Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2940
Peer-review started: December 2, 2016
First decision: December 28, 2016
Revised: February 15, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: April 28, 2017
To investigate the effects of nesfatin-1 on gastric function in obese rats.
The obese rat model was induced by a high-fat diet. The gastric emptying rate and gastric acid secretory capacity of the rats were determined after treatment with different drug concentrations of nesfatin-1 and administration routes. Based on this, the expression of H+/K+-ATPase was measured using RT-PCR and western blot to preliminarily explore the mechanism of gastric acid secretion changes.
Body weight, body length, and Lee’s index of the rats significantly increased in the high-fat diet-induced obese rat model. Two hours after lateral intracerebroventricular injection of nesfatin-1, the gastric emptying rate and gastric acid secretory capacity of rats decreased. Four hours after injection, both were restored to normal levels. In addition, the expression of H+/K+-ATPase decreased and moved in line with changes in gastric acid secretory capacity. This in vivo experiment revealed that intracerebroventricular injection of nesfatin-1, rather than intravenous injection, could suppress gastric function in obese rats. Moreover, its effect on the gastric emptying and gastric acid secretory capacity of rats is dose-dependent within a certain period of time.
Through this research, we provide a theoretical basis for further studies on nesfatin-1, a potential anti-obesity drug.
Core tip: The high-fat diet-induced obese rat model was used to study the effects of nesfatin-1 on gastric function. We found that intracerebroventricular injection of nesfatin-1, rather than intravenous injection, could suppress gastric function in a dose-dependent manner within a certain period of time. In addition, the expression of H+/K+ ATPase was down-regulated, which may explain the mechanisms of gastric acid secretion changes.
- Citation: Yang GT, Zhao HY, Kong Y, Sun NN, Dong AQ. Study of the effects of nesfatin-1 on gastric function in obese rats. World J Gastroenterol 2017; 23(16): 2940-2947
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2940
Obesity is a global public health priority. With the improvement of people’s standard of living, the number of obese Chinese has grown to an annual rate of 38.1% over the past several years[1-4]. It is therefore vital to carry out studies on mechanisms linked to obesity. Nesfatin-1, an anorectic peptide first discovered in 2006, has been proven as an inhibitor of food intake[5-8]. Nesfatin-1 can be expressed in peripheral organs, such as the gastrointestinal tract, and plays a role in the regulation of many physiological processes, such as carbohydrate metabolism, immunity, and the digestive tract[9-13]. Studies have shown that nesfatin-1 mRNA, together with ghrelin somatostatin and histidine decarboxylase, regulate gastric acid secretion and are co-expressed in oxyntic glands in the fundus of the stomach; this means that nesfatin-1 may be also involved in gastric acid secretion[14-16]. In vitro studies have shown that nesfatin-1 can inhibit the expression of H+/K+ ATPase to block gastric acid secretion induced by histamine, as oxyntic cells of the glands secrete gastric acid[17,18]. Researchers have also found that lateral intracerebroventricular injection of nesfatin-1 can reduce the gastrointestinal mobility of rats[19]. However, the clinical use of nesfatin-1 is limited, since it remains unknown whether the routes of nesfatin-1 administration influence gastric emptying, as well as how and when this would affect gastric functions. The present study therefore focuses on the effects of drug administration routes and drug concentration on gastric functions, as well as the potential mechanisms, in order to provide a preliminary theoretical basis for further studies for determining how nesfatin-1 acts and the development of possible drugs for the treatment of obesity.
Healthy and weaned Sprague Dawley (SD) rats weighing 50 g were provided by the experimental animal center of Anhui Medical University (Permit number: SYXK (e) 2012-002). Animals were housed in a controlled environment in collective cages (five rats in each cage) at 23 ± 2 °C with a 12-h light/dark cycle. Rats were allowed to acclimate to these conditions for a week before being randomized into experimental groups. Phenol red was purchased from Tiangen Biotech (Beijing). To produce methylcellulose-phenol red solution (100 mL, 50 mg/dL), 5 mg of phenol red was dissolved in 1.5% methylcellulose. Anti-nesfatin-1 polyclonal antibody (H-003-22) was obtained from American Phoenix Pharmaceuticals (United States), rabbit anti-rat histone polyclonal antibody (ab125260) was purchased from Abcam (United States), and rabbit anti-rat H+/K+ ATPase subunit-β antibody and rabbit anti-rat H+/K+ ATPase subunit-αantibody were obtained from Merk (Germany). These experiments were approved by the institute, and all efforts were made to minimize animal suffering.
Animals: After an adjustable feeding period of one week, 155 rats were randomly assigned into two groups: the control group (five normal rats) and the high-fat diet group (300 obese rats). In the control group, rats were fed with basic forage. In the high-fat diet group, rats were fed with high-fat forage (15 g of lard, 50 g of egg yolk, 15 g of whole milk, and 10 drops of concentrated cod liver oil were added into per 100 g basic forage). In order to assess the feasibility of the obese rat model, body length and body weight changes of the rats were recorded. Lee’s index and serum triglyceride levels were calculated after feeding for six weeks.
Implantation of the cannula into the lateral ventricle: Each rat was anesthetized using 7% chloral hydrate (0.5 mL/100 g) by intraperitoneal injection. The fur on the head was then shaved, with each rat being subsequently placed on the stereotaxic apparatus. According to the Paxinos-Watson rat brain stereotaxic coordinates, an incision was made into the skin above the animal’s skull, with a hole then being drilled into the skull. A thin catheter was slowly inserted into the right-cerebral ventricle and fixed with bone cement[20,21]. After surgery, each rat was fed separately and provided with 15000 units of penicillin to avoid infection. Follow-up experiments were carried out after they were fed normally for one week, when the stress reaction was reduced.
Experimental grouping: The 300 obese rats that had a cannula placed in the right lateral-cerebral ventricle were further randomly assigned into two groups: the intracerebroventricular injection group and the intravenous injection group. The treatment dose was as follows: intracerebroventricular injection group, 0 μg/5 μL of nesfatin-1, 0.5 μg/5 μL of nesfatin-1, and 1.0 μg/5 μL of nesfatin-1; intravenous injection group, 0 μg/5 μL of nesfatin-1, 0.5 μg/5 μL of nesfatin-1, and 1.0 μg/5 μL of nesfatin-1. Each group was comprised of 150 rats. Gastrointestinal mobility and gastric acid secretion in the rats were measured at 1, 2, 3, and 4 h after drug administration.
Determination of gastrointestinal mobility: Rats received intragastric administration of 2 mL of phenol red. After 20 min, the rats were anesthetized using diethyl ether and sacrificed. The stomach was then cut along the greater curvature, washed with distilled water to collect the stomach contents, and diluted with water to 20 mL. Next, 20 mL of 0.5 mol/L of NaOH was added into the suspension before standing for 0.5 h at room temperature. Then, 0.5 mL of trichloroacetic acid (200 g/L) was added into 2.5 mL of the supernatant to remove the proteins. The mixture was centrifuged at 3500 rpm for 10 min. The absorbance of the supernatant was measured at 560 mL. Gastric emptying rate was determined using the following formula: gastric emptying rate = (1- measured phenol red absorbance/ standard phenol red absorbance) × 100%[22].
Determination of gastric acid secretion: The principle of acid-base titrations was applied to determine gastric acid secretion. As previously described, gastric juice was collected from the stomach of rats. Then, 0.5 mL of gastric acid was pipetted into a clean beaker containing 2 mL of distilled water. Two drops of phenolphthalein was added, swirled gently to mix well, titrated by slowly adding the NaOH solution (0.5 mol/L), and then swirled in the beaker. Titration ended when the solution turned a faint and persistent color. The volume of NaOH dispensed was recorded in the following manner: volume of gastric acid secretion= (C [NaOH] ×V [NaOH] ×V [gastric juice] /0.5 mL).
Real-time polymerase chain reaction: Gastric mucosal tissues measuring 20 mg were randomly obtained from different parts. Total RNA was extracted using TRIZOL reagent and converted into cDNAs by reverse transcription. With β-actin as the internal control gene, target gene fragments were amplified by real-time polymerase chain reaction (PCR). RT-PCR products measuring 5 μL were subjected to 2% agarose gel electrophoresis. The absorbance of the target gene strands was detected. The relative mRNA expression level of H+/K+-ATP was determined as the ratio of the absorbance of the target gene to that of β-actin.
The real-time PCR primers are as follows: H+/K+-ATPase, 5’-CTCTGCTTTGCGGGACTT-3’ (forward) and 5’-CCTTGGCTGTGATGGGAT-3’(reverse); β-actin, 5’-AGCTGAGAGGGAAATCGTGCG-3’ (forward) and 5’-GTGCCACCAGACAGCACTGTG-3’ (reverse).
Western blot: Gastric mucosal tissues measuring 20 mg were randomly obtained from different parts and total proteins were extracted from the samples. Protein concentrations were detected using the BCA method. Protein extracts containing the same quality were subjected to SDS-PAGE using 12% polyacrylamide gel, followed by western blotting. Each membrane was blocked in 5% skim milk for 2 h at room temperature. The membrane was then incubated for one hour at room temperature with anti-H+/K+-ATPase antibody. After washing with phosphate-buffered saline/tween three times for five minutes each time, each membrane was incubated with a corresponding secondary fluorescein-labeled antibody for one hour at room temperature. The bands were then visualized and imaged using the Odyssey infrared imaging system (LI-COR, Germany).
Statistical analysis was performed using SPSS Statistics version 20. Data were expressed as means ± SD. Comparisons of categorical variables were carried out using one-way ANOVA. Dunnett’s t-test was employed to further analyze the differences between groups. P < 0.05 was considered statistically significant.
As shown in Table 1, the body weight, body length, Lee’s index, and triglyceride level of the rats were much higher in the high-fat diet group than in the control group; the differences were considered statistically significant (P < 0.05).
| Group | Body weight (g) | Body length (cm) | Lee’s index | Triglyceride (mmol/L) |
| Control (n = 5) | 329.76 ± 65.23 | 19.8 ± 0.8 | 335.59 ± 12.87 | 1.22 ± 0.24 |
| High-fat diet (n = 40) | 410 ± 53.34 | 21.02 ± 1.0 | 357.40 ± 13.98 | 1.56 ± 0.26 |
| t | -3.101 | -2.616 | -3.125 | -2.776 |
| P | 0.003 | 0.012 | 0.002 | 0.008 |
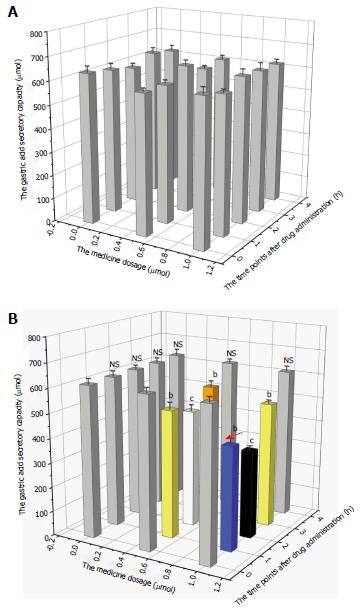
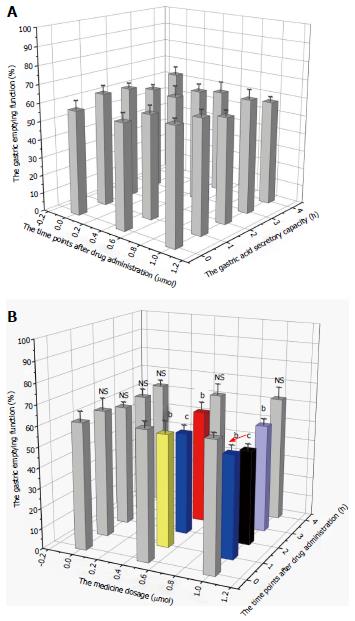
Changes in gastric acid secretion and gastrointestinal mobility are shown in Figures 1 and 2. The gastric acid secretory capacity and gastrointestinal mobility of rats that received nesfatin-1 by intravenous injection did not significantly change at each drug dosage and time point; each group was presented as a gray value histogram (Figure 1A and Figure 2A). However, the intracerebroventricular administration of nesfatin-1 induced a great difference in the gastric acid secretion of rats. While the gastric acid secretion level did not significantly change at any of the time points after the administration of nesfatin-1 at a dose of 0 μg/5 μL, the secretion levels of rats injected with nesfatin-1 at a dose of 0.5 μg/5 μL and 1.0 μg/5 μL initially decreased, returned to normal levels, and then returned to the lowest level two hours after administration (see black and white value histogram in Figure 1B). Changes in gastric emptying revealed similarities, as it reached its lowest level two hours after administration before returning to normal levels four hours after administration (Figure 2B).
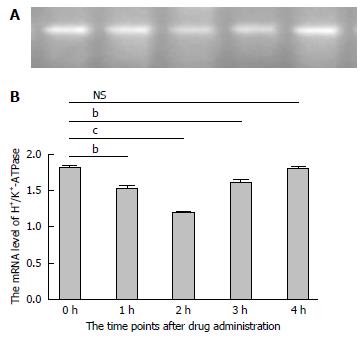
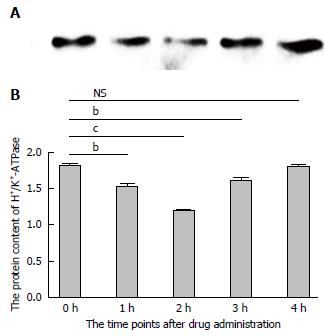
In order to further investigate the potential mechanism of gastric acid secretion induced by intracerebroventricularly administered nesfatin-1, gastric mucosal tissues that receiving 1.0 μg/5 μL of nesfatin-1 were obtained to study the expression of H+/K+-ATPase. A similar tendency was observed in the mRNA expression of H+/K+-ATPase detected by RT-PCR and western blot. The data revealed that intracerebroventricularly injected nesfatin-1 significantly decreased mRNA expression levels two hours after administration; this difference was statistically significant (P = 0.000). In addition, four hours after the injection, mRNA expression returned to normal levels (P = 0.792). The tendency of the protein level of H+/K+-ATPase was similar (Figures 3 and 4).
Due to lifestyle changes, obesity has become a major global public health issue that troubles many countries[23]. As shown in many epidemiologic studies, the morbidity of diabetes and cardiac disease in the obese is higher than that in the normal population, which can potentially lead to a heavy financial burden for both families and societies. Therefore, studying obesity is of great importance[24-27]. There is an apparent correlation between obesity and dieting status, with a person’s dietary behavior being tightly controlled by neural and humoral factors[28-30]. In 2006, Japanese scholars found a peptide involved in the regulation of energy homeostasis, as well as in food and water intake, with said peptide being able to regulate feeding behavior via the neuro humoral system[31-34]. This current study systematically studied the effects of drug-delivery methods and drug concentration on gastric functions, as well as the molecular mechanisms involved in changes in gastric acid secretory capacity. This research may provide a preliminary theoretical basis for the design of anti-obesity drugs.
Given the fact that the main cause of obesity is overconsumption of high-calorie food, it is of great importance to establish a rat model that has the same cause of obesity as humans[35-37]. Based on the dietary characteristics of some individuals (which are high in fat and sugar), the obese rat model was established in six weeks by adding moderate amounts of sugar, fat, and protein into the rat’s diets. Results revealed that body weight, body length, Lee’s index, and postprandial triglyceride levels significantly increased in the obese rat model induced by the high-fat diet. This indicates that the model was successfully established.
The precursor of nesfatin-1, NMCB-2, can be hydrolyzed into three parts by the action of prohormone convertase, namely nesfatin-1, nesfatin-2, and nesfatin-3[38-41]. Research carried by Pan et al[42] revealed that the permeation of nesfatin-1 between the blood and brain was a non-saturable process. Furthermore, Atsuchi et al[43] found that the vagal nerve may be involved in the process of nesfatin-1’s effect on gastric functions. However, our study revealed that intravenous injection of nesfatin-1 had no significant impact on the volume of gastric juice or gastric emptying rate. This indicates that nesfatin-1 in the brain effects gastric function via some method that includes the nervous system, rather than crossing the blood-brain barrier before taking effect.
Studies have found that the central administration of nesfatin-1 results in the inhibition of gastrointestinal mobility in rats in a dose-dependent manner. In addition, administration of nesfatin-1 antibody promotes food intake[44,45]. This indicates that nesfatin-1 can reduce food intake. Our study further revealed that intracerebroventricular injection of nesfatin-1, rather than intravenous injection, can slow down intestinal peristalsis and suppress gastric emptying and gastric acid production. Moreover, these effects are dependent on drug dosage within a certain period of time.
Previous studies have shown that a delay in gastric emptying leads to increased stomach content, thereby affecting food intake[46]. Hence, we postulate that nesfatin-1 may cause food refusal by affecting gastrointestinal peristalsis. Nesfatin-1 suppress gastric emptying and secretion of gastric acid within certain periods of time, which can prolong food stay in the stomach, slow down food digestion, and induce satiety, resulting in food refusal.
H+/K+-ATPase, a key enzyme in gastric acid production, promotes the secretion of gastric acid through phosphorylation in the process of extracellular K+ and intracellular H+ transmembrane transport[47,48]. H+/K+-ATPase serves as a key point in the signaling transduction pathway regulating gastric acid secretion. Its expression levels in cells located in the gastric mucosal gland have a direct effect on gastric acid production[49,50]. Therefore, in the present study, we observed the expression levels of H+/K+-ATPase to preliminarily explore the potential mechanism of centrally administered nesfatin-1 on gastric acid secretion. Western bolt and RT-PCR analyses demonstrated that the protein and mRNA expression levels of H+/K+-ATPase decreased after intracerebroventricular injection of nesfatin-1. These levels returned to normal after four hours, and moved in line with the changes in gastric acid production. Our study revealed that centrally administered nesfatin-1 may inhibit the expression of H+/K+-ATPase in gastric mucosal tissues, thereby suppressing the secretion of gastric acid.
Due to the limited time, we were unable to explore the relationship between gastric mobility and the vagus nerve, even though centrally administered nesfatin-1 has been reported to play a role in the regulation of gastrointestinal function through the vagus nerve. Further studies are needed to investigate whether the central administration of nesfatin-1 affects the expression of H+/K+-ATPase through the vagus nerve.
Overall, our in vivo experiment demonstrates that the central administration of nesfatin-1, rather than its peripheral administration, had an effect on gastrointestinal functions in obese rats. Centrally administered nesfatin-1 inhibited gastric mobility and gastric acid secretion in a dose-independent manner over a period of time. This study provides a preliminary theoretical basis for the development of nesfatin-1 for the treatment of obesity.
Obesity has become a major global public health issue that can lead to heavy financial burdens for both families and societies. Nesfatin-1, a new anorectic peptide, has been proven as an inhibitor of food intake and has the potential to be used in the treatment of obesity. However, the clinical use of nesfatin-1 is limited, since it remains unknown whether the routes of administration of nesfatin-1 influence gastric emptying, as well as how and when this would affect gastric functions.
Nesfatin-1 plays a role in the regulation of many physiological processes, such as carbohydrate metabolism, immunity, and the digestive tract. Studies have shown that nesfatin-1 mRNA, together with ghrelin somatostatin and histidine decarboxylase, regulate gastric acid secretion and are co-expressed in oxyntic glands in the fundus of the stomach. In vitro studies have shown that nesfatin-1 can inhibit the expression of H+/K+ ATPase to block gastric acid secretion. Researchers have also found that lateral intracerebroventricular injection of nesfatin-1 can reduce the gastrointestinal mobility of rats.
This in vivo experiment revealed that intracerebroventricular injection of nesfatin-1 could suppress gastric function in obese rats. Moreover, its effect on gastric emptying and the gastric acid secretory capacity of rats is dose-dependent within a certain period of time. Intracerebroventricular injection of nesfatin-1 could also suppress the expression of H+/K+ ATPase in vivo, which may be the mechanism of gastric acid secretion changes.
This study demonstrates that intracerebroventricular injection of nesfatin-1 could successful suppress gastric function in obese rats, which may indicate its potential use for the treatment of obesity. If further research finds the exact molecular mechanism of nesfatin-1, obesity may even be cured someday.
Although obesity is a major global public health issue, there is no drug cure for it. This study reveals that intracerebroventricular injection of nesfatin-1 could successful suppress gastric function in a dose-dependent manner within a certain period of time. A preliminary theoretical basis for the development of nesfatin-1 for the treatment of obesity is provided by this research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arredondo M, Berger BM S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Guo Y, Yue XJ, Li HH, Song ZX, Yan HQ, Zhang P, Gui YK, Chang L, Li T. Overweight and Obesity in Young Adulthood and the Risk of Stroke: a Meta-analysis. J Stroke Cerebrovasc Dis. 2016;25:2995-3004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Li Z, Guo X, Liu Y, Zhang N, Chang Y, Chen Y, Sun Y, Abraham MR. Metabolism rather than obesity is associated with ischemic stroke: a cross-sectional study in rural Northeastern China. Springerplus. 2016;5:1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Tao XG, Su PY, Yuspeh L, Lavin RA, Kalia-Satwah N, Bernacki EJ. Is Obesity Associated With Adverse Workers’ Compensation Claims Outcomes? J Occup Environ Med. 2016;58:880-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Zhang T, Cai L, Ma L, Jing J, Chen Y, Ma J. The prevalence of obesity and influence of early life and behavioral factors on obesity in Chinese children in Guangzhou. BMC Public Health. 2016;16:954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Algul S, Ozkan Y, Ozcelik O. Serum nesfatin-1 levels in patients with different glucose tolerance levels. Physiol Res. 2016;65:979-985. [PubMed] [Cited in This Article: ] |
| 6. | Gulcicek OB, Solmaz A, Yiğitbaş H, Ercetin C, Yavuz E, Ozdogan K, Arici S, Akkalp AK, Sarac T, Çelebi F. Comparison of the Effects of Glutamine, Curcumin, and Nesfatin-1 on the Gastric Serosal Surface Neomucosa Formation: An Experimental Rodent Model. Gastroenterol Res Pract. 2016;2016:2081962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Prinz P, Stengel A. Expression and regulation of peripheral NUCB2/nesfatin-1. Curr Opin Pharmacol. 2016;31:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Prinz P, Stengel A. Nesfatin-1: current status as a peripheral hormone and future prospects. Curr Opin Pharmacol. 2016;31:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Xiao C, Liu J, Tang Y, Chen J, Wu X, Bi F, Zhang J. Expression, purification, and characterization of mouse nesfatin-1 in Escherichia coli. Biotechnol Appl Biochem. 2017;64:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Blanco AM, Bertucci JI, Delgado MJ, Valenciano AI, Unniappan S. Tissue-specific expression of ghrelinergic and NUCB2/nesfatin-1 systems in goldfish (Carassius auratus) is modulated by macronutrient composition of diets. Comp Biochem Physiol A Mol Integr Physiol. 2016;195:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Burgos JR, Iresjö BM, Smedh U. MCG101-induced cancer anorexia-cachexia features altered expression of hypothalamic Nucb2 and Cartpt and increased plasma levels of cocaine- and amphetamine-regulated transcript peptides. Oncol Rep. 2016;35:2425-2430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Cao X, Zhou X, Cao Y, Liu XM, Zhou LH. Expression of NUCB2/nesfatin-1 in the taste buds of rats. Endocr J. 2016;63:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jiang S, Zhou W, Zhang X, Wang D, Zhu H, Hong M, Gong Y, Ye J, Fang F. Developmental expression and distribution of nesfatin-1/NUCB2 in the canine digestive system. Acta Histochem. 2016;118:90-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Chen X, Shu X, Cong ZK, Jiang ZY, Jiang H. Nesfatin-1 acts on the dopaminergic reward pathway to inhibit food intake. Neuropeptides. 2015;53:45-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Ozturk CC, Oktay S, Yuksel M, Akakin D, Yarat A, Kasimay Cakir O. Anti-inflammatory effects of nesfatin-1 in rats with acetic acid - induced colitis and underlying mechanisms. J Physiol Pharmacol. 2015;66:741-750. [PubMed] [Cited in This Article: ] |
| 16. | Watanabe A, Mochiki E, Kimura A, Kogure N, Yanai M, Ogawa A, Toyomasu Y, Ogata K, Ohno T, Suzuki H. Nesfatin-1 suppresses gastric contractions and inhibits interdigestive migrating contractions in conscious dogs. Dig Dis Sci. 2015;60:1595-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Jia FY, Li XL, Li TN, Wu J, Xie BY, Lin L. Role of nesfatin-1 in a rat model of visceral hypersensitivity. World J Gastroenterol. 2013;19:3487-3493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mohan H, Unniappan S. Phylogenetic aspects of nucleobindin-2/nesfatin-1. Curr Pharm Des. 2013;19:6929-6934. [PubMed] [Cited in This Article: ] |
| 19. | Tian ZB, Deng RJ, Sun GR, Wei LZ, Kong XJ, Ding XL, Jing X, Zhang CP, Ge YL. Expression of gastrointestinal nesfatin-1 and gastric emptying in ventromedial hypothalamic nucleus- and ventrolateral hypothalamic nucleus-lesioned rats. World J Gastroenterol. 2014;20:6897-6905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Peredery O, Persinger MA, Parker G, Mastrosov L. Temporal changes in neuronal dropout following inductions of lithium/pilocarpine seizures in the rat. Brain Res. 2000;881:9-17. [PubMed] [Cited in This Article: ] |
| 21. | Figini M, Zucca I, Aquino D, Pennacchio P, Nava S, Di Marzio A, Preti MG, Baselli G, Spreafico R, Frassoni C. In vivo DTI tractography of the rat brain: an atlas of the main tracts in Paxinos space with histological comparison. Magn Reson Imaging. 2015;33:296-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Sobczak M, Cami-Kobeci G, Sałaga M, Husbands SM, Fichna J. Novel mixed NOP/MOP agonist BU08070 alleviates pain and inhibits gastrointestinal motility in mouse models mimicking diarrhea-predominant irritable bowel syndrome symptoms. Eur J Pharmacol. 2014;736:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Novotny R, Li F, Fialkowski MK, Bersamin A, Tufa A, Deenik J, Coleman P, Guerrero RL, Wilkens LR. Prevalence of obesity and acanthosis nigricans among young children in the children’s healthy living program in the United States Affiliated Pacific. Medicine (Baltimore). 2016;95:e4711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Armstrong SC, Skinner AC. Defining “Success” in Childhood Obesity Interventions in Primary Care. Pediatrics. 2016;138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Macia E, Gueye L, Duboz P. Hypertension and Obesity in Dakar, Senegal. PLoS One. 2016;11:e0161544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Ruhstaller KE, Elovitz MA, Stringer M, Eppersonmd CN, Durnwald CP. Obesity and the Association with Maternal Mental Health Symptoms. J Matern Fetal Neonatal Med. 2016; Epub ahead of print: 1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2017;134:62-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13:11-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 212] [Article Influence: 30.3] [Reference Citation Analysis (1)] |
| 29. | Luchterhand KM, Silva PR, Chebel RC, Endres MI. Association between Prepartum Feeding Behavior and Periparturient Health Disorders in Dairy Cows. Front Vet Sci. 2016;3:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Mignon-Grasteau S, Chantry-Darmon C, Boscher MY, Sellier N, Le Bihan-Duval E, Bertin A. Genetic Determinism of Fearfulness, General Activity and Feeding Behavior in Chickens and Its Relationship with Digestive Efficiency. Behav Genet. 2017;47:114-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Li Z, Mulholland M, Zhang W. Regulation of gastric nesfatin-1/NUCB2. Curr Pharm Des. 2013;19:6981-6985. [PubMed] [Cited in This Article: ] |
| 32. | Sedbazar U, Maejima Y, Nakata M, Mori M, Yada T. Paraventricular NUCB2/nesfatin-1 rises in synchrony with feeding suppression during early light phase in rats. Biochem Biophys Res Commun. 2013;434:434-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Stengel A, Taché Y. New Developments On NUBC2/Nesfatin-1. Curr Pharm Des. 2013; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 34. | Stengel A, Taché Y. Role of brain NUCB2/nesfatin-1 in the regulation of food intake. Curr Pharm Des. 2013;19:6955-6959. [PubMed] [Cited in This Article: ] |
| 35. | Gzielo K, Kielbinski M, Ploszaj J, Janeczko K, Gazdzinski SP, Setkowicz Z. Long-Term Consumption of High-Fat Diet in Rats: Effects on Microglial and Astrocytic Morphology and Neuronal Nitric Oxide Synthase Expression. Cell Mol Neurobiol. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Lee JH, Lee JJ, Cho WK, Yim NH, Kim HK, Yun B, Ma JY. KBH-1, an herbal composition, improves hepatic steatosis and leptin resistance in high-fat diet-induced obese rats. BMC Complement Altern Med. 2016;16:355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Torres-Villalobos G, Hamdan-Pérez N, Díaz-Villaseñor A, Tovar AR, Torre-Villalvazo I, Ordaz-Nava G, Morán-Ramos S, Noriega LG, Martínez-Benítez B, López-Garibay A. Autologous subcutaneous adipose tissue transplants improve adipose tissue metabolism and reduce insulin resistance and fatty liver in diet-induced obesity rats. Physiol Rep. 2016;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Feijóo-Bandín S, Rodríguez-Penas D, García-Rúa V, Mosquera-Leal A, Otero MF, Pereira E, Rubio J, Martínez I, Seoane LM, Gualillo O. Nesfatin-1 in human and murine cardiomyocytes: synthesis, secretion, and mobilization of GLUT-4. Endocrinology. 2013;154:4757-4767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Nakata M, Yada T. Role of NUCB2/nesfatin-1 in glucose control: diverse functions in islets, adipocytes and brain. Curr Pharm Des. 2013;19:6960-6965. [PubMed] [Cited in This Article: ] |
| 40. | Vas S, Ádori C, Könczöl K, Kátai Z, Pap D, Papp RS, Bagdy G, Palkovits M, Tóth ZE. Nesfatin-1/NUCB2 as a potential new element of sleep regulation in rats. PLoS One. 2013;8:e59809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Yosten GL, Samson WK. Cardiovascular and antidipsogenic effects of nesfatin-1. Curr Pharm Des. 2013;19:6973-6975. [PubMed] [Cited in This Article: ] |
| 42. | Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223-2228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 43. | Atsuchi K, Asakawa A, Ushikai M, Ataka K, Tsai M, Koyama K, Sato Y, Kato I, Fujimiya M, Inui A. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport. 2010;21:1008-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 45. | Senin LL, Al-Massadi O, Barja-Fernandez S, Folgueira C, Castelao C, Tovar SA, Leis R, Lago F, Baltar J, Baamonde I. Regulation of NUCB2/nesfatin-1 production in rat’s stomach and adipose tissue is dependent on age, testosterone levels and lactating status. Mol Cell Endocrinol. 2015;411:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science. 1986;231:609-611. [PubMed] [Cited in This Article: ] |
| 47. | Qin H, Zhang Y, Wang R, Du X, Li L, Du H. Puerarin Suppresses Na+-K+-ATPase-Mediated Systemic Inflammation and CD36 Expression, and Alleviates Cardiac Lipotoxicity In Vitro and In Vivo. J Cardiovasc Pharmacol. 2016;68:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Walter C, Tanfous MB, Igoudjil K, Salhi A, Escher G, Crambert G. H,K-ATPase type 2 contributes to salt-sensitive hypertension induced by K(+) restriction. Pflugers Arch. 2016;468:1673-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Miyazaki H, Wangemann P, Marcus DC. The gastric H,K-ATPase in stria vascularis contributes to pH regulation of cochlear endolymph but not to K secretion. BMC Physiol. 2016;17:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Wyckelsma VL, McKenna MJ. Effects of Age on Na(+),K(+)-ATPase Expression in Human and Rodent Skeletal Muscle. Front Physiol. 2016;7:316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |