Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4211
Peer-review started: February 1, 2017
First decision: March 16, 2017
Revised: April 8, 2017
Accepted: June 1, 2017
Article in press: June 1, 2017
Published online: June 21, 2017
To compare liver proteolysis and proteasome activation in steatotic liver grafts conserved in University of Wisconsin (UW) and Institut Georges Lopez-1 (IGL-1) solutions.
Fatty liver grafts from male obese Zücker rats were conserved in UW and IGL-1 solutions for 24 h at 4 °Cand subjected to “ex vivo” normo-thermic perfusion (2 h; 37 °C). Liver proteolysis in tissue specimens and perfusate was measured by reverse-phase high performance liquid chromatography. Total free amino acid release was correlated with the activation of the ubiquitin proteasome system (UPS: measured as chymotryptic-like activity and 20S and 19S proteasome), the prevention of liver injury (transaminases), mitochondrial injury (confocal microscopy) and inflammation markers (TNF 1 alpha, high mobility group box-1 (HGMB-1) and PPAR gamma), and liver apoptosis (TUNEL assay, cytochrome c and caspase 3).
Profiles of free AA (alanine, proline, leucine, isoleucine, methionine, lysine, ornithine, and threonine, among others) were similar for tissue and reperfusion effluent. In all cases, the IGL-1 solution showed a significantly higher prevention of proteolysis than UW (P < 0.05) after cold ischemia reperfusion. Livers conserved in IGL-1 presented more effective prevention of ATP-breakdown and more inhibition of UPS activity (measured as chymotryptic-like activity). In addition, the prevention of liver proteolysis and UPS activation correlated with the prevention of liver injury (AST/ALT) and mitochondrial damage (revealed by confocal microscopy findings) as well as with the prevention of inflammatory markers (TNF1alpha and HMGB) after reperfusion. In addition, the liver grafts preserved in IGL-1 showed a significant decrease in liver apoptosis, as shown by TUNEL assay and the reduction of cytochrome c, caspase 3 and P62 levels.
Our comparison of these two preservation solutions suggests that IGL-1 helps to prevent ATP breakdown more effectively than UW and subsequently achieves a higher UPS inhibition and reduced liver proteolysis.
Core tip: Although several reports have confirmed that proteolytic activity during cold storage determines graft outcome after transplantation, the effect of preservation solution on steatotic liver graft proteolysis and on the activation of ATP-dependent proteasome during cold ischemia injury has not been fully investigated. Here, we compared the effect of two preservation solutions Institut Georges Lopez-1(IGL-1) and University of Wisconsin on liver proteolysis and ubiquitin-proteasome activation when steatotic liver grafts were subjected to cold storage. We provide evidence for a protective role of proteasome and proteolysis inhibition using IGL-1 during steatotic liver graft preservation.
- Citation: Zaouali MA, Panisello-Roselló A, Lopez A, Castro Benítez C, Folch-Puy E, García-Gil A, Carbonell T, Adam R, Roselló-Catafau J. Relevance of proteolysis and proteasome activation in fatty liver graft preservation: An Institut Georges Lopez-1 vs University of Wisconsin appraisal. World J Gastroenterol 2017; 23(23): 4211-4221
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4211
Functional graft recovery remains one of the major complications after liver surgery. Cold static preservation is an inherent feature of liver transplantation (LT) and is strongly associated with graft outcome after transplantation[1]. Despite continued attempts to improve preservation solutions, success in liver transplantation is always hampered by the complexity of ischemia reperfusion (I/R) injury[2,3]. In addition, exacerbated I/R injury is due, to a large extent, to the quality of the graft and to its conservation in preservation solutions[4,5]. In the liver, the presence of steatosis makes the graft more vulnerable to cold I/R injury[6] and thus aggravates the detrimental effects of cold I/R injury in fatty liver grafts preserved in commercial solutions.
University of Wisconsin (UW) solution is considered to be the standard solution for liver graft preservation. However, alternative preservation solutions have been used in clinical liver transplantation, such as Institut Georges Lopez-1 (IGL-1), histidine-tryptophan-ketoglutarate (HTK) and Celsior solutions. Briefly, IGL-1 is a new preservation solution whose differences vis-à-vis UW are the oncotic agent used (PEG35, instead of HES) and its lower potassium and lower viscosity. HTK and Celsior solutions have no oncotic agent[2,7].
The ubiquitin-proteasome system (UPS) is the principal non-lysosomal proteolytic system and is thought to contribute to a large variety of pathologies, including I/R injury associated with LT[8-10]. Recently, we showed that UPS modulation is a pharmacological target for improving graft preservation and for reducing I/R injury in the liver[10].
Moreover, it is has been well established that proteolysis is necessary to control protein concentration and to prevent its abnormal accumulation[8]. Proteasomes also perform multiple intracellular functions, such as the degradation of damaged proteins and the modulation of many regulatory proteins that are involved in inflammatory processes including the cell cycle, metabolism, growth and differentiation[8]. In fact, proteolytic activity is necessary for amino acid (AA) recycling of proteins that are no longer needed, thus preventing their accumulation in the cytoplasm[11,12].
The first evidence that proteolysis has a detrimental effect on liver graft out-come after transplantation was provided by Calmus et al[13] who showed that the degree of proteolytic activity detected by the free amino acids in the effluent of human liver grafts is a good predictive marker for postoperative graft function when using UW solution. Later, Upadhya et al[14] proved that the composition of the preservation solution may be relevant for the prevention of liver proteolysis. These authors demonstrated that lactobionate, a component of the UW solution, is a key factor for preventing the release of matrix metalloproteinases, particularly gelatinases, during cold preservation[15]. More recently, other solutions such as IGL-1 have also been considered as potential alternatives to UW[1,16]. Despite the proven efficiency of IGL-1, especially in steatotic liver preservation, its effects on graft proteolysis have not been investigated to date.
It is well known that energy breakdown following oxygen deprivation in liver graft is the main event during cold storage, and that its effects are concomitant with a significant decrease in ATP content which leads to severe graft damage[2]. It was recently reported that this ATP decline may activate a subset of 26S proteasomes, a cell-destructive protease that contributes to myocardial injury during cold ischemia[17,18]. Moreover, this proteasome inhibition contributes to prolonging myocardial viability in hypothermic preservation[19]. Recently, we demonstrated that proteasome inhibitors such as MG132 and bortezomib protected fatty liver grafts when they were used as additives to UW and IGL-1 solutions[10,20]. However, the role of the UPS system and liver proteolysis in fatty liver graft preservation has not been fully investigated.
The aim of this study is to assess the potential relationship between proteolysis, energy breakdown and liver injury using UW and IGL-1 solutions, in order to shed new light on the molecular and cellular mechanisms involved in liver cold I/R injury.
Homozygous (obese [Ob]) Zücker rats aged 16-18 wk were purchased from Iffa-Credo (L’Abresle, France). An “ex vivo” perfused rat liver model was used, as previously described. All procedures were performed under isofluorane inhalation anesthesia according to the European Union regulations (Directive 86/609 EEC) for animal experiments[21].
We used UW (gold standard) and IGL-1 solutions. IGL-1 solution is a modification of UW solution in which hydroxyethyl starch (HES) is substituted by polyethylene glycol 35 (PEG 35) and the ionic K/Na ratio is also reversed.
Briefly, 24 rats were randomly divided into three groups. The abdomen was opened by midline incision, following cannulation of the common bile duct, and the portal vein, the splenic and gastroduodenal veins were ligated. After organ recovery the livers were flushed with UW (UW group) and IGL-1 (IGL-1 group) preservation solutions respectively, and then stored in each solution for 24 h at 4 °C. Next, the preserved livers were flushed with a perfusion liquid consisting of a cell culture medium (William’s medium E, Bio Whitaker, Barcelona, Spain), with a Krebs-Henseleit-like electrolyte composition enriched with 5% albumin as osmotic support. For the reperfusion, livers were connected via the portal vein to a recirculating perfusion system for 2 h at 37 °C. The third study group was a Control group (Cont) in which livers were flushed and immediately perfused ex vivo without ischemic preservation. Time 0 was the point at which the portal catheter was satisfactorily connected to the circuit. During an initial equilibration period of 15 min of perfusion, the flow was progressively increased in order to stabilize the portal pressure at 12 mmHg (Pression Monitor BP-1, Instruments, Inc., Sarasota, FL, United States). In order to maintain the portal pressure at 12 mmHg, the flow rate was modified using a peristaltic pump (Minipuls 3, Gilson, France). The buffer was continuously ventilated with a 95% O2 and 5% CO2 gas mixture. It was subsequently passed through a heat exchanger (37 °C) and a bubble trap prior to entering the liver[21,22].
Protocol I Proteasome activity and ATP levels after 24 h cold storage: In order to evaluate the proteasome activity and ATP breakdown in steatotic liver grafts following 24 h-cold storage in UW or IGL-1, aliquots of the flush effluents and liver tissue samples were collected and stored at -80 °C for subsequent measurement. Control livers (Cont 1 group) were flushed with Ringer’s lactate solution via the portal vein without ischemic preservation.
Protocol II Evaluation of proteasome activity and liver viability after 2 h reperfusion: To examine the role of UW and IGL-1 solutions in proteasome activation and their subsequent effect on proteolysis, liver function and also liver damage, fatty livers were subjected to two hours of normoxic reperfusion. Then, the perfusate effluent and the liver tissue sample were collected and stored at -80 °C for later measurement. In control group (Cont 2) livers were flushed with Ringer’s lactate and immediately perfused ex vivo without ischemic preservation.
Nucleotide analysis and ATP content: Livers were homogenized in perchloric acid solution, and the adenine nucleotide pool was measured by high-performance liquid chromatography (HPLC) as previously reported[23,24].
Assessment of liver proteolysis[7]: Free amino acid content in ex vivo eluates and tissue specimens was measured by HPLC techniques, as previously described[25]. Briefly, effluent and tissue homogenization samples were first deproteinized by ultrafiltration and then derivatized with phenylisothiocyanate (PITC) to produce phenylthiocarbamyl (PTC) amino acids. Amino acids were determined by automated gradient reverse phase HPLC and ultraviolet detection at 254 nm. Quantitative analysis of total free amino acids was performed using the PICO.TAG Amino Acid Analysis System[25].
Transaminase assay: Hepatic injury was evaluated according to transaminase levels using a commercial kit from Boehringer Mannheim (Munich, Germany)[10].
Proteasome chymotryptic-like activity assay[9,10]: ATP-dependent chymotryptic activity of the proteasome was measured using the substrate N-Suc-Leu-Leu-Val-Tyr-aminomethylcoumarin (ENZO Life Sciences). The cleavage products AMC were analyzed in a fluorimeter (excitation/emission 380/460 nm). Product formation was linear with time (at least for 60 min). Background activity (caused by nonproteasomal degradation) was determined by the addition of the proteasome inhibitor epoxomicin at a final concentration of 20 μmol/L (ENZO Life Sciences).
Glutamate dehydrogenase activity[10]: Liver mitochondrial damage was measured by GLDH activity levels at the end of reperfusion, as previously reported.
TNF alpha levels were measured using a commercial immunoassay kit for rat TNF alpha from Biosource (Caramillo)[10,26]. IL-1 beta and IL10 were measured by enzyme-linked immunosorbent assay as previously reported[10,27]. Commercial kits from Amersham LifeScience (Amersham, United Kingdom) were used.
During 2 h of normothermic preservation, fatty livers were perfused with Krebs supplemented with rhodamine 123 (0.11 mg/L, Sigma, R8004) for mitochondrial membrane potential staining and 1% Evans blue dye used as a viability assay on the basis of its penetration into non-viable cells. Fatty livers were then carefully sectioned (0.5 cm3 fragments) and the internal side of the liver was exposed on the glass coverslip mounted on the stage of a Leica TCS SP5 resonant scan multiphoton confocal microscope (Leica Microsystems Heidelberg GmbH) equipped with a HCX IR APO L 25 × water immersion objective (Numerical Aperture 0.95), scanner at 400 lines/s, and a near infrared Titanium:Saphire laser (MaiTai, SpectraPhysics) for two-photon excitation running at 800 nm. Images were acquired with resonant scan at 8000 lines/s. Two-photon excitation was performed at 800 nm and emission of the different fluorescent dyes was captured at the following wavelength ranges: Evans blue dye (515-560 nm), and rhodamine 123 (500-550 nm)[28,29].
Liver tissue was homogenized as described elsewhere[30], and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were immunoblotted with antibodies against 20S5beta and 19S proteasome subunits (BML-PW 8895,and BML-PW8825 respectively, ENZO Life Sciences, Madrid, Spain), PPAR-γ and HMGB-1 (Abcam, United Kingdom), cleaved caspase 3 and cytochrome C (Cell Signaling, Beverly, MA, United States), and β-Actin (Sigma Chemical, St. Louis, MO, United States). Signals were detected by enhanced chemiluminescence and quantified by scanning densitometry[10].
To detect apoptotic cells, 16-μm-thick frozen sections from livers were collected on poly-L-lysine-coated glass slides, and the nuclear DNA fragmentation of apoptotic cells was labeled in situ by the TUNEL method using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Intergen Co. Purchase, NY, United States). Briefly, the sections were fixed in 1% paraformaldehyde in PBS, pH 7.4 for 10 min at room temperature and, after washing in PBS, they were post-fixed in precooled ethanol:acetic acid 2:1 for 5 min at -20 °C. After rinsing in distilled water, the sections were treated with 3% hydrogen peroxide in 10% methanol for 5 min, washed with distilled water and incubated in the equilibration buffer provided for 10 min. Then, the sections were incubated with terminal deoxynucleotide transferase (TdT) in the reaction buffer provided with digoxigenin-dUTP, in a humidifier chamber at 37 °C for 1 h. The incorporated digoxigenin-dUTP was detected by peroxidase-conjugated antidigoxigenin antibody and the signal developed by incubation with 3,3-diamino-benzidine (DAB) in the presence of H2O2. The slides were counterstained with Harris hematoxylin. Negative controls were prepared by replacing the antidigoxigenin antibody with phosphate saline buffer, and a case of breast carcinoma was included as positive control.
Data are expressed as means ± SD and were compared statistically by variance analysis, followed by the Student-Newman-Keuls test. P < 0.05 was considered significant.
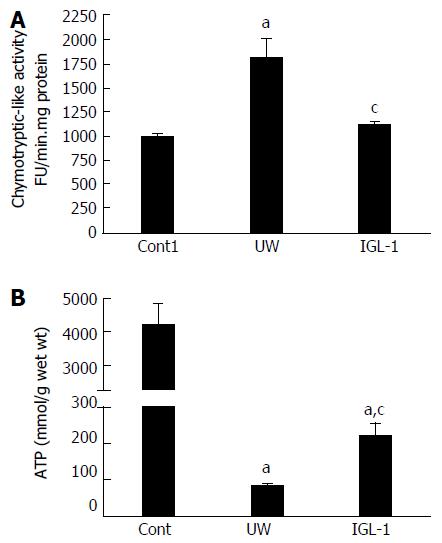
We evaluated the relevance of proteasome activity and proteolysis in fatty livers preserved in IGL-1 and UW solutions when subjected to normothermic reperfusion. As Figure 1A shows, chymotryptic-like proteasome activity increased in steatotic liver grafts during cold preservation in UW solution compared with control non-preserved livers. However, steatotic livers preserved in IGL-1 solution showed lower chymotryptic-like proteasome activity than those preserved in UW solution.
Given the close relationship between proteasome activity and the ATP contents during cold preservation[18], we next evaluated the ATP concentration during liver preservation. Lower ATP levels during cold storage were observed in steatotic livers preserved in UW solution than in non-preserved livers. ATP breakdown was more effectively prevented by the use of IGL-1 solution (Figure 1B).
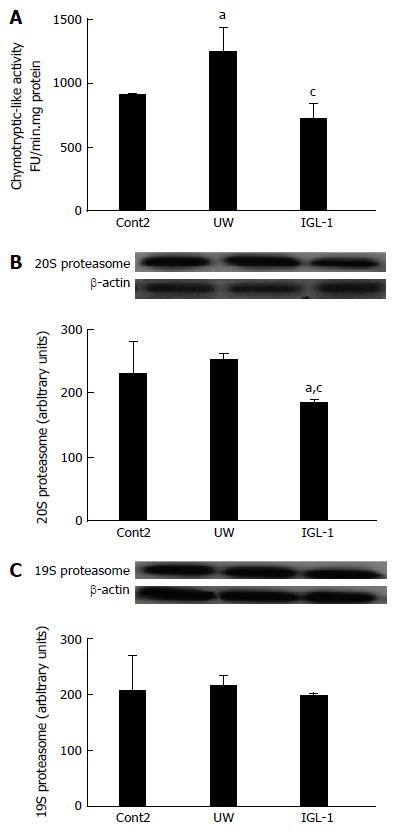
With these results in mind, we also evaluated the chymotryptic-like proteasome activity and the 19S and 20S proteasome protein levels after reperfusion. As indicated in Figure 2A, the chymotryptic-like proteasome activity after reperfusion follows the same pattern profile as those observed for cold storage. The 20S proteasome protein levels were reduced only when IGL-1 preservation solution was used. In contrast, the 19S subset protein levels remained unchanged across all experimental groups (Figure 2B and C).
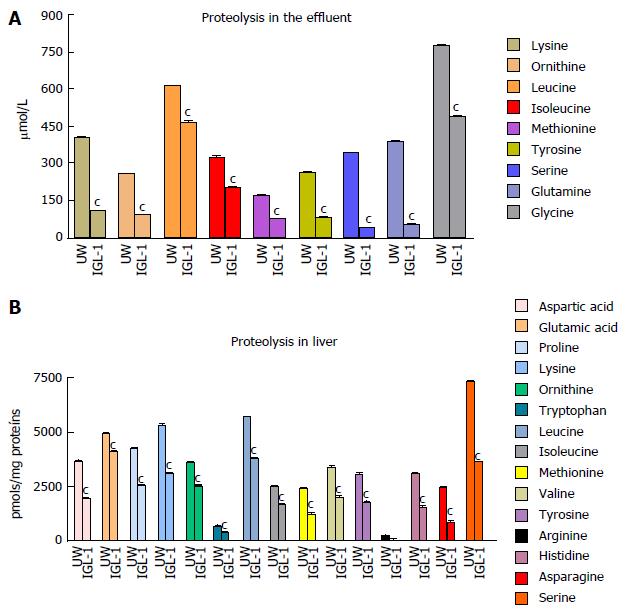
Also, the AA profiling studies confirmed that IGL-1 offered more efficient prevention of AA release in tissue graft specimens and effluents after 2 h-reperfusion at 37 °C. The AA profiles obtained in liver tissue (Figure 3B) and eluate samples (Figure 3A) were similar but were seen more in tissue samples than in ex vivo eluates, thus confirming the relevance of proteolysis (measured as free AA release) after cold I/R injury. The better prevention of liver proteolysis in grafts preserved in IGL-1 solution than in UW was consistent with significant reductions in other parameters associated with the pathophysiology of liver I/R injury, such as transaminases (ALT and AST) and GLDH release as sensitive and specific markers of mitochondrial damage (Table 1).
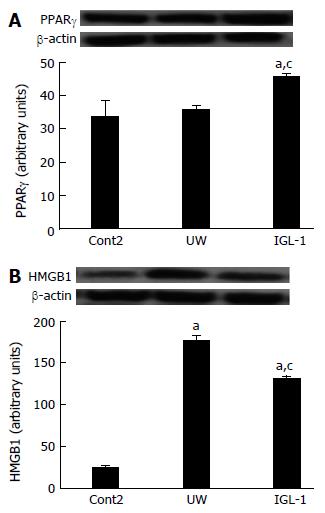
Given that proteasome activity plays a crucial role in the modulation of many of the regulatory proteins involved in inflammatory processes in fatty liver grafts[8], we evaluated the involvement of other inflammatory markers in fatty liver, such as PPARγ, in the proteasome changes and proteolysis inhibition in steatotic liver grafts subjected to cold I/R injury. As shown in Figure 4A, PPARγ protein levels in steatotic liver grafts preserved in UW solution remained unchanged compared with control non preserved grafts, but increased significantly in grafts preserved in IGL-1 preservation solution. We also measured the effect of preservation solution on other cytokines such as high mobility group box 1 (HMGB1) which was recently shown to be involved in fatty liver preservation and transplantation[32]. IGL-1 showed lower levels of HMGB-1 than UW (Figure 4B) concomitant with a significant reduction in the release of other inflammatory cytokines such as TNFα but not for IL1. IGL-1 also increased the concomitant release of anti-inflammatory IL-10 in fatty livers after reperfusion (Table 2).
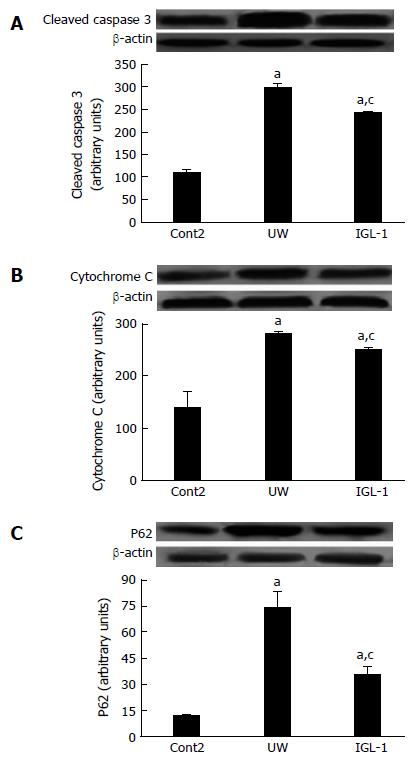
Next, we evaluated the effect of proteasome activity and proteolysis modulation on apoptosis and autophagy induction. Figure 5 shows a significant increase in cytochrome C and cleaved caspase 3 protein levels in steatotic livers preserved in UW solution compared with non-preserved ones. However, preservation in IGL-1 solution significantly reduced both apoptotic markers. In addition, the autophagy-related ubiquitin-binding protein SQSTM1/p62, which is involved in aggresome formation and degradation through autophagy, is increased in steatotic livers preserved in UW solution compared with those preserved in IGL-1 solution (Figure 5C).
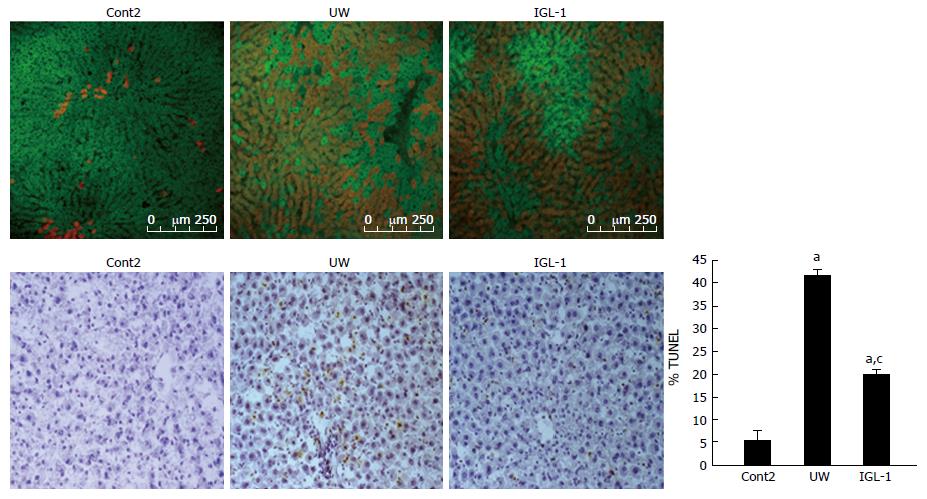
This effect on liver apoptosis in both IGL-1 and UW solutions was also corroborated by the percentage of TUNEL-positive hepatocytes (Figure 6). Only a few sinusoidal lining cells were positive to TUNEL staining in control non-preserved steatotic livers (Figure 6B). After preservation with UW and reperfusion, the number of positive cells significantly increased (Figure 6B). Preservation with IGL-1 reduced apoptotic cell death (Figure 6B). In all cases, single (but not clustered) TUNEL-stained cells were observed more extensively in periportal and mid-zonal areas. Finally, the confocal microscopic study confirmed that steatotic livers preserved in IGL-1 solution conserved the membrane potential of liver mitochondria more efficiently, as shown by an increase in the rhodamine 123 cell viability marker (in green) and a decrease in Evans blue labeling (in red), indicating the albumin content and the disrupted mitochondrial membranes (Figure 6A).
At present, a considerable number of fatty donor livers have to be discarded, a situation that accentuates even further the critical shortage of human donor livers. A better knowledge of the preservation mechanisms of steatotic liver grafts is urgently needed to reduce their high vulnerability to cold I/R injury, and thus to improve their viability after transplantation[33,34].
In this study, we investigated the involvement of UPS activation and liver proteolysis and their relationship with the breakdown energy metabolism in steatotic liver grafts preserved in different commercial preservation solutions such as IGL-1 and UW. We also associated the changes in UPS activation during cold I/R injury with the inflammatory events and liver apoptosis. Our data demonstrate that IGL-1 prevented liver proteolysis more effectively than UW. In all cases, free AA levels determined in tissue specimens and eluates were lower in IGL-1 than in UW after cold I/R. This improved prevention of liver proteolysis with IGL-1 is consistent with its more effective protection against I/R injury. This could be explained, in part, by the presence of different oncotic agents: PEG35 in IGL-1, and HES in UW. In fact, we have recently demonstrated that the addition of PEG35 to washout solution protects the liver against I/R injury by the inhibition of metalloproteinases MMP9 and MMP2, a finding that may explain its role in preventing liver proteolysis[21]. The presence of lactobionate (a common ingredient of the UW and IGL-1 solutions) may also help to prevent liver proteolysis due its strong inhibitory effect on gelatinases, presumably via calcium or zinc chelation[13,14]. This effective prevention of proteolysis is also consistent with the significant reduction in proteasome activity reflected by decreases in chymotryptic-like proteasome activity and 20S proteasome protein levels and the better prevention of energy metabolism breakdown with IGL-1 solution. In fact, a recent report established a functional link between 26S proteasome activity and ATP depletion in tissue during cold I/R injury[18]. Those authors advanced that ATP depletion during ischemic insult appears to activate the 26S proteasome which is formed from a multimeric proteasome core particle (20S proteasome) which is singly or doubly capped at its ends by a 19S regulator complex[17]. Taking this into account, we suggest that the reduced 20S proteasome protein levels in steatotic livers preserved in IGL-1 solution are to do some extent the consequence of the better preservation of ATP content in this group, which thus affects 26S assembly and activity. Furthermore, our results are in accordance with previous studies which have demonstrated the relevance of proteasome inhibition in protecting steatotic liver grafts against I/R injury when preservation solutions were supplemented with proteasome inhibitors MG132 and bortezomib[10,20].
In order to explain the mechanisms by which proteasome modulation and proteolysis inhibition protect steatotic livers against cold I/R injury, we also assessed levels of PPARγ and HMGB-1 proteins, which are both involved in the modulation of the inflammatory response after I/R injury[35-37]. It is clear that PPARγ belongs to the hormone nuclear receptor superfamily of ligand-activated transcription factors which are major regulators of post-ischemic liver injury[35]. Its protective effect is mediated by its anti-inflammatory properties via the inhibition of pro-inflammatory gene expression[35] in which the UPS has recently been implicated; the UPS is responsible for PPAR turnover and is also involved in the modulation of the ligand-dependent activity of these nuclear receptors[38].
The fact that the UPS is the major system for selective degradation of short-lived proteins in eukaryotic cells such as PPARγ[38] suggests that proteasome inhibition after steatotic liver graft preservation in IGL-1 solution may be responsible for the PPARγ accumulation induced, thus leading to a reduction in the expression of pro-inflammatory proteins[39]. These findings were also confirmed by the lower HMGB-1 protein levels in livers preserved in IGL-1 solution than in livers preserved in UW. HMGB-1 is a well-known extracellular signaling pro-inflammatory mediator which, when released from cells, leads to cell death in several pathologies including liver I/R[32]. Our results corroborate those of a previous study which demonstrated that PPARγ-mediated upregulation of miR-142-3p inhibits HMGB-1 expression, which, in turn, is a novel anti-inflammatory mechanism of PPARγ and plays an important role in the treatment of inflammatory diseases[40]. Moreover, HMGB-1 is associated with apoptotic cell death[41] and autophagy modulation[42,43].
Next we evaluated both parameters after cold I/R injury in steatotic livers preserved in UW and IGL-1 solutions. Our results demonstrated that the use of IGL-1 reduced apoptotic cell death, as reflected by decreases in cleaved caspase3 and cytochrome C protein levels when compared with UW solution. The relevance of cytochrome c as a reliable biomarker of mitochondrial damage in fatty liver disease was also reported by another study[44]. These results were correlated with a better prevention of liver mitochondrial damage and were also consistent with the finding that IGL-1 solution efficiently prevented liver apoptosis in rat liver transplantation[45].
Finally, in order to explore the effect of proteasome modulation on autophagy, we determined the levels of autophagy-related ubiquitin-binding protein SQSTM1/p62. This protein is involved in aggresome formation and degradation through autophagy which is associated with the liver graft self-response to cold I/R injury (the SQSTM/p62 substrate that accumulates in autophagy-deficient cells)[46,47]. Our results demonstrated that UW solution increased SQSTM1/p62 protein levels, which are inversely correlated to autophagy, while the use of IGL-1 solution reduced SQSTM1/p62 protein levels, thus showing autophagic activation, as a response to better preservation mechanisms. These results corroborate our previous finding that impaired autophagic clearance after steatotic liver preservation is correlated with increased liver injury[31].
In conclusion, we show that liver graft proteolysis and proteasome activation are dependent on the organ preservation solutions used for liver transplantation such as UW and IGL-1. Our results confirm the relevance of both markers for evaluating the graft damage caused by cold I/R injury in fatty liver preservation.
The authors would like to thank Michael Maudsley at the Language Advisory Service of the University of Barcelona for revising the English text.
Cold ischemia reperfusion (I/R) injury is a multifactorial process that can interfere with graft function after liver transplantation (LT). A better understanding of the pathophysiology of this injury is fundamental for the development of protective strategies able to improve the outcome of LT. The ischemic graft damage that occurs during cold storage of fatty liver grafts depends in part on the ATP-energy breakdown. It is well known that the prevention of ATP-breakdown during cold static preservation is associated with inhibition of ubiquitin-proteasome system (UPS) activity, which helps to protect the liver graft against reperfusion. In this context the selection of commercial organ solution seems crucial for modulating the UPS, as well as the graft proteolysis against cold I/R injury. We demonstrate that Institut Georges Lopez-1 (IGL-1) solution reduces cold I/R injury more efficiently by helping to prevent ATP- breakdown and subsequently achieving a higher UPS inhibition than University of Wisconsin (UW). Both these factors are modulated by the organ preservation solution and determine the degree of proteolysis in the liver graft.
The authors focused on strategies for interfering with the mechanisms responsible for hepatic I/R injury associated with LT, and on strategies for enhancing the endogenous mechanisms that protect against cold ischemic damage. UW and IGL-1 solutions are widely used in LT. They demonstrate that the nature of the oncotic agent present in UW and IGL-1 solutions is responsible, in part, for the modulation of energy breakdown and its subsequent inhibitory action on the UPS, which are key factors in graft protection against I/R insult. In the present study IGL-1 showed more hepato-protective effects than UW, due, in part, to the presence of the oncotic agent PEG-35.
This study demonstrates for the first time that UPS inhibition is a key factor in fatty liver preservation using different commercial organ preservation solutions such as UW and IGL-1. UPS inhibition may explain the better prevention of the proteolysis observed in IGL-1 than in UW, thus favoring the use of IGL-1 in fatty liver graft preservation.
UPS inhibition and the degree of proteolysis can be used to predict the viability of steatotic liver grafts after prolonged static preservation.
The UPS system and proteolysis are involved in the complex pathophysiology of hepatic cold I/R injury. Both are helpful for evaluating the fatty liver preservation using either static or dynamic preservation (with machine perfusion) strategies.
The manuscript is about Relevance of proteolysis and proteasome activation in fatty liver graft preservation. There must be possible prospective achievement in this basic studies. The authors try to solve the problem of preservation of fatty donor liver from ischemic injury in liver transplantation, and the study design was reasonable. The results were also good and provided some scientific hints.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chow WK, Tarantino G S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, Belghiti J, Pezet D, Castaing D, Le Treut YP. Compared efficacy of preservation solutions in liver transplantation: a long-term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Zaouali MA, Ben Abdennebi H, Padrissa-Altés S, Mahfoudh-Boussaid A, Roselló-Catafau J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin Pharmacother. 2010;11:537-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Salahudeen AK. Cold ischemic injury of transplanted organs: some new strategies against an old problem. Am J Transplant. 2004;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | McLaren AJ, Friend PJ. Trends in organ preservation. Transpl Int. 2003;16:701-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Jamieson RW, Friend PJ. Organ reperfusion and preservation. Front Biosci. 2008;13:221-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 520] [Cited by in F6Publishing: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Bejaoui M, Pantazi E, Folch-Puy E, Baptista PM, García-Gil A, Adam R, Roselló-Catafau J. Emerging concepts in liver graft preservation. World J Gastroenterol. 2015;21:396-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 55] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Padrissa-Altés S, Zaouali MA, Bartrons R, Roselló-Catafau J. Ubiquitin-proteasome system inhibitors and AMPK regulation in hepatic cold ischaemia and reperfusion injury: possible mechanisms. Clin Sci (Lond). 2012;123:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Padrissa-Altés S, Zaouali MA, Boncompagni E, Bonaccorsi-Riani E, Carbonell T, Bardag-Gorce F, Oliva J, French SW, Bartrons R, Roselló-Catafau J. The use of a reversible proteasome inhibitor in a model of Reduced-Size Orthotopic Liver transplantation in rats. Exp Mol Pathol. 2012;93:99-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Zaouali MA, Bardag-Gorce F, Carbonell T, Oliva J, Pantazi E, Bejaoui M, Ben Abdennebi H, Rimola A, Roselló-Catafau J. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Exp Mol Pathol. 2013;94:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1279] [Cited by in F6Publishing: 1279] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 12. | Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1530] [Cited by in F6Publishing: 1529] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 13. | Calmus Y, Cynober L, Dousset B, Lim SK, Soubrane O, Conti F, Houssin D, Giboudeau J. Evidence for the detrimental role of proteolysis during liver preservation in humans. Gastroenterology. 1995;108:1510-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Upadhya GA, Strasberg SM. Glutathione, lactobionate, and histidine: cryptic inhibitors of matrix metalloproteinases contained in University of Wisconsin and histidine/tryptophan/ketoglutarate liver preservation solutions. Hepatology. 2000;31:1115-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Upadhya AG, Harvey RP, Howard TK, Lowell JA, Shenoy S, Strasberg SM. Evidence of a role for matrix metalloproteinases in cold preservation injury of the liver in humans and in the rat. Hepatology. 1997;26:922-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Ben Mosbah I, Roselló-Catafau J, Franco-Gou R, Abdennebi HB, Saidane D, Ramella-Virieux S, Boillot O, Peralta C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006;12:1215-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Majetschak M. Regulation of the proteasome by ATP: implications for ischemic myocardial injury and donor heart preservation. Am J Physiol Heart Circ Physiol. 2013;305:H267-H278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Geng Q, Romero J, Saini V, Baker TA, Picken MM, Gamelli RL, Majetschak M. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem Biophys Res Commun. 2009;390:1136-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Baker TA, Geng Q, Romero J, Picken MM, Gamelli RL, Majetschak M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem Biophys Res Commun. 2010;401:548-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Bejaoui M, Zaouali MA, Folch-Puy E, Pantazi E, Bardag-Gorce F, Carbonell T, Oliva J, Rimola A, Abdennebi HB, Roselló-Catafau J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J Pharm Pharmacol. 2014;66:62-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Zaouali MA, Bejaoui M, Calvo M, Folch-Puy E, Pantazi E, Pasut G, Rimola A, Ben Abdennebi H, Adam R, Roselló-Catafau J. Polyethylene glycol rinse solution: an effective way to prevent ischemia-reperfusion injury. World J Gastroenterol. 2014;20:16203-16214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Zaouali MA, Ben Mosbah I, Boncompagni E, Ben Abdennebi H, Mitjavila MT, Bartrons R, Freitas I, Rimola A, Roselló-Catafau J. Hypoxia inducible factor-1alpha accumulation in steatotic liver preservation: role of nitric oxide. World J Gastroenterol. 2010;16:3499-3509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Ben Mosbah I, Casillas-Ramírez A, Xaus C, Serafín A, Roselló-Catafau J, Peralta C. Trimetazidine: is it a promising drug for use in steatotic grafts? World J Gastroenterol. 2006;12:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | v Frankenberg M, Stachlewitz RF, Forman DT, Frey W, Bunzendahl H, Lemasters JJ, Thurman RG. Amino acids in rinse effluents as a predictor of graft function after transplantation of fatty livers in rats. Transpl Int. 1999;12:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Zaouali MA, Padrissa-Altés S, Ben Mosbah I, Ben Abdennebi H, Boillot O, Rimola A, Saidane-Mosbahi D, Roselló-Catafau J. Insulin like growth factor-1 increases fatty liver preservation in IGL-1 solution. World J Gastroenterol. 2010;16:5693-5700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Zaouali MA, Ben Mosbah I, Padrissa-Altés S, Calvo M, Ben Abdennebi H, Saidane-Mosbahi D, Bjaoui M, Garcia-Gil FA, Panisello A, Roselló-Catafau J. Relevance of epidermal growth factor to improve steatotic liver preservation in IGL-1 solution. Transplant Proc. 2010;42:3070-3075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Bejaoui M, Pantazi E, Calvo M, Folch-Puy E, Serafín A, Pasut G, Panisello A, Adam R, Roselló-Catafau J. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid Med Cell Longev. 2016;2016:9096549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 30. | Massip-Salcedo M, Zaouali MA, Padrissa-Altés S, Casillas-Ramirez A, Rodés J, Roselló-Catafau J, Peralta C. Activation of peroxisome proliferator-activated receptor-alpha inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia-reperfusion. Hepatology. 2008;47:461-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Zaouali MA, Boncompagni E, Reiter RJ, Bejaoui M, Freitas I, Pantazi E, Folch-Puy E, Abdennebi HB, Garcia-Gil FA, Roselló-Catafau J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: a role for melatonin and trimetazidine cocktail. J Pineal Res. 2013;55:65-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, Mäkisalo H, Höckerstedt K, Isoniemi H. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver Transpl. 2008;14:1517-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation. 1999;67:195-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Xu C, Yu C, Li Y. Current studies on therapeutic approaches for ischemia/reperfusion injury in steatotic livers. Hepatol Res. 2008;38:851-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Elias-Miró M, Jiménez-Castro MB, Mendes-Braz M, Casillas-Ramírez A, Peralta C. The Current Knowledge of the Role of PPAR in Hepatic Ischemia-Reperfusion Injury. PPAR Res. 2012;2012:802384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Liu A, Dirsch O, Fang H, Dong W, Jin H, Huang H, Sun J, Dahmen U. HMGB1 translocation and expression is caused by warm ischemia reperfusion injury, but not by partial hepatectomy in rats. Exp Mol Pathol. 2011;91:502-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Liu A, Dirsch O, Fang H, Sun J, Jin H, Dong W, Dahmen U. HMGB1 in ischemic and non-ischemic liver after selective warm ischemia/reperfusion in rat. Histochem Cell Biol. 2011;135:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Genini D, Catapano CV. Control of peroxisome proliferator-activated receptor fate by the ubiquitinproteasome system. J Recept Signal Transduct Res. 2006;26:679-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Yuan ZY, Liu Y, Liu Y, Zhang JJ, Kishimoto C, Wang YN, Ma AQ, Liu ZQ. PPAR-gamma ligands inhibit the expression of inflammatory cytokines and attenuate autoimmune myocarditis. Chin Med J (Engl). 2004;117:1253-1255. [PubMed] [Cited in This Article: ] |
| 40. | Yuan Z, Luo G, Li X, Chen J, Wu J, Peng Y. PPARγ inhibits HMGB1 expression through upregulation of miR-142-3p in vitro and in vivo. Cell Signal. 2016;28:158-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Liu A, Jin H, Dirsch O, Deng M, Huang H, Bröcker-Preuss M, Dahmen U. Release of danger signals during ischemic storage of the liver: a potential marker of organ damage? Mediators Inflamm. 2010;2010:436145. [PubMed] [Cited in This Article: ] |
| 42. | Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 646] [Cited by in F6Publishing: 723] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 43. | Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1536] [Cited by in F6Publishing: 1754] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 44. | Tarantino G, Colao A, Capone D, Conca P, Tarantino M, Grimaldi E, Chianese D, Finelli C, Contaldo F, Scopacasa F. Circulating levels of cytochrome C, gamma-glutamyl transferase, triglycerides and unconjugated bilirubin in overweight/obese patients with non-alcoholic fatty liver disease. J Biol Regul Homeost Agents. 2011;25:47-56. [PubMed] [Cited in This Article: ] |
| 45. | Mosbah IB, Zaouali MA, Martel C, Bjaoui M, Abdennebi HB, Hotter G, Brenner C, Roselló-Catafau J. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death Dis. 2012;3:e279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Gustafsson AB, Gottlieb RA. Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4:416-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Korolchuk VI, Menzies FM, Rubinsztein DC. A novel link between autophagy and the ubiquitin-proteasome system. Autophagy. 2009;5:862-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |