Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3407
Peer-review started: December 28, 2016
First decision: February 9, 2017
Revised: February 28, 2017
Accepted: April 13, 2017
Article in press: April 13, 2017
Published online: May 21, 2017
Processing time: 147 Days and 11.5 Hours
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide. Its pathogenesis is complex and not yet fully understood. Over the years many studies have proposed various pathophysiological hypotheses, among which the currently most widely accepted is the “multiple parallel hits” theory. According to this model, lipid accumulation in the hepatocytes and insulin resistance increase the vulnerability of the liver to many factors that act in a coordinated and cooperative manner to promote hepatic injury, inflammation and fibrosis. Among these factors, adipose tissue dysfunction and subsequent chronic low grade inflammation play a crucial role. Recent studies have shown that vitamin D exerts an immune-regulating action on adipose tissue, and the growing wealth of epidemiological data is demonstrating that hypovitaminosis D is associated with both obesity and NAFLD. Furthermore, given the strong association between these conditions, current findings suggest that vitamin D may be involved in the relationship between adipose tissue dysfunction and NAFLD. The purpose of this review is to provide an overview of recent advances in the pathogenesis of NAFLD in relation to adipose tissue dysfunction, and in the pathophysiology linking vitamin D deficiency with NAFLD and adiposity, together with an overview of the evidence available on the clinical utility of vitamin D supplementation in cases of NAFLD.
Core tip: Obesity-associated chronic low-grade inflammation plays a pivotal role in the development of non-alcoholic fatty liver disease (NAFLD). Vitamin D deficiency is associated with both obesity and NAFLD, and its anti-inflammatory and immune-modulatory properties provided plausible mechanisms by which hypovitaminosis D may link adipose tissue dysfunction and NAFLD. Animal studies showed beneficial effect of vitamin D supplementation on systemic inflammation and NAFLD, but these data are not confirmed by the results of clinical trials so far conducted in humans.
- Citation: Cimini FA, Barchetta I, Carotti S, Bertoccini L, Baroni MG, Vespasiani-Gentilucci U, Cavallo MG, Morini S. Relationship between adipose tissue dysfunction, vitamin D deficiency and the pathogenesis of non-alcoholic fatty liver disease. World J Gastroenterol 2017; 23(19): 3407-3417
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3407.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3407
Non-alcoholic fatty liver disease (NAFLD) is a condition characterized by the accumulation of excessive fat in the liver in individuals with no history of alcohol abuse (< 30 g/d in men and < 20 g/d in women) and no competing etiologies for hepatic steatosis. NAFLD represents a spectrum of diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which may evolve into hepatic fibrosis, cirrhosis, and eventually hepatic carcinoma[1-5].
NAFLD is a global public health problem[6]: it is currently the most common chronic liver disease worldwide, affecting approximately 20%-35% of adults in the general population[7]. Furthermore, the number of patients affected is growing rapidly, and the disease has now reached epidemic proportions. The reported prevalence of NAFLD is 20%-30% in western countries and approximately 15% in Asian countries. In individuals who are of normal weight and who have no metabolic risk factors, the prevalence of NAFLD is about 16%. Though, it rises dramatically in high-risk individuals such as patients with diabetes (60%), hyperlipidemia (90%) and obese patients (91%)[8-12]. In addition to diabetes and obesity, other independent risk factors identified for the disease to progress toward NASH and to develop fibrosis and cirrhosis are age, female sex, Hispanic ethnicity, smoking[13,14].
Notably, 55% of patients with NAFLD have normal aminotransferase levels[15], suggesting that studies using liver enzymes as a surrogate for NAFLD significantly underestimate the prevalence of this condition. NAFLD diagnosis is usually made by ultrasonography, which enables moderate and severe steatosis to be detected with acceptable sensitivity but only once the level of fat accumulated in the liver exceeds 33%. More sensitive techniques, including nuclear magnetic resonance imaging and spectroscopy, are hindered by the high costs involved and the lack of feasibility in large populations. The American Association for the Study of Liver Diseases sets the limit for the diagnosis of NAFLD at a biopsy-proven hepatic fat content greater than 5%[7]. Liver biopsy is therefore still considered to be the gold standard, although its widespread use is restricted by a number of factors, including the cost and lack of feasibility in population-based studies on both ethical and practical grounds.
The clinical implications of the alarming prevalence of NAFLD, our limited knowledge of its underlying pathophysiologic mechanisms, and the difficulties in both its diagnosis and treatment explain why NAFLD is currently a field of such intensive research.
A histological grading and staging system for non-alcoholic steatohepatitis was proposed by Brunt et al[16] in 1999. The amount of fat, fibrosis and necro-inflammation were the parameters included in the Brunt’s criteria for grading and staging NASH. The presence of hepatic steatosis and inflammation with hepatocyte injury (ballooning) defined the diagnosis of NASH, according to recently recommended guideline of the American Association for the Study of Liver Disease[7].
In patients with NAFLD, studies have shown that the vast majority of hepatic fat (59%) originates from adipose tissue lipolysis, 26% comes from de novo lipogenesis and 15% originates from the diet[17]. Hepatic steatosis results when the balance between delivery and synthesis of free fatty acids exceeds the liver capacity to oxidize or export them. Accumulation of lipids can exert toxic effects on the liver by inefficient oxidation or by activation of inflammatory pathways. Further, increased lipid metabolites such as diacylglycerol and ceramides may themselves cause cell injury and insulin resistance (IR) by interfering with the ability of insulin to phosphorylate insulin receptor substrate-2 through activation of protein kinase C-epsilon [18-21].
In 1998, Day et al[22] presented the “two hits” hypothesis to describe the pathogenesis of NAFLD. They proposed that the “first hit” was represented by lipid accumulation in the hepatocytes and consequent IR, and that the “second hit” was represented by increased oxidative stress that resulted in hepatic inflammation, fibrosis and necrosis. This model is now considered obsolete, because it is inadequate to explain the several molecular and metabolic changes which lead to the development of NAFLD.
According to the current “multiple-hits” theory, the “first hit” sensitizes the liver to further insults, which are represented by a variable combination of different hits, such as oxidative stress and subsequent lipid peroxidation, mitochondrial dysfunction, gut microbiota, adipose tissue dysfunction, and adipokine secretion, all of which are ultimately capable of inducing hepatic injury[23-26].
In this context, novel data has unraveled the role of adipose tissue dysfunction as a central player in the ectopic fat distribution associated with obesity and dysmetabolic conditions. In this review, we are therefore focusing on recent findings that provide an insight into the role of adipose tissue dysfunction in the pathogenesis of NAFLD.
Obesity is a major risk factor for the development of NAFLD, but not all patients with obesity go on to develop NAFLD. In the National Health and Nutrition Examination Survey III, 7.4% of lean adults and 27.8% of overweight/obese adults had hepatic steatosis which could be detected by ultrasound[27].
One reason for this incomplete overlap between obesity and NAFLD is related to the use of Body Mass Index (BMI) to define obesity, meaning that, although BMI cut-off points have good specificity for detecting excess adiposity, they lack sensitivity[28] and also fail to provide information about the distribution, type and quality of body fat.
Traditionally, adipose tissue was regarded as an inert organ for the storage of energy, but in the last few years this conventional view has been radically altered. Currently, adipose tissue is considered to be the major, and possibly the largest, endocrine organ, having the ability to synthesize and release a variety of hormones, cytokines, both complement and growth factors, extracellular matrix proteins and vasoactive agents, collectively known as adipokines. Therefore, it has been shown that adipose tissue biology is much more complex than previously considered[29] and visceral adipose tissue (VAT) dysfunction has been proposed as a major contributor to NAFLD[30,31].
VAT consists of a loose connective tissue that is predominately populated with tightly packed adipocytes that are vascularized by a dense network of capillaries. A second component of VAT is represented by the stromal vascular fraction and includes pre-adipocytes, multi-potent stem cells, fibroblasts, vascular endothelial cells, and immune cells surrounded by the extracellular matrix (ECM). The ECM contains a variety of structural proteins and collagen networks that anchor adipocytes to maintain the structural and functional integrity of the tissue[32].
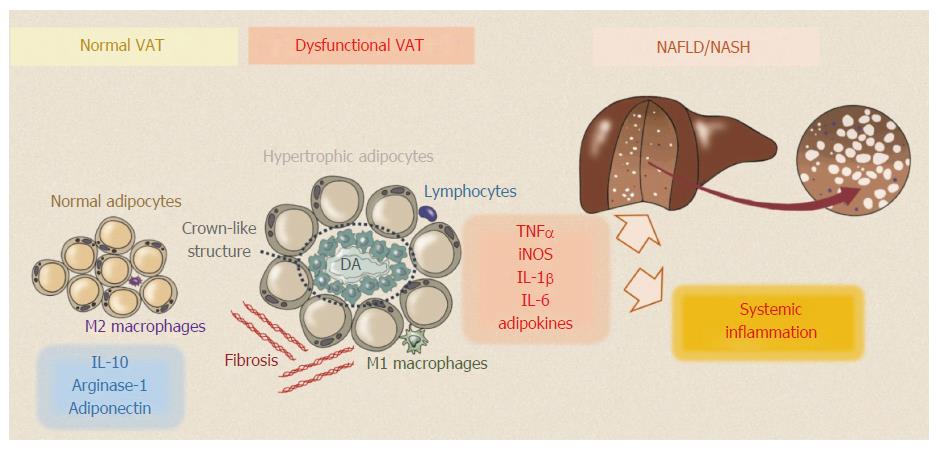
In obese subjects, excessive nutrient intake and the consequent accumulation of triglycerides result in an expansion of VAT that causes adipocytes hypertrophy and alters the stromal vascular compartment[33,34]. Progressive adipocytes hypertrophy is associated with increased adipokine and pro-inflammatory cytokine production[35] and leads to hypoxia and adipocyte cell death[36,37]. Dysfunctional VAT also undergoes excessive fibrosis by enhancing the expression of different ECM components such as collagen VI[32,38,39]; progressive fibrosis may also limit the amount of fat stored in the adipocytes, thus promoting the deposition of ectopic fat in liver and muscle[40].
One hallmark of adipose tissue dysfunction is the accumulation of inflammatory cells in the context of VAT; in particular, active macrophages infiltrate VAT[41,42] and surround dead adipocytes in typical “crown-like structures”[43] (Figure 1).
Two major subtypes of macrophage are found in adipose tissue: “alternatively activated” M2 macrophages and “classically activated” M1 macrophages, and the proportions of these cell populations in VAT are dependent on the tissue microenvironment. M2 macrophages maintain VAT homeostasis in lean individuals through the secretion of anti-inflammatory cytokines, such as IL-10, whereas in obese individuals, pro-M1 polarized macrophages secrete pro-inflammatory cytokines, including TNFα, IL-1β and IL-6, which can promote the proliferation of other inflammatory immune cells, chronic local and systemic inflammation, and can directly alter insulin receptor signaling in adipocytes, leading to IR[44,45]. The mechanisms leading to increased infiltration of macrophages into VAT are not entirely clear; however, it is known that in obese individuals adipocytes increase their expression of monocyte chemoattractant protein 1 (MCP-1) in order to recruit macrophages[46].
Moreover, adipose tissue secretes a large number of adipokines. These are delivered directly to the liver via its portal vein, and then exert local and peripheral effects. It is increasingly recognized that an impaired pattern of adipokines secretion could play a pivotal role in the development of NAFLD[47].
Several investigators have attempted to demonstrate a role for adipokines in the pathogenesis of NAFLD and in the progression to NASH, although the data on many of the adipokines apparently involved are sometimes controversial. Adipokines are characterized by complex interactions and the role they may exert in the pathogenesis of NAFLD is often difficult to interpret[48]. Those adipokines whose effects on the liver are defined and supported by solid data are adiponectin, leptin, TNF-α and IL-6.
TNF-α is the most commonly investigated and characterized. It is secreted by AT-associated macrophages as a response to chronic inflammatory activity. Human and experimental studies have suggested that TNF-α plays a role in all of the phases of fatty liver disease, from simple steatosis to steatohepatitis and cirrhosis; TNF-α also enhances IR[49-51] and promotes the development of IR complications in vitro[52,53]. Human studies have revealed that a high TNF-α level increases the risk of developing NAFLD in healthy individuals[49] and predicts the progression and severity of NASH[49,54-60].
In the pathogenesis and progression of IR and NAFLD, IL-6 exerts a role extensively investigated in both experimental models of steatosis and liver injury, and in humans. In vitro studies have shown that IL-6 promotes IR via several mechanisms[61-63], and in animal models this effect was evident in the liver[64-66]. Serum IL-6 levels were increased in subjects with biopsy-proven NAFLD compared to controls[67], and these levels correlated with the degree of inflammation, the stage of the fibrosis and with IR[68].
Leptin plays a role in both the development and progression of NAFLD, contributing to IR, steatosis and, through a pro-inflammatory role in the regulation of hepatic stellate cells, to hepatic fibrosis[69]. In NASH patients leptin levels were found to be increased and to be related to the grade of hepatic steatosis[70-72].
Adiponectin is produced specifically by differentiated adipocytes[73] and is an anti-inflammatory and insulin-sensitizing hormone[74]. It has been largely demonstrated that adiponectin prevents hepatocytes lipid accumulation by enhancing β-oxydation and by reducing synthesis of free fat acids[75-77], that it mediates anti-inflammatory activity by lowering NFκB action[78], and antagonizes leptin-induced STAT3 phosphorylation in activated hepatic stellate cells[79].
Certain in vivo studies have shown that low serum levels of adiponectin are associated with NAFLD[75,76,80-85] and that low adiponectin was an independent risk factor for NAFLD[86]; furthermore, that adiponectin is a good predictor of necro-inflammation and fibrosis in animal and in vitro models of NAFLD[78,87-89]. Moreover, studies in humans have shown reduction of adiponectin in the serum, as well as reduction of the expression of its receptor in the liver of patients with NASH when compared to BMI-matched patients with steatosis[54,83,90], providing robust evidence that decreased adipocyte production of adiponectin plays an important role in the progression of NAFLD.
The identification of serum adipokines associated with liver histology and, more specifically, with the severity of steatosis, fibrosis and inflammation might provide useful information about NAFLD pathogenesis and form the basis for new diagnostic and therapeutic approaches.
There is growing evidence to indicate that in addition to maintaining calcium and phosphorus homoeostasis and bone health[91], vitamin D also displays pleiotropic actions on several tissues, organs and metabolic processes[92-96]. Vitamin D deficiency is currently a global health issue and may contribute to the pathogenesis of many disorders, such as obesity, metabolic syndrome and type 2 diabetes[97-99].
Despite several epidemiological studies showing the existence of a close relationship between obesity and hypovitaminosis D[100-105], the mechanisms underlying this association are largely unknown. Interestingly, many studies have suggested that adipose tissue could be a direct target of vitamin D, and that this molecule might have a role in modulating adipose tissue pathophysiology[106-116].
Notwithstanding the inverse association between BMI and fat mass[114], higher plasma 25OHD has been associated with lower amounts of VAT and with reduced omental adipocyte size[115-119], suggesting a link between vitamin D status and fat distribution. This is further substantiated by reports of the regulatory effects of vitamin D on adipose tissue and lipid storage and by the fact that vitamin D receptor (VDR) is expressed in adipocytes both in animals models and in humans. In particular, the expression of VDR gene has been reported in cultured adipocytes[110], in human pre-adipocytes[111] and human subcutaneous and visceral adipose tissue[112,113].
Interestingly, it has been suggested that vitamin D may provide a protective effect in obese individuals who have healthy metabolic profiles as characterized by the absence of IR-related conditions and low systemic inflammation despite an increased body fat mass[120-122].
Thus, these data suggest an involvement of vitamin D in the regulation of adipose tissue inflammation. The transduction of inflammatory pathways in adipose tissue involves the activation of nuclear factor κ-β (NF-κB) that regulates the transcription of a wide range of inflammatory mediators. Several in vitro studies showed that vitamin D exerts an anti-inflammatory action on both mouse and human adipocytes by decreasing chemokines and cytokines expression via the involvement of p38 MAP kinase and the NF-κB classical inflammatory pathway[123-125]. Very recently, Karkeni et al[126] demonstrated that vitamin D modulates the expression of miRNAs in adipocytes in vitro and in adipose tissue in vivo through the NF-κB signaling pathway, representing, thus, a new mechanism of regulation of adipose tissue inflammation by vitamin D.
In line with these data, vitamin D supplementation has been recently demonstrate to decrease circulating pro-inflammatory adipokines, in particular IL-6 and TNF-α, in diet-induced obese mice[127,128]. Moreover, in a large cohort of human patients, serum 25OHD concentration correlated with low leptin[129] and high adiponectin levels, irrespective of their BMI[130].
The role of vitamin D in the pathogenesis of NAFLD is an active area of research[131]. The existence of an independent association between hypovitaminosis D and NAFLD has been largely demonstrated in studies conducted using liver imaging[132-136] and biopsy[137,138]. In particular, low vitamin D levels were associated with the histological severity of NAFLD/NASH[137-143] and with the prevalence of NAFLD among individuals with normal liver enzymes[131]. Overall, in the only meta-analysis available in the literature, a 26% additional risk of vitamin D deficiency has been reported in subjects with NAFLD compared to controls subjects[139].
Experimental studies have shown that vitamin D also directly exerts anti-inflammatory, anti-proliferative and anti-fibrotic activities in the liver[144,145] by linking VDR, widely expressed throughout the liver, in hepatocytes, cholangiocytes, and lymphocytes[146-148]. There is extensive evidence to show that the VDR function in the liver regulates not only the hepatic lipid metabolism but also hepatic necro-inflammation and fibrosis; notably, in chronic hepatic diseases VDR expression negatively correlates with the inflammatory damage[149].
In vitro and in vivo preclinical studies have found that vitamin D decreased hepatic stellate cell activation, suggesting it may have a potential role to protect against hepatic fibrosis[150,151].
Data from animal studies further support the notion that vitamin D plays an immunomodulatory role in NAFLD. Roth et al[152] showed that a lack of vitamin D intake in obese rats led to the progression of NAFLD with increased lobular inflammation and a higher NAFLD activity score as evaluated by liver histology; at the same time, mRNA levels of resistin, IL-6 and TNF-α, were increased in the liver. All the above markers are involved in oxidative stress and hepatic inflammation. Therefore, in another study on NASH rat, phototherapy, by increasing the serum active form of vitamin D, reduced hepatocyte inflammation and fibrosis, improved insulin resistance, and increased serum adiponectin, while at the same time reducing the hepatic expression of inflammatory genes TNF-α and TFG-β[153].
It has also been demonstrated that a vitamin D-deficient high-fat diet hampers the enterohepatic circulation of bile acids, leading to NASH[154]. Furthermore, a recent study evidenced that long-term dietary vitamin D depletion could generate spontaneous liver fibrosis in a mice model[155]. Han et al[156] demonstrated that vitamin D supplementation in mice with NASH reduced the hepatic levels of cytokeratin 18 apoptotic fragment M30, a widely validated marker of hepatic damage[157].
These observations regarding the link between vitamin D serum levels and the development and progression of NAFLD suggest that vitamin D supplementation might represent a new therapeutic option in the management of NAFLD. Nevertheless, controversies exist due to the limited number of studies and the conflicting results of prospective randomized clinical trials in humans designed to examine the role of vitamin D supplementation in NAFLD.
Our group has recently published the results of a randomized, double-blind, placebo-controlled trial involving 55 patients with type 2 diabetes and MRI-diagnosed NAFLD. In our study, the participants underwent a 24-wk course of high-dose oral vitamin D supplementation and no effect was shown on either the hepatic fat content or on markers of hepatic injury, i.e., serum transaminases, CK-18 and PIIINP[158].
Lorvand Amiri et al[159] conducted a randomized placebo-controlled double-blind clinical trial to evaluate the potential beneficial effects of oral calcium plus calcitriol supplementation versus calcitriol alone on liver enzymes and ultrasound-measured fat liver content in 120 patients with NAFLD, showing decreased improved serum ALT in the calcium plus calcitriol treated group.
Previously, a prospective small pilot study evaluated the impact of a 24-wk course of high-dose oral vitamin D supplementation on the liver histology of 12 non-cirrhotic NASH patients. The study found no beneficial effects of this treatment on hepatic damage or insulin sensitivity[160].
Sharifi et al[161] also investigated the effect of oral vitamin D supplementation in patients with NAFLD in a placebo-controlled trial and their results showed no effect on serum levels of hepatic enzymes, HOMA-IR, or on the degree of hepatic steatosis. However, their study did demonstrate the beneficial effects of vitamin D on serum malondialdehyde, a marker of lipid peroxidation, and on CRP levels.
Studies with a longer intervention period are warranted in order to explore the effects of long term exposure to vitamin D on NAFLD and on the associated systemic inflammation it causes, as well as on the prevention of NAFLD.
There is a well-established inverse relationship between vitamin D status and obesity, and hypovitaminosis D is associated with an unfavorable metabolic and inflammatory profile. Obesity-related systemic low grade inflammation characterized by alterations in levels of circulating adipokines is suggested to be involved in the pathogenesis of NAFLD and in its progression to NASH. Vitamin D deficiency is also associated with NAFLD and has even been correlated with the severity of the disease. Recent data has suggested that vitamin D’s anti-inflammatory and immune-modulatory properties provide plausible mechanisms by which hypovitaminosis D may link adipose tissue dysfunction to the various steps in the progression of NAFLD. Several animal studies have added further weight to this hypothesis by showing the beneficial effect of vitamin D supplementation on systemic inflammation and on NAFLD in a murine model, although these data are not yet confirmed by the results of the clinical trials conducted to date in humans. Further specifically-designed long-term randomized placebo-controlled trials are needed to clarify the therapeutic impact of vitamin D supplementation in NAFLD.
Thanks are due to Miss Costanza Crise for her help in the artwork generation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luyer M, Man IM S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582-589. [PubMed] |
| 2. | Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 4. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 5. | Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 Suppl:S186-S190. [PubMed] |
| 6. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 7. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2294] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 9. | Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106-1110. [PubMed] |
| 10. | Williamson RM, Price JF, Hayes PC, Glancy S, Frier BM, Johnston GI, Reynolds RM, Strachan MW. Prevalence and markers of advanced liver disease in type 2 diabetes. QJM. 2012;105:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Machado M, Cortez-Pinto H. Non-alcoholic steatohepatitis and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2006;9:637-642. [PubMed] |
| 12. | Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 606] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 13. | Attar BM, Van Thiel DH. Current concepts and management approaches in nonalcoholic fatty liver disease. ScientificWorldJournal. 2013;2013:481893. [PubMed] |
| 14. | Hamabe A, Uto H, Imamura Y, Kusano K, Mawatari S, Kumagai K, Kure T, Tamai T, Moriuchi A, Sakiyama T. Impact of cigarette smoking on onset of nonalcoholic fatty liver disease over a 10-year period. J Gastroenterol. 2011;46:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 653] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 16. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2888] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 17. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [PubMed] |
| 18. | Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711-725.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 19. | Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107-110. [PubMed] |
| 20. | Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab. 2012;15:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Popov VB, Lim JK. Treatment of Nonalcoholic Fatty Liver Disease: The Role of Medical, Surgical, and Endoscopic Weight Loss. J Clin Transl Hepatol. 2015;3:230-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 23. | Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1822] [Article Influence: 121.5] [Reference Citation Analysis (0)] |
| 25. | Vespasiani-Gentilucci U, Carotti S, Perrone G, Mazzarelli C, Galati G, Onetti-Muda A, Picardi A, Morini S. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015;35:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Carotti S, Guarino MP, Vespasiani-Gentilucci U, Morini S. Starring role of toll-like receptor-4 activation in the gut-liver axis. World J Gastrointest Pathophysiol. 2015;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism. 2013;62:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 796] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 29. | Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1781] [Article Influence: 161.9] [Reference Citation Analysis (0)] |
| 30. | Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, Kim CY, Cho YM, Kim SH, Lee KB. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol. 2008;23:900-907. [PubMed] |
| 31. | van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, Peduto T, Chisholm DJ, George J. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 32. | Divoux A, Clément K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev. 2011;12:e494-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471-482. [PubMed] |
| 34. | Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T. Dynamics of fat cell turnover in humans. Nature. 2008;453:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1591] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 35. | Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023-1033. [PubMed] |
| 36. | Giordano A, Murano I, Mondini E, Perugini J, Smorlesi A, Severi I, Barazzoni R, Scherer PE, Cinti S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J Lipid Res. 2013;54:2423-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901-911. [PubMed] |
| 38. | Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, Ravussin E, Bray GA, Smith SR. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155-5162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 244] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 399] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 40. | Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50-56. [PubMed] |
| 41. | Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2108] [Article Influence: 140.5] [Reference Citation Analysis (1)] |
| 42. | Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B. The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 43. | Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347-2355. [PubMed] |
| 44. | Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-184. [PubMed] |
| 45. | Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278. [PubMed] |
| 46. | Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494-1505. [PubMed] |
| 47. | Tilg H. Adipocytokines in nonalcoholic fatty liver disease: key players regulating steatosis, inflammation and fibrosis. Curr Pharm Des. 2010;16:1893-1895. [PubMed] |
| 48. | Polyzos SA, Kountouras J, Zavos C, Stergiopoulos C. Adipocytokines in insulin resistance and non-alcoholic fatty liver disease: the two sides of the same coin. Med Hypotheses. 2010;74:1089-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Seo YY, Cho YK, Bae JC, Seo MH, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. Tumor Necrosis Factor-α as a Predictor for the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Follow-Up Study. Endocrinol Metab (Seoul). 2013;28:41-45. [PubMed] |
| 50. | Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939-2945. [PubMed] |
| 51. | Basaranoglu M, Basaranoglu G, Sentürk H. From fatty liver to fibrosis: a tale of “second hit”. World J Gastroenterol. 2013;19:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol. 2009;1:72-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Dela Peña A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663-1674. [PubMed] |
| 54. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 692] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 55. | Tokushige K, Takakura M, Tsuchiya-Matsushita N, Taniai M, Hashimoto E, Shiratori K. Influence of TNF gene polymorphisms in Japanese patients with NASH and simple steatosis. J Hepatol. 2007;46:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Abiru S, Migita K, Maeda Y, Daikoku M, Ito M, Ohata K, Nagaoka S, Matsumoto T, Takii Y, Kusumoto K. Serum cytokine and soluble cytokine receptor levels in patients with non-alcoholic steatohepatitis. Liver Int. 2006;26:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158-1163. [PubMed] |
| 58. | Manco M, Marcellini M, Giannone G, Nobili V. Correlation of serum TNF-alpha levels and histologic liver injury scores in pediatric nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;127:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Alaaeddine N, Sidaoui J, Hilal G, Serhal R, Abedelrahman A, Khoury S. TNF-α messenger ribonucleic acid (mRNA) in patients with nonalcoholic steatohepatitis. Eur Cytokine Netw. 2012;23:107-111. [PubMed] |
| 60. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 61. | Kanemaki T, Kitade H, Kaibori M, Sakitani K, Hiramatsu Y, Kamiyama Y, Ito S, Okumura T. Interleukin 1beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology. 1998;27:1296-1303. [PubMed] |
| 62. | Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391-3399. [PubMed] |
| 63. | Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777-45784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 781] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 64. | Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 65. | Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 66. | Wunderlich FT, Ströhle P, Könner AC, Gruber S, Tovar S, Brönneke HS, Juntti-Berggren L, Li LS, van Rooijen N, Libert C. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 67. | Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 460] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 68. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 444] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 69. | Tsochatzis E, Papatheodoridis GV, Archimandritis AJ. The evolving role of leptin and adiponectin in chronic liver diseases. Am J Gastroenterol. 2006;101:2629-2640. [PubMed] |
| 70. | Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403-409. [PubMed] |
| 71. | Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, Deveci MS, Bagci S, Gulsen M, Karaeren N. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:3584-3589. [PubMed] |
| 72. | Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015;64:60-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 73. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [PubMed] |
| 74. | Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442-2450. [PubMed] |
| 75. | Anania FA. Adiponectin and alcoholic fatty liver: Is it, after all, about what you eat? Hepatology. 2005;42:530-532. [PubMed] |
| 76. | You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568-577. [PubMed] |
| 77. | Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 78. | Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem. 2002;277:29359-29362. [PubMed] |
| 79. | Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, Anania FA. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J Cell Biochem. 2010;110:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Nannipieri M, Cecchetti F, Anselmino M, Mancini E, Marchetti G, Bonotti A, Baldi S, Solito B, Giannetti M, Pinchera A. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obes Surg. 2009;19:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Ma H, Gomez V, Lu L, Yang X, Wu X, Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:233-237. [PubMed] |
| 82. | Uribe M, Zamora-Valdés D, Moreno-Portillo M, Bermejo-Martínez L, Pichardo-Bahena R, Baptista-González HA, Ponciano-Rodríguez G, Uribe MH, Medina-Santillán R, Méndez-Sánchez N. Hepatic expression of ghrelin and adiponectin and their receptors in patients with nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:67-71. [PubMed] |
| 83. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [PubMed] |
| 84. | Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, Bastard JP, Poupon R, Housset C, Capeau J. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 85. | Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 2007;47:556-564. [PubMed] |
| 86. | Matsuzawa Y. Adiponectin: a key player in obesity related disorders. Curr Pharm Des. 2010;16:1896-1901. [PubMed] |
| 87. | Musso G, Gambino R, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438-2446. [PubMed] |
| 88. | Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 89. | Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19:802-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 144] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (4)] |
| 90. | Arvaniti VA, Thomopoulos KC, Tsamandas A, Makri M, Psyrogiannis A, Vafiadis G, Assimakopoulos SF, Labropoulou-Karatza C. Serum adiponectin levels in different types of non alcoholic liver disease. Correlation with steatosis, necroinflammation and fibrosis. Acta Gastroenterol Belg. 2008;71:355-360. [PubMed] |
| 91. | Tanaka Y, Frank H, DeLuca HF. Biological activity of 1,25-dihydroxyvitamin D3 in the rat. Endocrinology. 1973;92:417-422. [PubMed] |
| 92. | Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. [PubMed] |
| 93. | Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909-2912. [PubMed] |
| 94. | Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 95. | Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 96. | Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): is it more than just an association? Hepatology. 2013;58:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 97. | Hyppönen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298-305. [PubMed] |
| 98. | Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes. 2008;57:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 99. | Barchetta I, De Bernardinis M, Capoccia D, Baroni MG, Fontana M, Fraioli A, Morini S, Leonetti F, Cavallo MG. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One. 2013;8:e68689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370-373. [PubMed] |
| 101. | Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690-693. [PubMed] |
| 102. | Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J, Yanovski JA. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196-1199. [PubMed] |
| 103. | Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119-4123. [PubMed] |
| 104. | Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573-580. [PubMed] |
| 105. | Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 106. | Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, Saltzman E, Dawson-Hughes B. Vitamin D(3) in fat tissue. Endocrine. 2008;33:90-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 107. | Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, Brady MJ, Li YC. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286:33804-33810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 108. | Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, Burgess JR, Teegarden D. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 109. | Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916-E924. [PubMed] |
| 110. | Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E. Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1993;193:948-955. [PubMed] |
| 111. | Trayhurn P, O’Hara A, Bing C. Interrogation of microarray datasets indicates that macrophage-secreted factors stimulate the expression of genes associated with vitamin D metabolism (VDR and CYP27B1) in human adipocytes. Adipobiology. 2011;3:29-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 113. | Nimitphong H, Holick MF, Fried SK, Lee MJ. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7:e52171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Stokić E, Kupusinac A, Tomic-Naglic D, Smiljenic D, Kovacev-Zavisic B, Srdic-Galic B, Soskic S, Isenovic ER. Vitamin D and Dysfunctional Adipose Tissue in Obesity. Angiology. 2015;66:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 115. | Stokić E, Kupusinac A, Tomić-Naglić D, Zavišić BK, Mitrović M, Smiljenić D, Soskić S, Isenović E. Obesity and vitamin D deficiency: trends to promote a more proatherogenic cardiometabolic risk profile. Angiology. 2015;66:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Soskić S, Stokić E, Isenović ER. The relationship between vitamin D and obesity. Curr Med Res Opin. 2014;30:1197-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond). 2012;36:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 300] [Article Influence: 21.4] [Reference Citation Analysis (2)] |
| 118. | Caron-Jobin M, Morisset AS, Tremblay A, Huot C, Légaré D, Tchernof A. Elevated serum 25(OH)D concentrations, vitamin D, and calcium intakes are associated with reduced adipocyte size in women. Obesity (Silver Spring). 2011;19:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 119. | Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, Robins SJ, O’Donnell CJ, Hoffmann U, Jacques PF. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 120. | Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 121. | Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond). 2011;35:971-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 122. | Esteghamati A, Aryan Z, Esteghamati A, Nakhjavani M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabetes Metab. 2014;40:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, Amiot MJ, Landrier JF. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012;56:1771-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 124. | Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Aström G, Sjölin E, Wåhlén K, Carlberg C, Laurencikiene J. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2012;51:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring). 2008;16:932-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 126. | Karkeni E, Bonnet L, Marcotorchino J, Tourniaire F, Astier J, Ye J, Landrier JF. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 127. | Karkeni E, Marcotorchino J, Tourniaire F, Astier J, Peiretti F, Darmon P, Landrier JF. Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology. 2015;156:1782-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 128. | de Souza WN, Norde MM, Oki É, Rogero MM, Marchioni DM, Fisberg RM, Martini LA. Association between 25-hydroxyvitamin D and inflammatory biomarker levels in a cross-sectional population-based study, São Paulo, Brazil. Nutr Res. 2016;36:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 129. | Vilarrasa N, Vendrell J, Maravall J, Elío I, Solano E, San José P, García I, Virgili N, Soler J, Gómez JM. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010;38:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 130. | Vaidya A, Williams JS, Forman JP. The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring). 2012;20:186-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 131. | Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol. 2015;21:1718-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 132. | Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 133. | Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, Chonchol M. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, Kang MI, Park SW, Kim SW, Oh KW. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60:743-752. [PubMed] |
| 135. | Wang D, Lin H, Xia M, Aleteng Q, Li X, Ma H, Pan B, Gao J, Gao X. Vitamin D Levels Are Inversely Associated with Liver Fat Content and Risk of Non-Alcoholic Fatty Liver Disease in a Chinese Middle-Aged and Elderly Population: The Shanghai Changfeng Study. PLoS One. 2016;11:e0157515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Zhai HL, Wang NJ, Han B, Li Q, Chen Y, Zhu CF, Chen YC, Xia FZ, Cang Z, Zhu CX. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: a cross-sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China)). Br J Nutr. 2016;115:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 137. | Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517-524. [PubMed] |
| 138. | Manco M, Ciampalini P, Nobili V. Low levels of 25-hydroxyvitamin D(3) in children with biopsy-proven nonalcoholic fatty liver disease. Hepatology. 2010;51:2229; author reply 2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 139. | Shoupe D, Mishell DR, Page MA, Madkour H, Spitz IM, Lobo RA. Effects of the antiprogesterone RU 486 in normal women. II. Administration in the late follicular phase. Am J Obstet Gynecol. 1987;157:1421-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Nobili V, Giorgio V, Liccardo D, Bedogni G, Morino G, Alisi A, Cianfarani S. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol. 2014;170:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 141. | Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, Khiyami A, McCullough AJ, Dasarathy S. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:e118-e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 142. | Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, Whalen E, Hoofnagle A, Mason M, Gersuk V. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-κB? Am J Gastroenterol. 2016;111:852-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 143. | Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, Koteish AA, Clark JM, Guallar E, Hernaez R. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 144. | Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 145. | Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 146. | Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034-1042. [PubMed] |
| 147. | Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 148. | Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125-25132. [PubMed] |
| 149. | Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 150. | Ding N, Liddle C, Evans RM, Downes M. Hepatic actions of vitamin D receptor ligands: a sunshine option for chronic liver disease? Expert Rev Clin Pharmacol. 2013;6:597-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | Beilfuss A, Sowa JP, Sydor S, Beste M, Bechmann LP, Schlattjan M, Syn WK, Wedemeyer I, Mathé Z, Jochum C. Vitamin D counteracts fibrogenic TGF-β signalling in human hepatic stellate cells both receptor-dependently and independently. Gut. 2015;64:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 152. | Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 153. | Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, Huang YZ, Honda H, Chen KD, Wang CC. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol. 2011;55:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 154. | Kong M, Zhu L, Bai L, Zhang X, Chen Y, Liu S, Zheng S, Pandol SJ, Han YP, Duan Z. Vitamin D deficiency promotes nonalcoholic steatohepatitis through impaired enterohepatic circulation in animal model. Am J Physiol Gastrointest Liver Physiol. 2014;307:G883-G893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 155. | Zhu L, Kong M, Han YP, Bai L, Zhang X, Chen Y, Zheng S, Yuan H, Duan Z. Spontaneous liver fibrosis induced by long term dietary vitamin D deficiency in adult mice is related to chronic inflammation and enhanced apoptosis. Can J Physiol Pharmacol. 2015;93:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 156. | Han H, Cui M, You X, Chen M, Piao X, Jin G. A role of 1,25(OH)2D3 supplementation in rats with nonalcoholic steatohepatitis induced by choline-deficient diet. Nutr Metab Cardiovasc Dis. 2015;25:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 157. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 516] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 158. | Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 159. | Lorvand Amiri H, Agah S, Tolouei Azar J, Hosseini S, Shidfar F, Mousavi SN. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin Nutr. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 160. | Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High-dose vitamin D supplementation and liver histology in NASH. Gut. 2016;65:717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 161. | Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |