anticancer agents. Toxicol In Vitro. 2014;28:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Kello M, Drutovic D, Chripkova M, Pilatova M, Budovska M, Kulikova L, Urdzik P, Mojzis J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules. 2014;19:10877-10897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Mezencev R, Updegrove T, Kutschy P, Repovská M, McDonald JF. Camalexin induces apoptosis in T-leukemia Jurkat cells by increased concentration of reactive oxygen species and activation of caspase-8 and caspase-9. J Nat Med. 2011;65:488-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Pilatova M, Ivanova L, Kutschy P, Varinska L, Saxunova L, Repovska M, Sarissky M, Seliga R, Mirossay L, Mojzis J. In vitro toxicity of camalexin derivatives in human cancer and non-cancer cells. Toxicol In Vitro. 2013;27:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Chripkova M, Zigo F, Mojzis J. Antiproliferative Effect of Indole Phytoalexins. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47161] [Article Influence: 3368.6] [Reference Citation Analysis (5)] |
| 25. | Izutani Y, Yogosawa S, Sowa Y, Sakai T. Brassinin induces G1 phase arrest through increase of p21 and p27 by inhibition of the phosphatidylinositol 3-kinase signaling pathway in human colon cancer cells. Int J Oncol. 2012;40:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Parsons MJ, Green DR. Mitochondria in cell death. Essays Biochem. 2010;47:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115-128. [PubMed] |
| 29. | Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Lakhani SA, Masud A, Kuida K, Porter GA, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 933] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 31. | Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833:3481-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 818] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 32. | Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 714] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 33. | Basu A, DuBois G, Haldar S. Posttranslational modifications of Bcl2 family members--a potential therapeutic target for human malignancy. Front Biosci. 2006;11:1508-1521. [PubMed] |
| 34. | Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H, Nowakowski GS, Dai H, Kaufmann SH. Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment. Biochim Biophys Acta. 2015;1853:1658-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Tamura Y, Simizu S, Osada H. The phosphorylation status and anti-apoptotic activity of Bcl-2 are regulated by ERK and protein phosphatase 2A on the mitochondria. FEBS Lett. 2004;569:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469-8478. [PubMed] |
| 37. | Yancey D, Nelson KC, Baiz D, Hassan S, Flores A, Pullikuth A, Karpova Y, Axanova L, Moore V, Sui G. BAD dephosphorylation and decreased expression of MCL-1 induce rapid apoptosis in prostate cancer cells. PLoS One. 2013;8:e74561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1432] [Cited by in RCA: 1826] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 39. | Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1289] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 40. | De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S. Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353-21361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Bononi A, Agnoletto C, De Marchi E, Marchi S, Patergnani S, Bonora M, Giorgi C, Missiroli S, Poletti F, Rimessi A. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011;2011:329098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 42. | Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 43. | Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 764] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 44. | Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 45. | Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 917] [Article Influence: 91.7] [Reference Citation Analysis (0)] |