Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3111
Peer-review started: December 23, 2016
First decision: January 10, 2017
Revised: February 8, 2017
Accepted: March 31, 2017
Article in press: March 31, 2017
Published online: May 7, 2017
Processing time: 136 Days and 8.3 Hours
To evaluate whether pathologically early hepatocellular carcinoma (HCC) exhibited local tumor progression after radiofrequency ablation (RFA) less often than typical HCC.
Fifty pathologically early HCCs [tumor diameter (mm): mean, 15.8; range, 10-23; follow-up days after RFA: median, 1213; range, 216-2137] and 187 typical HCCs [tumor diameter (mm): mean, 15.6; range, 6-30; follow-up days after RFA: median, 1116; range, 190-2328] were enrolled in this retrospective study. The presence of stromal invasion (namely, tumor cell invasion into the intratumoral portal tracts) was considered to be the most important pathologic finding for the diagnosis of early HCCs. Typical HCC was defined as the presence of a hyper-vascular lesion accompanied by delayed washout using contrast-enhanced computed tomography or contrast-enhanced magnetic resonance imaging. Follow-up examinations were performed at 3-mo intervals to monitor for signs of local tumor progression. The local tumor progression rates of pathologically early HCCs and typical HCCs were then determined using the Kaplan-Meier method.
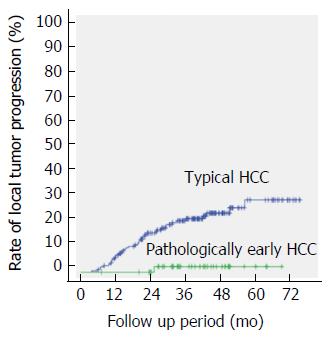
During the follow-up period for the 50 pathologically early HCCs, 49 (98%) of the nodules did not exhibit local tumor progression. However, 1 nodule (2%) was associated with a local tumor progression found 636 d after RFA. For the 187 typical HCCs, 46 (24.6%) of the nodules exhibited local recurrence after RFA. The follow-up period until the local tumor progression of typical HCC was a median of 605 d, ranging from 181 to 1741 d. Among the cases with typical HCCs, local tumor progression had occurred in 7.0% (7/187), 16.0% (30/187), 21.9% (41/187) and 24.6% (46/187) of the cases at 1, 2, 3 and 4 years, respectively. Pathologically early HCC was statistically associated with a lower rate of local tumor progression, compared with typical HCC, when evaluated using a log-rank test (P = 0.002).
The rate of local tumor progression for pathologically early HCCs after RFA was significantly lower than that for typical HCCs.
Core tip: This retrospective study evaluated whether pathologically early hepatocellular carcinoma (HCC) exhibited local tumor progression after radiofrequency ablation (RFA) less often than typical HCC. Among the 50 pathologically early HCCs, 49 (98%) of the nodules did not exhibit local tumor progression. However, 1 nodule (2%) was associated with a local tumor progression found 636 d after RFA. Among the 187 typical HCCs, 46 (24.6%) of the nodules exhibited local tumor progression after RFA. Pathologically early HCC was significantly associated with a lower rate of local tumor progression, compared with typical HCC, when evaluated using a log-rank test (P = 0.002).
- Citation: Hao Y, Numata K, Ishii T, Fukuda H, Maeda S, Nakano M, Tanaka K. Rate of local tumor progression following radiofrequency ablation of pathologically early hepatocellular carcinoma. World J Gastroenterol 2017; 23(17): 3111-3121
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3111
Depending on whether the term “early” is used pathologically or clinically, “early” hepatocellular carcinoma (HCC) can have different definitions. Clinically, the term “early” HCC refers to tumors that are smaller than 3 cm and three or fewer in number at stage A (early stage) according to the Barcelona Clinic Liver Cancer (BCLC) staging, although it sometimes includes solitary HCC ≤ 5 cm as defined according to the Milan criteria. In BCLC staging, very early and early stage tumors are relatively small, but both lesions are hyper-vascular during the arterial phase and wash out during the hepatic venous phase or the equilibrium phase of contrast-enhanced computed tomography (CT). On the other hand, pathologically “early” HCC defined as HCC in the early stage of carcinogenesis[1-3] appears as hypo-vascular or iso-vascular lesions with irregular boundaries using contrast-enhanced CT[4] and contains stromal invasion, namely tumor cell invasion into the intratumoral portal tracts without any significant effect on the original structure of the liver[1]. Accordingly, the accurate diagnosis and adequate treatment of pathologically early HCC, which is a precursor of typical HCC, is very important[5].
The development of modern imaging methods, especially contrast-enhanced ultrasonography (US) with perflubutane microbubble agent (Sonazoid; Daiichi Sankyo, Tokyo, Japan) and contrast-enhanced magnetic resonance imaging (MRI) with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA; Primovist; Bayer Schering Pharma AG, Berlin, Germany), have played a very important role in the imaging of multistep hepatocarcinogenesis[4,6]. Pathologically early HCCs are often recognized as hypo-intense masses during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA[4].
Radiofrequency ablation (RFA) is low-invasive percutaneous local treatment for patients with early-stage HCC, and this procedure can be expected to achieve complete necrosis. RFA is increasingly being used when surgical resection or liver transplantation is not feasible or available. Adequate tumor ablation significantly improves the survival outcomes of patients[7,8]. However, the 10-year cumulative rate of local tumor progression after RFA has been reported to be 3.2% or 36.9%[9,10]. Previous studies have reported possible risk factors for local tumor progression, including a pathological diagnosis of poorly differentiated tumor, a large tumor diameter, the presence of vessels adjacent to the tumor, the lack of an ablative margin (AM), a high degree of arterial enhancement, and an elevated tumor marker level[11-14].
Early-stage typical HCC lesions are often accompanied by micrometastases and microvascular invasion, despite their small size[15]. In contrast, pathologically early HCC lesions are generally not accompanied by these features[1]. Therefore, pathologically early HCC lesions are expected to exhibit a longer period until local tumor progression after curative treatment, such as resection or RFA, compared with typical HCC lesions. However, Midorikawa et al[16] reported that the survival benefit of resection for pathologically early HCCs is marginal because of a long lead-time bias; therefore, they suggested that surgery is not indicated for pathologically early HCC. In contrast, RFA is less invasive than resection for the curative treatment of small HCCs. To our knowledge, however, the local tumor progression rate of pathologically early HCC after adequate RFA, compared with that of typical HCC, has not been previously studied.
The purpose of the present study was, therefore, to evaluate whether local tumor progression after adequate RFA occurs less frequently for pathologically early HCC than for typical HCC.
Institutional review board approval and informed consent from all the patients were obtained for this retrospective study. From December 2009 to July 2014, we performed percutaneous RFA for the treatment of 221 consecutive patients with 480 HCCs at our institution. Overall, 136 patients with 237 HCCs were enrolled in this retrospective study.
The inclusion criteria for this study were as follows: (1) a maximum of three HCC lesions with diameters within 3 cm detected using contrast-enhanced CT or contrast-enhanced MRI with Gd-EOB-DTPA; (2) a platelet count of more than 5 × 104/mL; (3) hepatitis or cirrhosis with Child-Pugh grade A or B; (4) no eligibility for surgical resection or refusal of surgery; (5) evaluation of AM 1 d after RFA using fusion image combining contrast-enhanced US and contrast-enhanced CT or contrast-enhanced MRI with Gd-EOB-DTPA; and (6) treatment with RFA alone.
The exclusion criteria for this study were as follows: (1) patients who were unable to undergo contrast-enhanced CT or contrast-enhanced MRI with Gd-EOB-DTPA because of a contraindication for the use of intravenous contrast agents (allergic reaction or impaired renal function); (2) patients in whom contrast-enhanced US was impossible because of insufficient breath holding or contraindications for contrast-enhanced US with Sonazoid (e.g., egg allergy, severe pulmonary or cardiac disease); (3) lesions for which detailed evaluations using contrast-enhanced US were difficult because of non-visualization as a result of bowel gas or a location more than 12 cm from the skin surface; and (4) incomplete ablation of HCC, defined as the absence of an AM at any point surrounding the tumor as evaluated using contrast-enhanced US.
Overall, 243 HCCs that were treated with RFA during the registration period were excluded for the following reasons: the AM had not been evaluated using contrast-enhanced US after treatment (n = 203), the follow-up period was less than 6 mo because the patient had moved or died and had been lost to follow-up (n = 20), the use of an intravenous contrast agent was contraindicated (n = 12), or tumor ablation was incomplete because the HCC was located close to the lung, gallbladder, liver surface, or a combination of these areas, requiring the use of additional therapies (n = 8). Finally, 139 patients with 237 HCCs underwent technically successful and adequate ablation procedures and were included in this study.
The baseline characteristics of the study population are summarized in Table 1. The follow-up days, age, Child-Pugh score, tumor diameter, sex, and etiology did not differ significantly between patients with pathologically early HCC and patients with typical HCC. None of the patients experienced major complications after ablation.
| eHCC | tHCC | P value | |
| Number of nodules | 50 | 187 | |
| Follow-up days1, median (range) | 1213 (216-2137) | 1116 (181-2328) | 0.1582 |
| Age, mean (range) | 69.8 (50-87) | 71.6 (54-84) | 0.2002 |
| Child-Pugh score, A/B | 49/1 | 179/8 | 0.0992 |
| Tumor diameter in mm, mean (range) | 15.8 (10-23) | 15.6 (6-30) | 0.1542 |
| Sex, male/female | 35/15 | 125/58 | 0.8343 |
| Etiology, HCV/HBV/alcohol/others4 | 41/4/6/1 | 139/29/8/11 | 0.1985 |
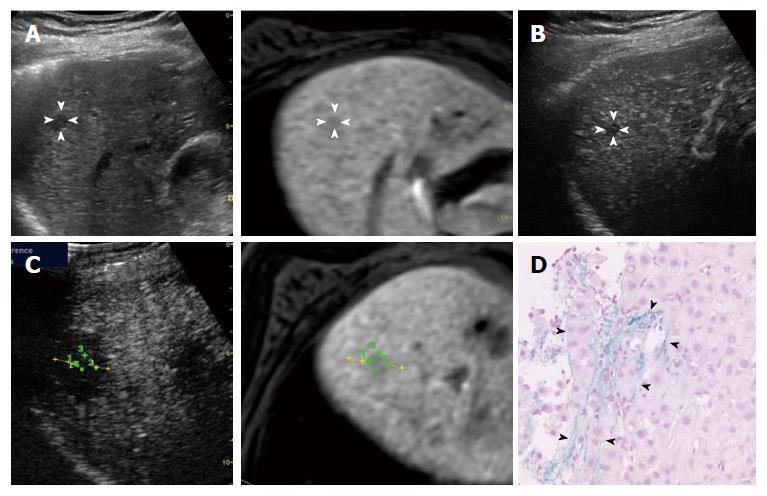
Diagnosis of pathologically early HCC: A diagnosis of early HCC was based on pathologic features. All the biopsies were obtained after contrast-enhanced US. At least two specimens were taken from each of the nodules to ensure an accurate histological diagnosis using a 21-gauge fine needle biopsy (SONOPSY; Hakko, Tokyo, Japan) or an 18-gauge biopsy needle (Biopty-Cut; Bard, Covington, GA, United States) under US guidance[17]. The international consensus group for hepatocellular neoplasia stated that the presence of stromal invasion (namely, tumor cell invasion into the intratumoral portal tracts)[18] should be recognized as the most important pathologic finding for the diagnosis of early HCCs. The diagnosis of stromal invasion is subjective and may require the assistance of Victoria blue staining[19] and immunohistochemical stains (cytokeratin 7)[19,20] to differentiate stromal invasion from pseudo-invasion. Thirty-nine patients with 54 nodules were diagnosed as having pathologically early HCC between December 2009 and July 2014, and 35 patients with 50 nodules who meet the study criteria were enrolled in the present study.
Diagnosis of typical HCC: When a lesion was visualized as a hyper-vascular area during the arterial phase of contrast-enhanced CT or dynamic contrast-enhanced MRI with Gd-EOB-DTPA and as a hypo-vascular area (washout) during the hepatic venous phase or equilibrium phase or during both phases of contrast-enhanced CT or the delayed phase of dynamic contrast-enhanced MRI with Gd-EOB-DTPA, we considered this enhancement pattern to represent a vascular pattern characteristic of typical HCC. The diagnosis of typical HCC was established based on these radiologic features[21].
CT: CT was performed using a 16-MDCT scanner (Aquilion 16; Toshiba Medical, Otawara, Japan) with a tube voltage of 120 kV, a tube current setting at the automatic milliampere exposure setting, a reconstruction section and interval thickness of 5 mm, a pitch of 15, and a gantry speed of 0.5 seconds per rotation. A nonionic contrast agent [iopamidol (Iopamiron 300 or 370; Bayer HealthCare, Berlin, Germany)] was injected. Patients weighing less than 70 kg received 300 mgI/mL, whereas those weighing 70 kg or more received 370 mgI/mL. After a power injector (Dual Shot GX; Nemoto Kyorindo) was used to inject 100 mL of iopamidol at 3 mL/s through a catheter placed in the antecubital vein, the scanning time in the arterial phase was confirmed using an automatic bolus-tracking program (RealPrep; Toshiba Medical). The trigger point for starting arterial phase scanning was set at an attenuation of 230 HU from the baseline attenuation of the abdominal aorta. Hepatic venous phase scanning was performed 70 s after contrast injection, and equilibrium phase images were acquired 180 s after injection. CT data were transferred to a computer workstation (Zio M900; Zio Software, San Francisco, CA, United States).
MRI: MRI was performed using a 1.5-T whole-body imager (Avant; Siemens Medical System, Erlangen, Germany). At the same time as the arrival of Gd-EOB-DTPA in the celiac artery, a power injector (Spectris Solaris EP; MEDRAD, Bayer Schering Pharma AG, Berlin, Germany) was used to inject 0.1 mmol/kg of Gd-EOB-DTPA at 1 mL/s through a catheter placed in the antecubital vein, followed by flushing with 20 mL of sterile saline solution at 2 mL/s. Arterial phase scanning was performed 13 s after contrast injection, portal phase scanning was performed 70-85 s after contrast injection, delay phase scanning was performed 180 s after contrast injection, and hepatobiliary phase scanning was performed 20 min after contrast injection. The images were obtained using fat-suppressed volumetric interpolated breath-hold examination (FS VIBE) T1-weighted sequences (TR, 6.2 ms; TE, 3.15 ms; flip angle, 20°; band width, 260 Hz/pix; matrix, 166 × 320; acquisition time, 20 s). In addition, a fast low angle shot (FLASH) T1-weighted sequence (TR, 115 ms; TE, 4.76 ms; flip angle, 70°; band width, 260 Hz/pix; matrix, 192 × 256; acquisition time, 20 s × 3) and turbo spin-echo (TSE) pace respiratory-triggered T2-weighted sequence as well as an echo planar imaging (EPI) diffusion-weighted sequence were also obtained.
US imaging: (1) Conventional US. First, we assessed the detection of hepatic lesions using the LOGIQ 7 or LOGIQ E9 ultrasound system (GE Healthcare, Milwaukee, WI, United States) with native tissue harmonic gray-scale imaging using a convex probe with a frequency of 2-5 MHz and a micro-convex probe with a frequency of 2-5 MHz (hereafter referred to as conventional US). If the detection of hepatic lesions by the procedures described above was difficult, we used fusion imaging combining conventional US and contrast-enhanced CT or the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA[21]; and (2) Contrast-enhanced US procedures. The procedures used for both contrast-enhanced US with a low mechanical index (MI) mode and that with a high MI mode were essentially the same. A 0.2-mL dose of Sonazoid was injected into an antecubital vein at 0.2 mL/sec via a 24-gauge cannula followed by 2 mL of 5% glucose after the Sonazoid injection. Contrast-enhanced US images were acquired during three contrast phases, consisting of an arterial phase (about 10-50 s after injection), a portal phase (about 80-120 s after injection), and a post-vascular phase (about 10 min after injection).
Contrast-enhanced US using a low MI mode at a low MI (0.2-0.3) can provide a real-time evaluation of tumor vessels and tumor enhancement at 11 frames per second during the arterial phase and tumor enhancement during the portal and post-vascular phases. On the other hand, when contrast-enhanced US was performed using the coded harmonic angio (CHA) mode at a high MI (0.7-1.0), the lesion was scanned at 2-8 frames per s after the injection. We usually used 8 frames per s to observe the tumor vessels by eliminating microbubbles in the microvessels but not in relatively large vessels, such as the tumor vessels and portal veins, thereby prolonging the observation time for the tumor vessels during the arterial phase. A rate of 2 frames per second was used to observe tumor enhancement as a result of microbubble destruction within and around the tumor during the three phases. The transmission power was 70%-100%, and the MI ranged from 0.7-1.0. Using the CHA mode at a high MI at 2 frames per s with the focus point just beneath the lesion, we manually scanned the whole lesion to destroy any microbubbles within or around the tumor. We called this method “high MI intermittent imaging”[22]. This procedure enabled the evaluation of tumor vascularity in hyper-echoic nodules or lesions located deep within the liver (between 10 and 12 cm from the skin surface) during the three phases[22].
Fusion imaging: We used a fusion imaging system to detect the precise location of HCCs[17,23]. As mentioned in previous reports[17,23], after successful registration of the conventional US and MR images obtained during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA, the results of real-time ultrasound scanning were then viewed simultaneously with the corresponding multiplanar reconstruction slice from the pre-acquired volumetric MR DICOM data. These procedures were also used to perform fusion imaging, in which contrast-enhanced US was combined with the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA.
In addition, a subset of the fusion imaging modality, known as the “overlay image” tool, enabled a reference MR image to be overlaid on the US image. After the correct registration of the US image and the MR image, we overlaid the MR image on the US image. This method can display the location of a tumor as it appears on a MR image directly on a US image. Moreover, another subset of the fusion imaging modality, known as the global positioning system (GPS) tool, enables a marked position to be visually tracked during scanning. For example, after the registration of the US image and the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA, we set the GPS mark at an HCC lesion, which appeared as a low-intensity area on the hepatobiliary phase of contrast-enhanced MRI. This mark then simultaneously appeared as a small green cross graphic on the contrast-enhanced MR image and the US image. Both the “overlay image” and the “GPS mark” were used to confirm the location of the HCC lesions[23].
RFA was performed under real-time US guidance using the LOGIQ 7 or LOGIQ E9 ultrasound system and a convex probe with a frequency of 2.5 MHz and a micro-convex probe with a frequency of 2.5 MHz. One physician who had 10 years of experience performing RFA for the treatment of HCC performed all the procedures. To ablate all the tumors, a 20-cm-long, 17-gauge cool-tip radio frequency electrode with a 2- or 3-cm-long exposed metallic tip (Cool-tip Needle; COVIDIEN Valleylab, Boulder, CO, United States) was promptly inserted into the targeted tumor, and RFA was performed using an RFA generator system (COVIDIEN Valleylab). RFA was performed under local anesthesia in all the cases. The electrode was inserted at different sites and overlapping ablations were performed until the entire lesion was ablated, as determined using fusion imaging. The ablation algorithm was based on elevations of tissue impedance. Successful ablations usually increased the temperature of the ablated tissue to above 60 °C. Among the 136 patients with 237 HCCs enrolled in this study, fever, abdominal pain and elevated liver enzyme levels were observed after treatment, but no serious complications requiring an extended hospital stay occurred.
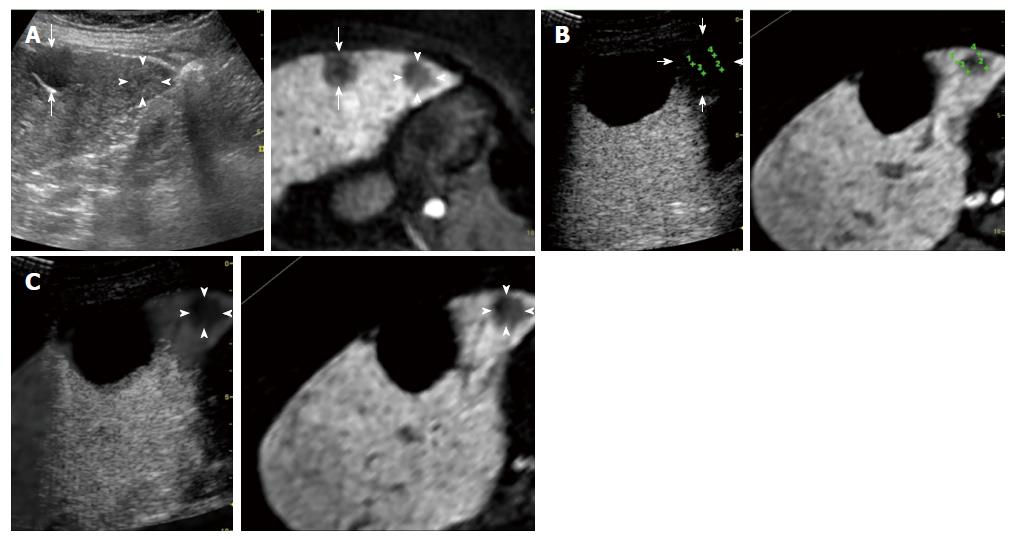
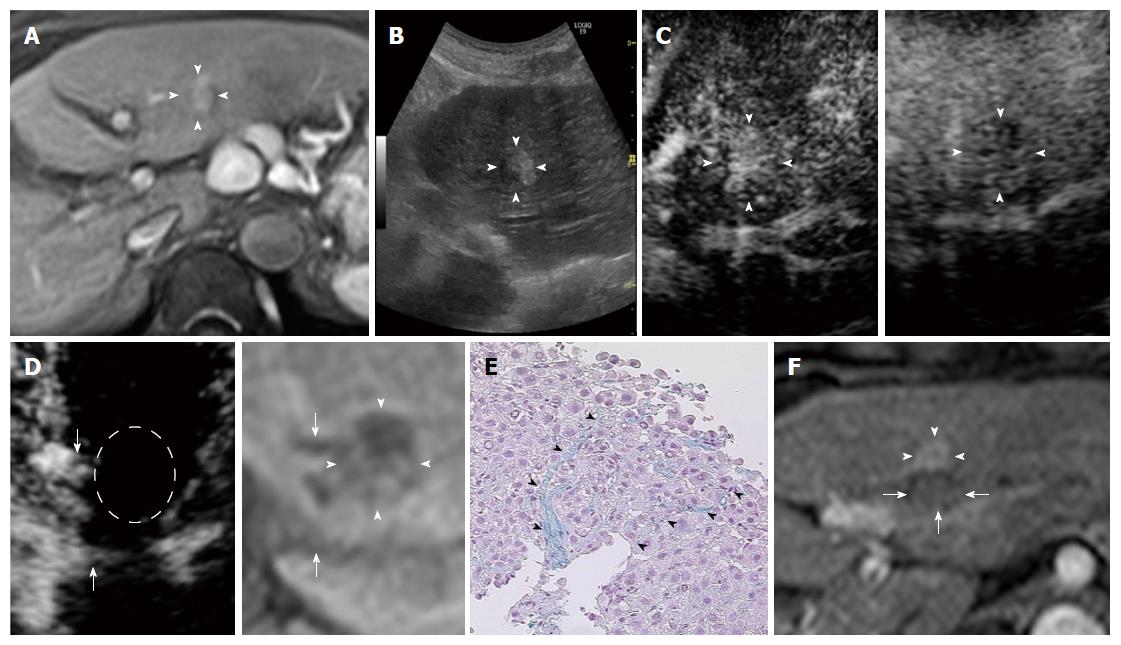
Evaluation of AM using fusion imaging: At 1 d after ablation, the location of the HCCs could not be precisely confirmed using contrast-enhanced US because of the disappearance of the enhancement of the HCC lesion itself and the surrounding non-tumor area. Using fusion imaging with overlay imaging and GPS marks, we were able to confirm the location of the ablated HCC, enabling the ablated HCC to be differentiated from the ablated adjacent liver parenchyma and allowing the thinnest AM to be measured (Figure 1).
The thinnest AM was classified into two groups with reference to a previous report[13]: (1) AM of 0 to < 5 mm: although complete tumor ablation had been achieved, the AM was less than 5 mm; (2) AM of ≥ 5: an AM of 5 mm or more. When the HCC lesions were located on the liver surface or adjacent to relatively large vessels, such as the portal or hepatic vein, we considered the thinnest AM to be 0 to < 5 mm even if the other AMs were more than 5 mm.
Follow-up protocol: Subsequent diagnostic findings using either contrast-enhanced CT or contrast-enhanced MRI with Gd-EOB-DTPA were obtained after 1 mo.
The follow-up protocol for early HCC included the acquisition of contrast-enhanced MRI with Gd-EOB-DTPA at 3-mo intervals to monitor for signs of local tumor recurrence. Meanwhile, typical HCC was followed by the acquisition of either contrast-enhanced CT or contrast-enhanced MRI with Gd-EOB-DTPA at 3-mo intervals.
The follow-up period was considered to last until local tumor recurrence. The follow-up period of this study was considered to end with the incidence of local tumor recurrence or the last examination using either contrast-enhanced CT or contrast-enhanced MRI as of June 2016.
Local tumor progression: The local tumor progression of pathologically early HCC was defined as the incidence of a hypo-intense lesion occurring adjacent to the ablation zone as evaluated using contrast-enhanced MRI with Gd-EOB-DTPA during the hepatobiliary phase. The local tumor progression of typical HCC was defined as the presence of a hyper-vascular lesion accompanied by delayed washout and occurring adjacent to the ablation zone as evaluated using either contrast-enhanced CT or contrast-enhanced MRI[12,13].
The image evaluations were performed independently by four experienced radiologists who were blinded to the final diagnoses; each of the radiologists had at least 5 years of clinical experience performing US, CT, and MRI. The first and second radiologists reviewed all the US images, including the conventional US and contrast-enhanced US findings recorded on still images and cine clips. The third and fourth radiologists reviewed all the contrast-enhanced CT and contrast-enhanced MRI findings using a commercially available viewer system or a picture archiving and communication system (Synapse; Fujifilm Medical, Tokyo, Japan). Each of the two groups of readers met to arrive at a consensus for the image evaluation.
The Mann-Whitney U test and χ2 test were used to compare the clinical characteristics of patients with pathologically early HCCs and those with typical HCCs. The χ2 test was also used to compare the AMs between pathologically early HCCs and typical HCCs. The Fisher’s exact test was used to compare the frequency of local tumor progression classified into two groups according to the AM for pathologically early HCCs and typical HCCs. The Kaplan-Meier method was used to estimate the interval from RFA until local tumor progression. The independent risk factors as determinants of local tumor progression were analyzed using the log-rank test. A P value < 0.05 was considered to indicate a statistically significant difference. The statistical analyses were performed using SPSS, version 22 (IBM SPSS, Inc., Chicago, IL, United States) for Windows (Microsoft).
During the follow-up period for the 50 pathologically early HCCs (median, 1213 d; range, 216-2137 d), 49 (98%) of the nodules did not exhibit local tumor progression. However, 1 nodule (2%) was associated with a local recurrence found 636 d after ablation.
During the follow-up period for the 187 typical HCCs (median, 1116 d; range, 190-2328 d), 46 (24.6%) of the nodules exhibited local tumor progression after ablation. The follow-up period until the local tumor progression of typical HCC was a median of 605 d, ranging from 181 to 1741 d. Local tumor progression had occurred in 7.0%, 16.0%, 21.9% and 24.6% of the cases at 1, 2, 3 and 4 years, respectively.
Table 2 shows the thinnest AM for each pathologically early HCC and typical HCC, as evaluated using contrast-enhanced US. Among the 50 pathologically early HCCs, 10 (20.0%) had an AM ≥ 5 mm (Figures 2 and 3). Among the 187 typical HCCs, 27 (14.4%) had an AM ≥ 5 mm. The percentage of lesions with an AM ≥ 5 mm was not significantly different between the pathologically early HCCs and the typical HCCs when evaluated using the χ2 test (P = 0.336).
| eHCC | tHCC | P value1 | |
| AM 0 to < 5 mm | 80% (40/50) | 85.5% (160/187) | NS |
| AM ≥ 5 mm | 20% (10/50) | 14.4% (27/187) | NS |
The frequencies of local tumor progression after classifying both the pathologically early HCCs and the typical HCCs into two groups according to the acquisition of an ablative margin are shown in Table 3. Whether a pathologically early HCC versus a typical HCC status influenced the risk of local tumor progression after RFA was examined using a log-rank test (Figure 4), and a significant difference (P = 0.002) was observed.
| AM 0 to < 5 mm | AM ≥ 5 mm | |
| Local tumor progression | ||
| eHCC | 2.5% (1/40) | 0% (0/10) |
| tHCC | 27.5% (44/160) | 7.4% (2/27) |
| P value1 | 0.01 | NS |
Among the pathologically early HCCs, an AM ≥ 5 mm versus an AM of 0 to < 5 mm was compared to determine whether such factors influenced the rate of local tumor progression when examined using a log-rank test. However, no significant difference was observed (P = 0.338). Among the typical HCCs, however, an AM ≥ 5 mm versus an AM of 0 to < 5 mm was associated with a lower rate of local tumor progression (P = 0.032).
We performed adequate RFA for 50 pathologically early HCCs and demonstrated that the frequency of local tumor progression of pathologically early HCCs after adequate RFA was significantly lower than that of typical HCCs, although the present study was a pilot study, and a multicenter study is needed to confirm the presently reported results.
For the differential diagnosis of pathologically early HCC and dysplastic nodules (DN), the current algorithm used for the diagnosis of HCC in Japan recommends that a biopsy be performed for 1-1.5 cm or larger lesions appearing as hypo-intense masses during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA when the mass is hypo-vascular during the arterial phase of contrast-enhanced US and iso-vascular during the post-vascular phase of contrast-enhanced US[21]. Meanwhile, the optimal timing for the treatment of pathologically early HCC currently remains unclear. However, we have been aggressively using RFA for pathologically early HCC after providing an adequate explanation and obtaining the patient’s agreement, since these nodules are known to progress to typical HCC relatively frequently[24-26].
RFA is required to acquire an AM, since the liver tissues surrounding typical HCC can include micrometastases and microvascular invasion[15,27]. An AM of 5 mm or more for typical HCC is reportedly associated with a lower rate of local tumor progression[13]. Similarly, in our study, an AM of 5 mm or more for typical HCCs was associated with a lower rate of local tumor progression during the follow-up period. However, in assessments made using Sonazoid-enhanced three-dimensional US, many HCCs are located close to surrounding vessels[28]. In fact, an AM of 5 mm or more (as evaluated using contrast-enhanced US) was only secured in 15.6% of the cases in the present study. Therefore, RFA for pathologically early HCC lesions without micrometastases and microvascular invasion[1] may contribute to a reduction in local tumor progression after ablation. Nakazawa et al[13] reported that an ablation zone that included an AM of less than 5 mm from the tumor border and the presence of blood vessels contiguous with the tumors was significantly correlated with local tumor progression. If an AM of 5 mm or more is difficult to acquire because of the presence of blood vessels contiguous with the tumors, the benefit of early treatment for pathologically early HCC may be even more significant.
Hypo-vascular masses with hypo-intensity during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA have a high risk of progressing to typical HCC[26]. While pathologically early HCC itself is not a target lesion for hepatic resection[16], identifying the risk factors for the hypervascularization of these nodules, most of which are pathologically early HCCs, is important for making decisions regarding the timing of treatment[29]. Although the risk of hyper-vascularization of pathologically early HCC has not been previously studied, a risk of hypo-vascular masses with hypo-intensity during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA has been reported in some studies[24,25,30-33]. The findings of the diagnostic performance analysis showed that hypo-intensity during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA had an excellent sensitivity (97%) for the detection of early HCC and an exceptional specificity (100%) for distinguishing early HCC from DN[4]. Motosugi et al[24] reported that nodules that were more than 10 mm in diameter and contained fat were associated with a higher risk of developing hyper-vascularization. In addition, a maximum diameter of more than 10 mm[30] or 15 mm or greater[25], hyper-intensity on T1-weighted images[31], hyper-intensity on T2-weighted and diffusion-weighted images[32], and a tumor volume doubling time of less than 542 d[33] have been identified as risk factors for the hyper-vascularization of lesions appearing as hypo-intense masses during the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA. Of course, the detection of early hyper-vascularization in pathologically early HCC is important for identifying nodules requiring early treatment. Statistically, contrast-enhanced US (32.7%) was more sensitive than contrast-enhanced CT (21.2%) for the detection of hyper-vascularity in pathologically early HCCs[34].
In this study, only one pathologically early HCC was associated with a local tumor progression (Figure 3). Similar to typical HCC, pathologically early HCC also exhibits an internal histological heterogeneity[2], and the pathological diagnosis based on needle biopsied specimens might have resulted in an underestimation of the histological grade. This recurrent case might have contained internal dedifferentiated foci, and the needle biopsied specimens might not have been sufficiently acquired from these areas, leading to an underestimated diagnosis of pathologically early HCC. Furthermore, the thinnest AM of this recurrent case was less than 5 mm. To prevent local tumor progression, a larger AM might have been preferable in this case.
Contrast-enhanced CT is the most widely used imaging technique for evaluating the efficacy of HCC ablation[35,36]. Otherwise, contrast-enhanced US is useful for the early evaluation of the therapeutic response to RFA[28]. However, the boundary of the tumor is obfuscated after RFA in some cases[37]. Fusion imaging combining contrast-enhanced US images and the arterial-phase of contrast-enhanced CT images is useful for evaluating the efficacy of RFA for the treatment of hyper-vascular HCC[23]. The AM could be evaluated in detail using fusion imaging with GPS marks and an overlay image. Meanwhile, pathologically early HCCs are often recognized as an absence of hyper-vascularity during the arterial-phase of contrast-enhanced CT or MR images[4]. In the present study, we used fusion imaging combining contrast-enhanced US images and the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB-DTPA obtained before RFA to evaluate the efficacy of RFA for pathologically early HCCs.
Our study had several limitations. First, as mentioned above, the pathological diagnosis of early HCC was based on criteria obtained using needle biopsied specimens. Second, different definitions for evaluating local tumor progression after RFA were used for pathologically early HCC and typical HCC, since we wished to avoid a long lead-time bias. Third, patients who had both pathologically early HCC and typical HCC simultaneously were not excluded, and only 10 of the 50 pathologically early HCC cases had primary pathologically early HCC; therefore, the rates of intra-hepatic recurrence-free survival and of overall survival after ablation were not evaluated for cases with primary pathologically early HCC or for those with primary typical HCC. Further studies examining overall survival will be essential for defining the survival benefit of RFA for pathologically early HCC.
In conclusion, pathologically early HCC was rarely associated with local tumor progression after adequate RFA, compared with typical HCC, in the present study. We hope that the lower local tumor progression rate of pathologically early HCC after adequate RFA may lead to an improved patient prognosis.
The International Consensus Group for Hepatocellular Neoplasia announced a consensus on the pathological criteria for early hepatocellular carcinoma (HCC) (small well-differentiated HCC of the vaguely nodular type) in 2009. Midorikawa et al reported that the survival benefit associated with the resection of pathologically early HCC was marginal because of a long lead-time bias; therefore, they suggested that surgery is not indicated for pathologically early HCC. However, radiofrequency ablation (RFA) is expected to be an effective treatment for pathologically early HCC, which is known to progress to typical HCC relatively frequently. In this study, we evaluated whether pathologically early HCC exhibited local tumor progression after RFA less often than typical HCC.
Early-stage typical HCC lesions are often accompanied by micrometastases and microvascular invasion, despite their small size. In contrast, pathologically early HCC lesions are not accompanied by these features. Therefore, pathologically early HCC lesions are expected to exhibit a longer period until local tumor progression after RFA than typical HCC lesions. However, to our knowledge, the local tumor progression rate of pathologically early HCC after adequate RFA, compared with that of typical HCC, has not been previously studied.
In this study, 1/50 pathologically early HCC and 46/187 typical HCC exhibited local tumor progression after adequate RFA. Pathologically early HCC was statistically associated with a lower rate of local tumor progression after adequate RFA, compared with typical HCC.
Although further studies examining overall survival will be essential for defining the survival benefit of RFA for pathologically early HCC, the rate of local tumor progression after adequate RFA was relatively low when pathologically early HCC was treated before progression to typical HCC.
Early HCC was characterized by various combinations of the following major pathological features: (1) a cell density more than 2-fold higher than that of the surrounding tissue, with a higher nuclear/cytoplasm ratio and irregularly thin trabecular pattern; (2) varying numbers of portal tracts within the nodule (intratumoral portal tracts); (3) a pseudoglandular pattern; (4) diffuse fatty changes; and (5) varying numbers of unpaired arteries.
This is an interesting paper on an important argument. Very early HCC defined by Barcelona Clinic Liver Cancer, commonly known as BCLC, staging classification could be also an invasive tumor. On the other hand, pathologically early HCC should represent a less invasive neoplastic lesion. RFA could be the ideal treatment.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Joko K, Santambrogio R, Tsoulfas G S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | International Consensus Group for Hepatocellular Neoplasia The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 589] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 2. | Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44 Suppl 19:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y. Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology. 2009;252:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011;261:834-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 5. | Kudo M. Early hepatocellular carcinoma: definition and diagnosis. Liver Cancer. 2013;2:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Ohama H, Imai Y, Nakashima O, Kogita S, Takamura M, Hori M, Seki Y, Sawai Y, Igura T, Fukuda K. Images of Sonazoid-enhanced ultrasonography in multistep hepatocarcinogenesis: comparison with Gd-EOB-DTPA-enhanced MRI. J Gastroenterol. 2014;49:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia. 2013;29:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-577; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 10. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (& gt; 2 and & lt; 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Kawamura Y, Ikeda K, Seko Y, Hosaka T, Kobayashi M, Saitoh S, Kumada H. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR Am J Roentgenol. 2011;197:W665-W673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 271] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 14. | Park Y, Kim YS, Rhim H, Lim HK, Choi D, Lee WJ. Arterial enhancement of hepatocellular carcinoma before radiofrequency ablation as a predictor of postablation local tumor progression. AJR Am J Roentgenol. 2009;193:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26:142-147. [PubMed] |
| 16. | Midorikawa Y, Takayama T, Shimada K, Nakayama H, Higaki T, Moriguchi M, Nara S, Tsuji S, Tanaka M. Marginal survival benefit in the treatment of early hepatocellular carcinoma. J Hepatol. 2013;58:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Kunishi Y, Numata K, Morimoto M, Okada M, Kaneko T, Maeda S, Tanaka K. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Nakano M, Saito A, Yamamoto M, Doi M, Takasaki K. Stromal and blood vessel wall invasion in well-differentiated hepatocellular carcinoma. Liver. 1997;17:41-46. [PubMed] |
| 19. | Kobayashi S, Kim SR, Imoto S, Ando K, Hirakawa M, Saito J, Fukuda K, Otono Y, Sakaki M, Tsuchida S. Histopathological diagnosis of early HCC through biopsy: efficacy of Victoria blue and cytokeratin 7 staining. Dig Dis. 2012;30:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Park YN, Kojiro M, Di Tommaso L, Dhillon AP, Kondo F, Nakano M, Sakamoto M, Theise ND, Roncalli M. Ductular reaction is helpful in defining early stromal invasion, small hepatocellular carcinomas, and dysplastic nodules. Cancer. 2007;109:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 22. | Numata K, Luo W, Morimoto M, Kondo M, Kunishi Y, Sasaki T, Nozaki A, Tanaka K. Contrast enhanced ultrasound of hepatocellular carcinoma. World J Radiol. 2010;2:68-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Numata K, Fukuda H, Morimoto M, Kondo M, Nozaki A, Oshima T, Okada M, Takebayashi S, Maeda S, Tanaka K. Use of fusion imaging combining contrast-enhanced ultrasonography with a perflubutane-based contrast agent and contrast-enhanced computed tomography for the evaluation of percutaneous radiofrequency ablation of hypervascular hepatocellular carcinoma. Eur J Radiol. 2012;81:2746-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Motosugi U, Ichikawa T, Sano K, Sou H, Onohara K, Muhi A, Amemiya F, Enomoto N, Matsuda M, Fujii H. Outcome of hypovascular hepatic nodules revealing no gadoxetic acid uptake in patients with chronic liver disease. J Magn Reson Imaging. 2011;34:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Kumada T, Toyoda H, Tada T, Sone Y, Fujimori M, Ogawa S, Ishikawa T. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2011;197:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Kobayashi S, Matsui O, Gabata T, Koda W, Minami T, Ryu Y, Kawai K, Kozaka K. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic Acid-enhanced magnetic resonance imaging findings of borderline lesions at high risk for progression to hypervascular classic hepatocellular carcinoma. J Comput Assist Tomogr. 2011;35:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US, CT and MRI. World J Gastroenterol. 2014;20:4160-4166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Luo W, Numata K, Morimoto M, Oshima T, Ueda M, Okada M, Takebayashi S, Zhou X, Tanaka K. Role of Sonazoid-enhanced three-dimensional ultrasonography in the evaluation of percutaneous radiofrequency ablation of hepatocellular carcinoma. Eur J Radiol. 2010;75:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Matsuda M. Clinical value of gadoxetic acid-enhanced magnetic resonance imaging in surgery for hepatocellular carcinoma - with a special emphasis on early hepatocellular carcinoma. World J Hepatol. 2015;7:2933-2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Takechi M, Tsuda T, Yoshioka S, Murata S, Tanaka H, Hirooka M, Mochizuki T. Risk of hypervascularization in small hypovascular hepatic nodules showing hypointense in the hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with chronic liver disease. Jpn J Radiol. 2012;30:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Higaki A, Ito K, Tamada T, Teruki S, Yamamoto A, Higashi H, Kanki A, Sato T, Noda Y. High-risk nodules detected in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging in cirrhosis or chronic hepatitis: incidence and predictive factors for hypervascular transformation, preliminary results. J Magn Reson Imaging. 2013;37:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012;265:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Hyodo T, Murakami T, Imai Y, Okada M, Hori M, Kagawa Y, Kogita S, Kumano S, Kudo M, Mochizuki T. Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology. 2013;266:480-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Numata K, Fukuda H, Miwa H, Ishii T, Moriya S, Kondo M, Nozaki A, Morimoto M, Okada M, Takebayashi S. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur J Radiol. 2014;83:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, Lim JH, Kim SA. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, Lee JH, Lim JH, Choo IW. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Zhou P, Kudo M, Minami Y, Chung H, Inoue T, Fukunaga T, Maekawa K. What is the best time to evaluate treatment response after radiofrequency ablation of hepatocellular carcinoma using contrast-enhanced sonography? Oncology. 2007;72 Suppl 1:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |