Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3488
Peer-review started: January 3, 2017
First decision: February 10, 2017
Revised: February 25, 2017
Accepted: April 21, 2017
Article in press: April 21, 2017
Published online: May 21, 2017
Processing time: 141 Days and 23.1 Hours
To assess the relationship between serum levels of insulin-like growth factor-1 (IGF1)/IGF-binding protein-3 (IGFBP3) and the risk of esophageal carcinoma.
We assessed the relationship between the serum levels of these molecules and the risk of esophageal cancer in a prospective, nested case-control study of participants from the Japan Collaborative Cohort Study. A baseline survey was conducted from 1988 to 1990. Of the 110585 enrolled participants, 35% donated blood samples. Those who had been diagnosed with esophageal cancer were considered cases for nested case-control studies. A conditional logistic model was used to estimate odds ratios for the incidence of esophageal cancer associated with serum IGF1 and IGFBP3 levels.
Thirty-one cases and 86 controls were eligible for the present assessment. The molar ratio of IGF1/IGFBP3, which represents the free and active form of IGF1, was not correlated with the risk of esophageal carcinoma. A higher molar difference between IGFBP3 and IGF1, which estimates the free form of IGFBP3, was associated with a decreased risk of esophageal carcinoma (P = 0.0146), and people in the highest tertile had the lowest risk (OR = 0.107, 95%CI: 0.017-0.669). After adjustment for body mass index, tobacco use, and alcohol intake, the molar difference of IGFBP3-IGF1 was inversely correlated with the risk of esophageal carcinoma (P = 0.0150).
The free form of IGFBP3, which is estimated by this molar difference, may be inversely associated with esophageal cancer incidence.
Core tip: Insulin-like growth factor-1 (IGF1) is a potent mitogen, whereas IGF-binding protein-3 (IGFBP3) binds and inhibits IGF1. High circulating IGF1 and low IGFBP3 are associated with increased risk of several cancers. Here we assessed the relationship between these molecules and the risk of esophageal carcinoma in a prospective, nested case-control study from the Japan Collaborative Cohort Study. Free IGF1, represented by the molar ratio of IGF1/IGFBP3, was not correlated with the risk of esophageal carcinoma. The free form of IGFBP3, which is estimated by the molar difference of IGFBP3-IGF1, may be inversely associated with esophageal cancer incidence.
- Citation: Adachi Y, Nojima M, Mori M, Yamashita K, Yamano HO, Nakase H, Endo T, Wakai K, Sakata K, Tamakoshi A. Insulin-like growth factor-1, IGF binding protein-3, and the risk of esophageal cancer in a nested case-control study. World J Gastroenterol 2017; 23(19): 3488-3495
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3488
Esophageal carcinoma is one of the worst prognostic neoplasms internationally[1,2]. The two main types of esophageal neoplasms are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). When diagnoses were made, most patients have either metastatic or unresectable cancers. Even if the patient received curative-intent surgery, the rest of time is still limited, and therapies for inoperable esophageal tumors are not so effective. Therefore, we must search for new risk factors for esophageal neoplasms to identify people who may develop early stages of the disease whose cancer is thus more readily treatable.
Several growth factor signals are needed for tumorigenesis and cancer progression[3,4]. The insulin-like growth factor (IGF) system, which includes both the IGF1 and IGF2 ligands and the type 1 insulin-like growth factor receptor (IGF1R), may be an important molecular target for cancer therapy[4-6]. After ligands bind to IGF1R, the receptor is autophosphorylated, and then several downstream signal pathways are activated[7]. IGFs are secreted by hepatocytes and by several extra-hepatic components, such as cancer and stromal cells[8]. In the homeostatic condition, the IGF axis is tightly regulated by multiple ways[9]. The production of IGFs and IGF-binding proteins (IGFBPs) 1-6 is regulated by growth hormone, which is produced in the pituitary gland. IGF1R activation is controlled by the quantity of the free IGFs, which is modulated by those binding proteins and the nonstimulatory type 2 IGF receptor[7,10]. In serum, almost all of IGF1 is inactive and bound with IGFBPs, which make a complex with IGF in a 1:1 molar ratio. IGFBP3 is the most plentiful binding protein and occupies around 80% of all IGF complexes. The IGF-IGFBP complex balance is controlled by proteases, including matrix metalloproteinase[11]. Furthermore, IGFBPs have IGF-independent activities, however, these actions are not understood well[10,12].
Normal esophageal epithelium express IGF1R, and IGFs could induce both cellular proliferation and DNA synthesis[13-15]. Salivary IGF1 is in the free form (not bound to IGFBP, unlike the serum pool) and continuously bathes the esophageal epithelial cells[16]. These data might suggest that the IGF system plays important parts not only in homeostasis but also premalignancy[15]. Both IGF1R and its ligands are overexpressed in esophageal tumor components compared to normal ones[17-19], and both serum levels of IGF1 and IGFBP3 are significantly elevated in patients with esophageal cancer compared with healthy subjects[20,21]. Previously we revealed that expressions of IGF2 and IGF1R were detected in 50% and 60% of ESCC, respectively, and both molecules were related with advanced tumor stage, invasion depth, metastasis, and recurrence[22]. Multivariate analysis revealed that patients with ESCC expressing both IGF2 and IGF1R show a significantly shorter survival than those expressing only one molecule or neither. A meta-analysis revealed that the expression of IGF1R is correlated with unfavorable prognosis in patients with EAC[23]. IGF1R blockers, including antibodies, tyrosine kinase inhibitors, IGF1R dominant negative, and IGFBP3, suppress proliferation and up-regulate apoptosis[22,24-26].
Elevated serum concentration of total-IGF1 or free-IGF1 levels, which could be calculated by the molar ratio of IGF1/IGFBP3, increase the future risk of several malignancies, including breast, colon, and prostate[27-29]. Additionally, low serum levels of total-IGFBP3 or free-IGFBP3, which can be approximated by the molar difference (IGFBP3-IGF1), upregulate the future risk of neoplasms[29,30]. However, the association between the risk of esophageal carcinoma and serum levels of IGF related compounds has not been reported. Although several correlations between the risk of cancer death and IGF axial proteins from the Japan Collaborative Cohort (JACC) Study were published[30-32], the incidence of esophageal carcinoma has not been reported. Therefore, we assessed relationships between these parameters and esophageal tumor risk in a nested, case-control study in a prospective cohort study of participants in the JACC study.
We assessed data of the JACC Study, in which cancer risk associated with lifestyle factors in a Japanese population was evaluated. The study was described in detail elsewhere[33-35]. Briefly, a baseline survey was conducted between 1988 and 1990. A total of 110,585 participants aged 40 to 79 years from 45 areas throughout Japan were enrolled in the study and completed a self-administered questionnaire.
Informed consent was obtained from all participants. The ethical board of the Nagoya University School of Medicine approved this study.
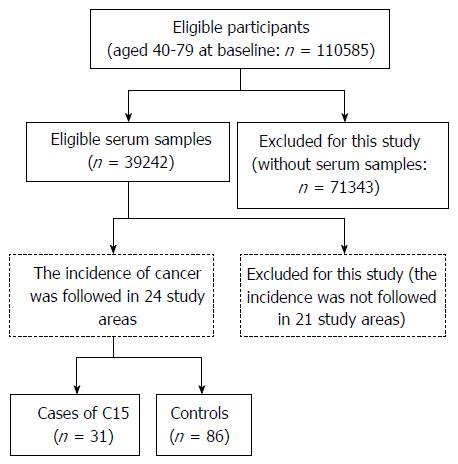
Approximately 35% of the cohort participants (39242 subjects) provided blood samples, which were stored at -80 °C until analyzed.
The incidence of cancer was followed in subjects living in 24 study areas beginning at the time of the baseline survey (Figure 1). Individuals who moved away from the study district were treated as dropouts, because deaths after such moves could not be detected in our system for follow-up. Participants with a history of malignant tumor at baseline were excluded. The malignancy was confirmed in population-based cancer registries or by reviewing the records of local major hospitals. We defined esophageal cancer as C15 (malignant neoplasm of esophagus) according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (http://www.who.int/classifications/icd/en/). Subjects diagnosed with esophageal carcinoma by 1997 were regarded as cases for this nested case-control study. For each case, we randomly selected three or two controls that were matched for residential area, gender, and age (as near as possible). A total of 31 cases and 86 control participants were eligible for this study.
The trained staff who blinded to case-control status, measured all samples at a single laboratory (SRL, Tokyo, Japan) in 1999 and 2000. Serum levels of IGF1 and IGFBP3 were analyzed with an immunoradiometric assay using commercially available kits (Daiichi Radioisotope Lab., Tokyo, Japan). Details of the assay were reported previously[36].
Proportions and mean values of baseline characteristics between cases and controls were assessed by a t-test or Fisher’s exact test. The cross-sectional relationship between serum IGF1 and IGFBP3 was examined using the Spearman correlation coefficient. Serum values were divided into tertiles based on the distribution of serum values in all control subjects, with the first tertile used as a reference. IGF1 tertile values for tertiles 1, 2, and 3 were < 120; 120-150; and > 150.0 ng/mL, respectively. IGFBP3 tertile values for tertiles 1, 2, and 3 were < 2.88; 2.88-3.55; and > 3.55 ng/mL, respectively.
The odds ratios (ORs) for the incidence of esophageal carcinoma associated with serum IGF-related protein levels were estimated using conditional logistic regression. ORs were adjusted for alcohol intake, body mass index (BMI, computed as weight in kilograms divided by the square of the height in meters), and tobacco smoking habit. The statistical significance of trends across exposure tertiles was assessed by including ordinal terms for each serum level tertile and entering the variable as a continuous term in the model. All P values and 95%CI presented in the tables were based on two-sided tests.
Because the molar ratio of IGF1 to IGFBP3 is believed to stand for free form IGF1, we also assessed the molar ratio IGF1/IGFBP3 (for conversion, 1 ng/mL is 0.130 nmol/L for IGFI and 0.036 nmol/L for IGFBP3)[29]. In addition, we assessed the molar difference between IGFBP3 and IGF1, which is considered to represent free IGFBP3[30].
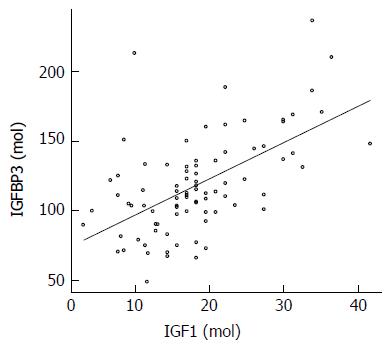
Baseline characteristics are shown in Table 1. No differences were found in height, weight, BMI, smoking habits, or alcohol consumption between case and control groups. The mean concentration of IGF1 tended to be higher in the control group, but the difference was not significant. The mean concentration of IGFBP3 was significantly higher in the control group than in the cases. Spearman correlation coefficient between IGFBP3 and IGF1 among controls is 0.541 (P < 0.0001; Figure 2).
| Cases | Controls | P value | |
| Number of subjects | 31 | 86 | |
| Age | 61.5 ± 7.3 | 61.3 ± 7.2 | 0.811 |
| Male | 25 (80.6) | 75 (87.2) | 0.3831 |
| Height | 162.5 ± 10.5 | 161.5 ± 5.3 | 0.495 |
| Weight | 57.0 ± 12.9 | 59.0 ± 9.1 | 0.352 |
| BMI (kg/m2) | 21.5 ± 4.2 | 22.7 ± 3.1 | 0.112 |
| Cigarette smoking | 30 | 77 | 0.3841 |
| Never | 6 (20.0) | 25 (32.5) | |
| Past | 5 (16.7) | 14 (18.2) | |
| Current | 19 (63.3) | 38 (49.3) | |
| Alcohol intake | 29 | 84 | 0.4101 |
| Never | 6 (20.7) | 21 (25.0) | |
| Past | 3 (10.3) | 3 (3.6) | |
| Current | 20 (69.0) | 60 (71.4) | |
| IGF1 | 122.4 ± 59.9 | 140.8 ± 59.8 | 0.145 |
| IGFBP3 | 2.82 ± 0.95 | 3.30 ± 0.97 | 0.020 |
Both concentrations of total IGF1 and total IGFBP3 were inversely associated the risk of esophageal carcinoma (P = 0.036 and 0.024, respectively, Table 2). After adjusted for BMI, cigarette smoking, and alcohol intake, the latter still showed inverted relation with the risk of esophageal tumor but the former did not (P = 0.042 and 0.052, respectively). After adjusted for the concentration of IGFBP3, total IGF1 was not associated with the risk of esophageal carcinoma. After adjusted for the concentration of IGF1, total IGFBP3 was not associated with the risk of esophageal carcinoma. After adjustment for alcohol consumption, BMI, and smoking status, and each other, both serum levels of IGF1 and IGFBP3 were not also related with the risk of esophageal cancer.
| Tertile | P value | |||
| 1 (referent) | 2 | 3 | ||
| IGF1 | ||||
| ng/mL (range) | < 120 | 120-150 | > 150 | |
| No. of case/control | 17/27 | 8/33 | 6/26 | |
| OR (95%CI) | 1 | 0.300 (0.098-0.913) | 0.263 (0.068-1.011) | 0.036 |
| OR adjusted 1 (95%CI) | 1 | 0.292 (0.087-0.982) | 0.265 (0.064-1.092) | 0.052 |
| OR adjusted 2 (95%CI) | 1 | 0.281 (0.077-1.023) | 0.387 (0.071-2.112) | 0.247 |
| OR adjusted 3 (95%CI) | 1 | 0.298 (0.080-1.109) | 0.417 (0.074-2.348) | 0.249 |
| IGFBP3 | ||||
| ng/mL (range) | < 2.88 | 2.88-3.55 | > 3.55 | |
| No. of case/control | 18/30 | 9/27 | 4/29 | |
| OR (95%CI) | 1 | 0.518 (0.171-1.564) | 0.133 (0.023-0.786) | 0.024 |
| OR adjusted 1 (95%CI) | 1 | 0.527 (0.155-1.792) | 0.137 (0.021-0.911) | 0.042 |
| OR adjusted 4 (95%CI) | 1 | 0.914 (0.241-3.468) | 0.182 (0.025-1.329) | 0.140 |
| OR adjusted 5 (95%CI) | 1 | 0.853 (0.210-3.468) | 0.192 (0.023-1.585) | 0.196 |
A lower molar ratio of IGF1/IGFBP3, which means free IGF1, tended to be correlated with a decreased risk of esophageal cancer but the difference was not statistically significant (P = 0.273; Table 3). After adjustment for BMI, smoking status, and alcohol intake, the association was not observed (P = 0.408).
| Tertile | P value | |||
| 1 (referent) | 2 | 3 | ||
| IGF1/IGFBP3 | ||||
| Molar ratio | < 0.137 | 0.137-0.177 | > 0.177 | |
| No. of case/control | 9/29 | 11/28 | 11/29 | |
| OR (95%CI) | 1 | 1.733 (0.517-5.851) | 2.127 (0.554-8.164) | 0.273 |
| OR adjusted (95%CI) | 1 | 1.486 (0.416-5.309) | 1.810 (0.453-7.226) | 0.408 |
| IGFBP3-IGF1 | ||||
| Molar difference | < 87.77 | 87.77-108.14 | > 108.14 | |
| No. of case/control | 18/29 | 9/28 | 4/29 | |
| OR (95%CI) | 1 | 0.432 (0.137-1.262) | 0.107 (0.017-0.669) | 0.015 |
| OR adjusted (95%CI) | 1 | 0.380 (0.115-1.250) | 0.100 (0.015-0.674) | 0.015 |
A lower molar difference of (IGFBP3-IGF1), which means free-form IGFBP3, was associated with an increased risk of esophageal carcinoma (P = 0.015; Table 3). After adjustment for BMI, smoking habit, and drinking status, the association was also observed (P = 0.015). The highest tertile of this molar difference showed the lowest risk of esophageal neoplasm (OR = 0.100, 95%CI: 0.015-0.674). Thus, the molecular difference of (IGFBP3-IGF1) might most precisely stand for the future risk of esophageal carcinoma in the current study.
In order to assess the interaction with age and gender, ORs were analyzed in subgroups. Higher free IGFBP3 was also related to a decreased risk of esophageal cancer in the male population (P = 0.011; Table 4). After adjustment for BMI, tobacco use, and alcohol intake, this association was confirmed (P = 0.004). However, we found no association between free IGFBP3 and the risk of esophageal cancer in the female population (P = 0.517), as the number of cases was too low. In non-elderly participants (population ≤ 65 years old), a higher molar difference (IGFBP3-IGF1) was related to a reduced risk of esophageal carcinoma (P = 0.007). After adjustment for BMI, tobacco use, and alcohol intake, this relationship was confirmed (P = 0.007). However, this correlation was not observed in elderly individuals (population > 65 years old, P = 0.696).
| Tertile | P value | |||
| 1 (referent) | 2 | 3 | ||
| Molar difference | < 87.77 | 87.77-108.14 | > 108.14 | |
| Male | ||||
| No. of case/control | 15 / 28 | 7/22 | 3/25 | |
| OR (95%CI) | 1 | 0.339 (0.085-1.349) | 0.044 (0.004-0.527) | 0.011 |
| OR adjusted (95%CI) | 1 | 0.186 (0.034-1.015) | 0.022 (0.001-0.319) | 0.004 |
| ≤ 65 years old | ||||
| No. of case/control | 11/14 | 7/21 | 3/27 | |
| OR (95%CI) | 1 | 0.260 (0.052-1.300) | 0.031 (0.025-0.400) | 0.007 |
| OR adjusted (95%CI) | 1 | 0.226 (0.042-1.224) | 0.028 (0.002-0.389) | 0.007 |
High serum concentrations of IGF1 and low IBFBP3 levels are risk factors for several malignancies[27-29]. Furthermore, IGFs take several parts in tumorigenesis and cancer development of esophageal carcinoma, and IGFBPs could inhibit those effects of IGF ligands[4,5,22,26]. In the present analysis, neither serum levels of IGF1 nor IGFBP3 were related to the OR for esophageal cancer, after adjustment for each other. Moreover, serum free IGF1 did not show any association with esophageal tumor risk.
Salivary IGF1 continuously bathes the esophageal lumen and exists in a free and active form[16]. Thus, the local concentration of IGF ligands around the esophageal mucosal cells may be high, even though the serum concentrations are low. Salivary IGF1 could have higher binding ability to IGF1R on esophageal mucosal cells. This may be a reason why serum concentrations of IGF1 did not show significant association with the risk of esophageal carcinoma.
In serum, IGF and the binding protein make a complex in a 1:1 molar ratio, and the molar level of IGFBP3 is more than that of IGF1. Hence, the molar difference of (IGFBP3-IGF1) could represent the level of the free type of IGFBP3[30]. The molar difference of IGFBP3-IGF1 was inversely and significantly correlated with the future risk of esophageal cancer. After adjustment for alcohol intake, BMI, and cigarette smoking, this molar difference was still significantly correlated with an inverse risk of esophageal carcinoma. Analyses of non-elderly or male subgroups might confirm that a high molar difference of (IGFBP3-IGF1) showed a low risk of esophageal tumors. Therefore, this parameter may be a candidate predictive marker of esophageal cancer. Moreover, we have reported that free IGFBP3 showed a reversed risk for hepatic cancer[30]. Thus, the molar difference of IGFBP3-IGF1 may be an important parameter.
As serum IGF1 levels were higher in viscerally obese patients with esophageal cancer than non-obese patients[21,37], visceral obesity may influence the IGF axis. Although this observation is seen in EAC in particular, more people have ESCC than EAC in Japan, as in Eastern Asia. Although our current study did not collect data about visceral obesity, free IGFBP3 was inversely associated with the risk of esophageal cancer after adjustment including BMI.
In a population-based case-control study, three polymorphisms in IGF and related genes such as IGF1 (CA)17 185-bp allele and two single-nucleotide polymorphisms, IGF1 rs6214 and growth hormone receptor rs6898743, were associated with EAC or its precursors, including reflux esophagitis and Barrett esophagus[38]. These results also suggest that the IGF pathway may be involved in EAC development.
The advantage of the current analysis is that the subjects were from a large-scale JACC study of 110792 participants. One limitation is, however, that some data about alcohol intake, BMI, and smoking habit were lost due to the self-administered survey[33]. Another is that both the numbers of serum samples and esophageal tumor cases were not so large (39242 subjects and 31 cases, respectively).
Our result might suggest that low free IGFBP3, estimated as the molar difference of IGFBP3-IGF1, represents an important predictive marker of a high incidence of esophageal carcinoma.
Insulin-like growth factor-1 (IGF1) is a potent mitogen, whereas IGF-binding protein-3 (IGFBP3) binds and inhibits IGF1. High circulating IGF1 and low IGFBP3 are associated with increased risk of several cancers. However there are limited information about the relationship between the serum levels of these molecules and the risk of esophageal carcinoma.
The authors assessed the relationship between the serum levels of IGF1 and IGFBP3 and the risk of esophageal carcinoma in a prospective, nested case-control study. They introduced a new concept of the free IGFBP3, which is estimated by molar difference IGFBP3 from IGF1.
The free form of IGFBP3, which is estimated by the molar difference of IGFBP3-IGF1, may be inversely correlated with the incidence of esophageal cancer. However, free IGF1, which is represented by the molar ratio of IGF1/IGFBP3, was not associated with esophageal carcinoma risk.
People with low free form of IGFBP3 might have higher future risk of esophageal carcinoma. However, the number of case was not so much, thus larger studies are needed. Moreover, some intervention studies for people with low free form of IGFBP3 are needed.
The free form of IGFBP3 is estimated by the molar difference of IGFBP3-IGF1. The free form of IGF1 is represented by the molar ratio of IGF1/IGFBP3.
This is an interesting and valuable article in exploring the association between esophageal carcinoma, IGF1 and IGFBP3. This is the merit and value of the paper that can be referred and cited by other studies in future when the results are found. However, there are several concerns that should be clarified. It is the concern on the matter for the interested readers who can be involved in this research and can repeatedly practice it in future. I here illustrate some that are unclear, non-understandable, and non-readable letting shortcomings clearly limit the contribution of the paper.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chien TWS S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [PubMed] |
| 2. | Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933-7943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 554] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (15)] |
| 4. | Adachi Y, Yamamoto H, Ohashi H, Endo T, Carbone DP, Imai K, Shinomura Y. A candidate targeting molecule of insulin-like growth factor-I receptor for gastrointestinal cancers. World J Gastroenterol. 2010;16:5779-5789. [PubMed] [DOI] [Full Text] |
| 5. | Adachi Y, Yamamoto H, Imsumran A, Oka T, Oki M, Nosho K, Min Y, Shinomura Y, Lee CT, Carbone DP. Insulin-like growth factor-I receptor as a candidate for a novel molecular target in the gastrointestinal cancers. Dig Endosc. 2006;18:245-251. |
| 6. | Yee D. A tale of two receptors: insulin and insulin-like growth factor signaling in cancer. Clin Cancer Res. 2015;21:667-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145-153. [PubMed] |
| 8. | Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324-7329. [PubMed] |
| 9. | Gu F, Schumacher FR, Canzian F, Allen NE, Albanes D, Berg CD, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Buring JE. Eighteen insulin-like growth factor pathway genes, circulating levels of IGF-I and its binding protein, and risk of prostate and breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2877-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1316] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 11. | Miyamoto S, Nakamura M, Yano K, Ishii G, Hasebe T, Endoh Y, Sangai T, Maeda H, Shi-Chuang Z, Chiba T. Matrix metalloproteinase-7 triggers the matricrine action of insulin-like growth factor-II via proteinase activity on insulin-like growth factor binding protein 2 in the extracellular matrix. Cancer Sci. 2007;98:685-691. [PubMed] |
| 12. | Takaoka M, Kim SH, Okawa T, Michaylira CZ, Stairs DB, Johnstone CN, Andl CD, Rhoades B, Lee JJ, Klein-Szanto AJ. IGFBP-3 regulates esophageal tumor growth through IGF-dependent and independent mechanisms. Cancer Biol Ther. 2007;6:534-540. [PubMed] |
| 13. | Vinayek R, Pichney LS, Tantry U, Dutta SK, Resau J, Vengurlekar S. Characterization of insulin-like growth factor I receptors in human esophageal epithelial cells. Am J Physiol. 1994;267:G105-G114. [PubMed] |
| 14. | Qureshi FG, Tchorzewski MT, Duncan MD, Harmon JW. EGF and IGF-I synergistically stimulate proliferation of human esophageal epithelial cells. J Surg Res. 1997;69:354-358. [PubMed] |
| 15. | Tchorzewski MT, Qureshi FG, Duncan MD, Duncan KL, Saini N, Harmon JW. Role of insulin-like growth factor-I in esophageal mucosal healing processes. J Lab Clin Med. 1998;132:134-141. [PubMed] |
| 16. | Costigan DC, Guyda HJ, Posner BI. Free insulin-like growth factor I (IGF-I) and IGF-II in human saliva. J Clin Endocrinol Metab. 1988;66:1014-1018. [PubMed] |
| 17. | Mori M, Inoue H, Shiraishi T, Mimori K, Shibuta K, Nakashima H, Mafune K, Tanaka Y, Ueo H, Barnard GF. Relaxation of insulin-like growth factor 2 gene imprinting in esophageal cancer. Int J Cancer. 1996;68:441-446. [PubMed] |
| 18. | Liu YC, Leu CM, Wong FH, Fong WS, Chen SC, Chang C, Hu CP. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J Biomed Sci. 2002;9:665-674. [PubMed] |
| 19. | Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803-808. [PubMed] |
| 20. | Sohda M, Kato H, Miyazaki T, Nakajima M, Fukuchi M, Manda R, Fukai Y, Masuda N, Kuwano H. The role of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 in human esophageal cancer. Anticancer Res. 2004;24:3029-3034. [PubMed] |
| 21. | Doyle SL, Donohoe CL, Finn SP, Howard JM, Lithander FE, Reynolds JV, Pidgeon GP, Lysaght J. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol. 2012;107:196-204. [PubMed] |
| 22. | Imsumran A, Adachi Y, Yamamoto H, Li R, Wang Y, Min Y, Piao W, Nosho K, Arimura Y, Shinomura Y. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis. 2007;28:947-956. [PubMed] |
| 23. | Nagaraja V, Eslick GD. Forthcoming prognostic markers for esophageal cancer: a systematic review and meta-analysis. J Gastrointest Oncol. 2014;5:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 24. | Chen SC, Chou CK, Wong FH, Chang CM, Hu CP. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and autocrine stimulation in human esophageal carcinoma cells. Cancer Res. 1991;51:1898-1903. [PubMed] |
| 25. | Takaoka M, Harada H, Andl CD, Oyama K, Naomoto Y, Dempsey KL, Klein-Szanto AJ, El-Deiry WS, Grimberg A, Nakagawa H. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711-7723. [PubMed] |
| 26. | Adachi Y, Ohashi H, Imsumran A, Yamamoto H, Matsunaga Y, Taniguchi H, Nosho K, Suzuki H, Sasaki Y, Arimura Y. The effect of IGF-I receptor blockade for human esophageal squamous cell carcinoma and adenocarcinoma. Tumour Biol. 2014;35:973-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563-566. [PubMed] |
| 28. | Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393-1396. [PubMed] |
| 29. | Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620-625. [PubMed] |
| 30. | Adachi Y, Nojima M, Mori M, Matsunaga Y, Akutsu N, Sasaki S, Endo T, Kurozawa Y, Wakai K, Tamakoshi A. Insulin-like growth factor-related components and the risk of liver cancer in a nested case-control study. Tumour Biol. 2016;37:15125-15132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Lin Y, Tamakoshi A, Kikuchi S, Yagyu K, Obata Y, Ishibashi T, Kawamura T, Inaba Y, Kurosawa M, Motohashi Y. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the risk of pancreatic cancer death. Int J Cancer. 2004;110:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Sakauchi F, Nojima M, Mori M, Wakai K, Suzuki S, Tamakoshi A, Ito Y, Watanabe Y, Inaba Y, Tajima K. Serum insulin-like growth factors I and II, insulin-like growth factor binding protein-3 and risk of breast cancer in the Japan Collaborative Cohort study. Asian Pac J Cancer Prev. 2009;10 Suppl:51-55. [PubMed] |
| 33. | Ohno Y, Tamakoshi A. Japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (JACC study). J Epidemiol. 2001;11:144-150. [PubMed] |
| 34. | Tamakoshi A, Yoshimura T, Inaba Y, Ito Y, Watanabe Y, Fukuda K, Iso H. Profile of the JACC study. J Epidemiol. 2005;15 Suppl 1:S4-S8. [PubMed] |
| 35. | Tamakoshi A, Ozasa K, Fujino Y, Suzuki K, Sakata K, Mori M, Kikuchi S, Iso H, Sakauchi F, Motohashi Y. Cohort profile of the Japan Collaborative Cohort Study at final follow-up. J Epidemiol. 2013;23:227-232. [PubMed] |
| 36. | Ito Y, Nakachi K, Imai K, Hashimoto S, Watanabe Y, Inaba Y, Tamakoshi A, Yoshimura T. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble Fas, and superoxide dismutase activity for the JACC study. J Epidemiol. 2005;15 Suppl 1:S67-S73. [PubMed] |
| 37. | Donohoe CL, Doyle SL, McGarrigle S, Cathcart MC, Daly E, O’Grady A, Lysaght J, Pidgeon GP, Reynolds JV. Role of the insulin-like growth factor 1 axis and visceral adiposity in oesophageal adenocarcinoma. Br J Surg. 2012;99:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | McElholm AR, McKnight AJ, Patterson CC, Johnston BT, Hardie LJ, Murray LJ. A population-based study of IGF axis polymorphisms and the esophageal inflammation, metaplasia, adenocarcinoma sequence. Gastroenterology. 2010;139:204-212.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |