Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2443
Peer-review started: October 30, 2016
First decision: December 19, 2016
Revised: December 23, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: April 7, 2017
Processing time: 159 Days and 6 Hours
Hepatic angiosarcoma is a mesenchymal tumor originating from liver sinusoidal endothelial cells. It is an extremely rare malignant neoplasm accounting for less than 1% of primary malignant liver tumors. The deregulated coagulopathy that can be seen in hepatic angiosarcoma fulfills the clinical diagnostic criteria of disseminated intravascular coagulation. However, the mechanism that governs this coagulopathy has been poorly understood. This case report provides histological evidence of the consumption of coagulation factors along with trapped platelets occurring within the tumor, which is the foundation for the concept of Kasabach-Merritt syndrome (KMS). KMS is characterized by thrombocytopenia and hyperconsumption of coagulation factors within a vascular tumor. However, KMS associated with angiosarcoma has not been well recognized. This case report describes, for the first time, the histological evidence of KMS that occurred in an extremely rare mesenchymal malignant tumor of the liver.
Core tip: Kasabach-Merritt syndrome (KMS) is characterized by thrombocytopenia and hyperconsumption of coagulation factors within a vascular tumor. KMS is typically seen in the pediatric population however there have been reports of KMS occurring in association with adult vascular tumors. Based on laboratory findings, it is hard to differentiate KMS from disseminated intravascular coagulation. Here, we describe, for the first time the histological evidence validating the concept of KMS.
- Citation: Wadhwa S, Kim TH, Lin L, Kanel G, Saito T. Hepatic angiosarcoma with clinical and histological features of Kasabach-Merritt syndrome. World J Gastroenterol 2017; 23(13): 2443-2447
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2443.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2443
Hepatic angiosarcoma arises from vascular endothelial cells within the liver[1]. It is believed that in the past, the malignant transformation of vascular endothelial cells was mediated or triggered by environmental or industrial toxins, such as vinyl chloride, arsenic, and thorium dioxide, however despite tighter regulations of these toxins, there is a still a constant number of reports of angiosarcoma without any such association
The clinical entity of hepatic angiosarcoma has been well known to have an extremely poor prognosis. The disease presentation typically manifests as abdominal distension from large hepatic mass effect or by its complication of tumor rupture into abdominal cavity or intra-tumor bleeding. Subsequently, the average lifespan after the diagnosis has been reported to be 6 mo[2]. To date, there is no definitive or effective treatment that has been established. An attempt of surgical resection or liver transplant does not provide significant advantage in extending life as the tumor recurs in nearly all cases reported[3]. This resulted in the median life expectancy of 16 and 5 mo in partial hepatectomy and liver transplant, respectively[3,4]. In addition, there are no well-established chemotherapy regimens and accordingly, this has been primarily utilized as a palliative measure[3].
The unique clinical characteristic of angiosarcoma is the pronounced dysregulation of the coagulation system seen in a subset of patients[5,6]. This is featured by significant thrombocytopenia, prolonged prothrombin time (PT), and activated partial thromboplastin (aPTT) time with significant elevation of fibrin net degradation products, such as d-dimer. These abnormalities are congruent with the diagnostic criteria of disseminated intravascular coagulation (DIC). However, the site and mechanism of the clinical feature of this abnormal coagulopathy associated with angiosarcoma have never been well explained. The clinical entity of Kasabach-Merritt syndrome (KMS) is indistinguishable from DIC by blood-based laboratory findings. The coagulopathy associated with KMS is characterized by hyper-activation of the coagulation cascade and entrapment of platelets within dilated sinusoids of the vascular tumor[7]. The concept of KMS is well described in the pediatric population with Kaposi hemangioendothelioma, and to a much lesser extent seen in giant hemangioma in the adult population[8]. Here we describe a case of primary hepatic angiosarcoma with the clinical and for the first time, histological evidence of KMS.
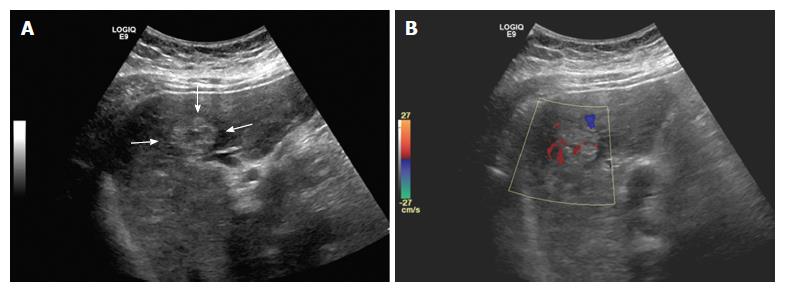
A 44-year-old Hispanic male was admitted to our hospital for worsening abdominal pain and jaundice. Abdominal ultrasound demonstrated multiple masses with heterogeneous echogenicity (Figure 1). Color doppler study revealed hypervascularity within the tumors.
The patient had no significant past medical, family, or social history. He had no prior exposures to vinyl chloride, arsenic, or thorium dioxide. The physical examination was significant for scleral icterus and a distended abdomen with diffuse tenderness. The exam was negative for spider angiomata, palmar erythema, shifting dullness, hepatic bruit, caput medusae, and asterixis.
Pertinent laboratory values on admission were as follows: white blood cell count 13400/mm3, hemoglobin 8.8 g/dL, mean corpuscular volume 99 fL, platelets 57000/mm3, alkaline phosphatase 118 IU/L, total protein 5.4 g/dL, albumin 2.7 g/dL, total bilirubin 6.4 mg/dL, direct bilirubin 3.1 mg/dL, aspartate aminotransferase 42 IU/L, alanine transaminase 72 IU/L, prothrombin time 29.4 s, INR 2.88, PTT 40.7 s, fibrinogen less than 60 mg/dL, and d-dimer greater than 9.999 mg/dL. Serologies for viral hepatitis and auto-immune liver disease were negative. Tumor markers such as AFP, CA 19-9, and CEA were all within normal limits.
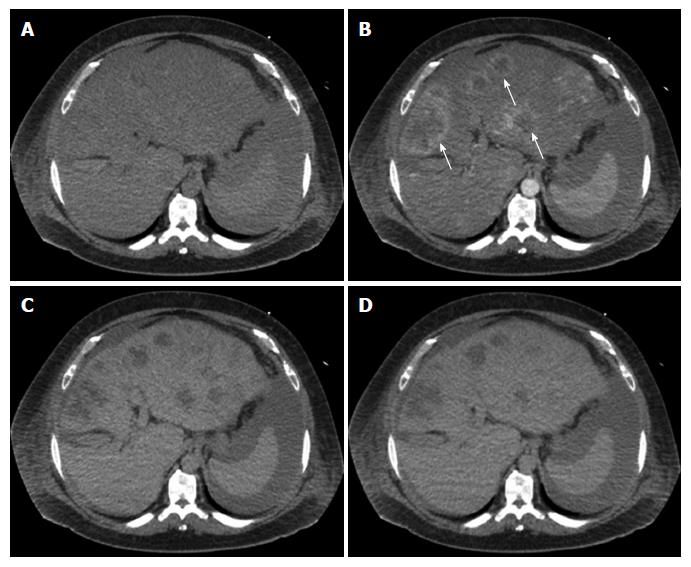
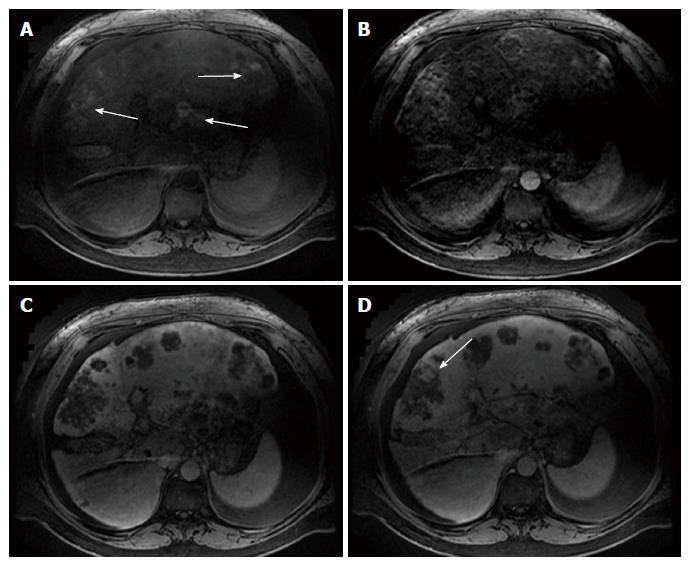
Multiphase computerized tomography and magnetic resonance imaging of the abdomen revealed discrete, multifocal, and isodense masses in precontrast images involving all segments of the liver, with the largest measuring 6.5 cm (Figures 2 and 3). Peripheral enhancement was seen in the arterial phase, but not in portal and delayed phases. Of note, there was no definite washout. These images did not demonstrate features of commonly identified hepatic malignancies, such as hepatocellular carcinoma and intrahepatic cholangiocarcinoma. The patient subsequently underwent liver needle biopsy.
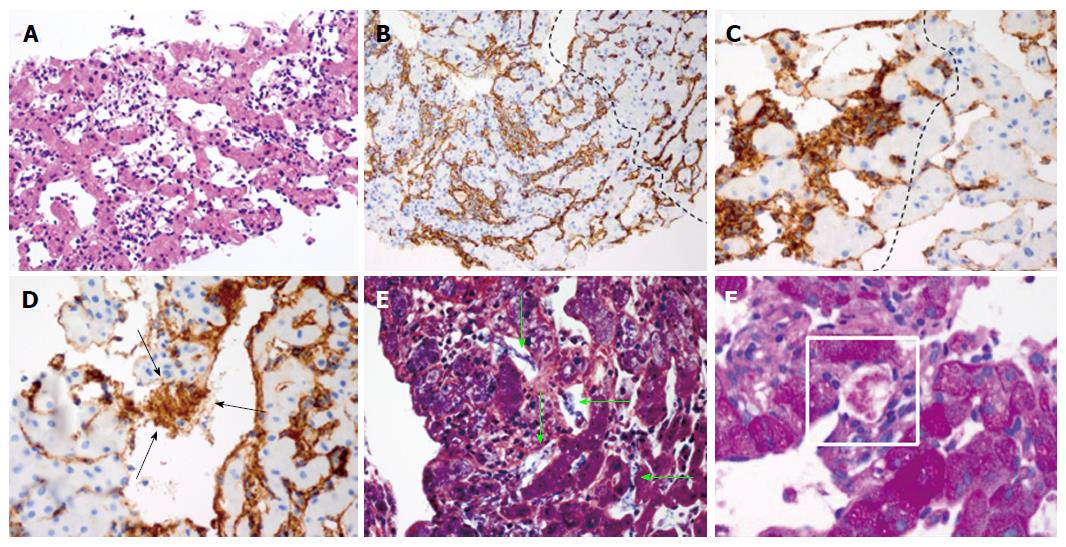
The tissue demonstrated a mesenchymal tumor infiltrating the sinusoids with anastomosing, dilated vascular channels lined by atypical cells (Figure 4A). Immunohistochemical studies demonstrated tumor cells strongly expressed CD34, suggesting a vascular endothelial origin (Figure 4B). Of note, the uninvolved region showed no evidence of chronic liver disease. Based on these findings, the diagnosis of angiosarcoma was made.
In this case report we described a case of hepatic angiosarcoma in a young male with no evidence of cirrhosis and no prior history of exposure to the aforementioned chemicals through his occupation or medical use. In addition, this patient had no history of use of anabolic steroids or conditions that were associated with the onset of angiosarcoma. Therefore, the diagnosis of idiopathic hepatic angiosarcoma was made. Consistent with other reports, this case also manifested with significant coagulopathy which is a unique feature of hepatic angiosarcoma. As described in our case, the results of the blood tests fit well into the diagnostic criteria of DIC.
Our histological investigation found increased expression of von-Willebrand factor (vWF)/factor VIII within the tumor cells, the formation of fibrin nets, and platelet aggregation within the dilated sinusoids of angiosarcoma (Figure 4C-F). Of importance, there was no histological evidence of cirrhosis. These findings strongly suggest hyper-activation of the coagulation cascade as well as entrapment of platelets within the tumor. Therefore, our histological findings are congruent with the proposed concept of KMS.
There are 72 case reports of KMS to date. Of those, 43 cases were associated with hemangioma, 16 with Kaposi hemangioendothelioma/tufted angioma, 8 with angiosarcoma, 2 with lymphangioma, 2 with angiolipoma, and 1 with Merkel cell carcinoma. These cases demonstrated marked abnormalities in coagulation and thrombocytopenia. However, none of these reports provided histological validation of KMS and therefore it remains indistinguishable from tumor-associated DIC.
Our histological investigation proposed the potential mechanism of hyper-activation of the coagulation cascade via up-regulation of vWF/Factor VIII within the dilated sinusoid of the tumor. Moreover, we speculate that the upregulated vWF/Factor VIII results in the downstream formation of fibrin nets and subsequent entrapment of platelets within the tumor. Our findings highly suggest that this is the potential explanation for the significant thrombocytopenia and deregulated coagulation cascade. Thus, our report provides a conceptual advancement for the differentiation of tumor-associated DIC from the systemic manifestation of coagulopathy occurring within the vascular tumor. In conclusion, we report a case of idiopathic hepatic angiosarcoma with features of KMS with clinical and histological evidences.
All of the authors would like to thank Dr. Neil Kaplowitz for his critical review of the manuscript.
A 40-year-old man with no past medical history presented with a 2 mo history of worsening abdominal pain and jaundice.
Distended abdomen with diffuse tenderness and scleral icterus without signs of chronic liver disease.
Hepatocellular carcinoma, metastatic adenocarcinoma, cirrhosis, disseminated intravascular coagulation (DIC).
Anemia, thrombocytopenia, decreased albumin, elevated bilirubin, elevated INR, decreased fibrinogen, elevated d-dimer.
Multiphase computerized tomography and magnetic resonance imaging showed discrete, multifocal, and isodense masses involving all segments of liver, largest measuring 6.5 cm.
Angiosarcoma with tumor cells strongly expressing CD34 with uninvolved region showing no evidence of chronic liver disease.
Kasabach-Merritt syndrome (KMS) has been reported before to occur in association with some adult vascular tumors. However, none of these reports provides histological validation of KMS and thus the disease entity remained indistinguishable from tumor-associated DIC.
KMS is characterized by thrombocytopenia and hyperconsumption of coagulation factors within a vascular tumor. KMS has been well described in the pediatric population but rare in vascular tumors of the adult population.
This entity can be confused for DIC due to similar abnormalities in coagulopathy. However, by histological investigation KMS can be differentiated from DIC.
It is really the first time to describe a case of primary hepatic angiosarcoma with the clinical and histological evidence of KMS.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Doganay L, Pan GD S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Molina E, Hernandez A. Clinical manifestations of primary hepatic angiosarcoma. Dig Dis Sci. 2003;48:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Mani H, Van Thiel DH. Mesenchymal tumors of the liver. Clin Liver Dis. 2001;5:219-257, viii. [RCA] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Zheng YW, Zhang XW, Zhang JL, Hui ZZ, Du WJ, Li RM, Ren XB. Primary hepatic angiosarcoma and potential treatment options. J Gastroenterol Hepatol. 2014;29:906-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Husted TL, Neff G, Thomas MJ, Gross TG, Woodle ES, Buell JF. Liver transplantation for primary or metastatic sarcoma to the liver. Am J Transplant. 2006;6:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Farid M, Ahn L, Brohl A, Cioffi A, Maki RG. Consumptive coagulopathy in angiosarcoma: a recurrent phenomenon? Sarcoma. 2014;2014:617102. [PubMed] |
| 6. | Truell JE, Peck SD, Reiquam CW. Hemangiosarcoma of the liver complicated by disseminated intravascular coagulation. A case report. Gastroenterology. 1973;65:936-942. [PubMed] |
| 7. | O’Rafferty C, O’Regan GM, Irvine AD, Smith OP. Recent advances in the pathobiology and management of Kasabach-Merritt phenomenon. Br J Haematol. 2015;171:38-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Sidbury R. Update on vascular tumors of infancy. Curr Opin Pediatr. 2010;22:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |