Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2385
Peer-review started: January 12, 2017
First decision: February 23, 2017
Revised: March 1, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 84 Days and 22.8 Hours
To determine the relationship between F-18 fluorodeoxyglucose (FDG) uptake of bone marrow (BM) on positron emission tomography/computed tomography (PET/CT) and clinical factors and to assess the prognostic value of FDG uptake of BM in gastric carcinoma.
We retrospectively enrolled 309 gastric cancer patients who underwent staging FDG PET/CT and curative surgical resection. FDG uptake of primary tumor was visually classified as positive or negative FDG uptake. Mean FDG uptake of BM (BM SUV) and BM-to-liver uptake ratio (BLR) were measured. The relationships of BM SUV or BLR with clinical factors were evaluated. The prognostic values of BM SUV, BLR, and other clinical factors for predicting recurrence-free survival (RFS) and overall survival (OS) were assessed.
Of 309 patients, 38 patients (12.3%) experienced cancer recurrence and 18 patients (5.8%) died. Patients with advanced gastric cancer, positive FDG uptake, and recurrence had higher values of BM SUV and BLR than those with early gastric cancer, negative FDG uptake, and no recurrence (P < 0.05). BM SUV and BLR were significantly correlated with hemoglobin level, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio (P < 0.05). On multivariate analysis, multiple tumors, T stage, lymph node metastasis, tumor involvement of resection margin, and BLR were significantly associated with RFS (P < 0.05). T stage, lymph node metastasis, hemoglobin level, and BLR were significantly associated with OS (P < 0.05).
BLR on PET/CT was an independent prognostic factor for RFS and OS in gastric cancer patients with curative surgical resection.
Core tip: This study evaluated the prognostic value of F-18 fluorodeoxyglucose (FDG) uptake of bone marrow (BM) on positron emission tomography/computed tomography (PET/CT) in gastric cancer patients with curative surgical resection. FDG uptake of BM was correlated with serum inflammatory markers. It was also significantly associated with worse prognosis. FDG uptake of BM on PET/CT could reflect the degree of systemic inflammatory response to cancer and provide information on the prognosis of patients with gastric cancer after curative surgical resection.
- Citation: Lee JW, Lee MS, Chung IK, Son MW, Cho YS, Lee SM. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J Gastroenterol 2017; 23(13): 2385-2395
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2385
Although the incidence of gastric cancer has gradually decreased and various treatment modalities have advanced in recent years, the prognosis of gastric cancer is still poor, with 5-year survival rate of 20%-25%[1-3]. Because of dismal prognosis, various prognostic biomarkers have been evaluated to identify patients with high risk for disease recurrence and progression. Tumor stage, presence of lymph node metastasis, tumor size, and lymphovascular invasion have been found to be significant prognostic factors[4-6]. Recently, systemic inflammatory response has been shown to play critical roles in carcinogenesis and tumor metastasis[7,8]. Several serum inflammatory markers have been assessed as prognostic factors and neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), albumin, and C-reactive protein (CRP) have been suggested as significant prognostic factors for predicting clinical outcomes of patients with gastric cancer[6,9-11].
F-18 fluorodexoyglucose (FDG) positron emission tomography/computed tomography (PET/CT) has been widely used to assess various types of malignancy. In patients with gastric cancer, the clinical role of FDG PET/CT has been limited because its sensitivity for detecting primary tumor and lymph node metastasis depends on tumor stage and pathologic subtypes of gastric cancer[12-14]. On the other hand, FDG PET/CT has shown high diagnostic ability for detecting cancer recurrence and is significantly associated with recurrence-free survival (RFS) and overall survival (OS) in gastric cancer patients with curative surgical resection[13,15,16]. In patients with malignancy, FDG uptake of bone marrow (BM) on PET/CT has been shown to be significantly associated with serum inflammatory markers such as NLR, PLR, CRP, and albumin, suggesting that FDG uptake of BM has significant relationship with systemic inflammatory response[17-19]. Since serum inflammatory markers are associated with the prognosis of gastric cancer patients, FDG uptake of BM could also show significant association with clinical outcomes. However, clinical implication of FDG uptake of BM in patients with gastric cancer has not been reported yet.
Therefore, the objective of this study was to evaluate the relationship of FDG uptake of BM on PET/CT with serum inflammatory markers and tumor factors and to assess the role of FDG uptake of BM as a prognostic factor in predicting RFS and OS of gastric cancer patients with curative surgical resection.
We retrospectively reviewed medical records of 332 patients with gastric cancer who underwent preoperative FDG PET/CT and subsequent curative surgical resection between March 2011 and January 2014 in our medical center. Of these patients, we excluded patients who had distant metastasis on staging work-up, had any neo-adjuvant treatment before surgical resection, were lost to follow-up within 12 mo after operation, had acute inflammatory disease or liver disease, or had a history of another malignancy. One patient who had a rare pathological type of gastric cancer (adenosquamous carcinoma) was also excluded for statistical analysis. After all, a total of 309 patients with gastric cancer were enrolled in the study. This study was approved by the Institutional Review Board of Soonchunhyang University Cheonan Hospital in accordance with the Helsinki Declaration.
All enrolled patients underwent preoperative staging work-up consisting of blood tests including hemoglobin, blood cell counts, and serum albumin and CRP, gastroduodenoscopy, contras-enhanced abdominopelvic CT, and FDG PET/CT. The interval between blood tests and FDG PET/CT was within two days. After staging work-up, all patients underwent curative subtotal or total gastrectomy with lymph node dissection of at least D1 dissection according to the treatment guidelines of the Japanese Gastric Cancer Association (JGCA)[20]. The interval between FDG PET/CT and operation was within three weeks for all patients. For surgical specimens, the T and N histopathological stages were assessed according to the 7th edition of the American Joint Committee on Cancer staging guidelines and the size of primary tumor was measured. The pathological subtypes of gastric cancer were categorized into papillary adenocarcinoma (PAC), well-differentiated and moderately-differentiated tubular adenocarcinoma (TAC), poorly-differentiated adenocarcinoma (PDAC), signet-ring cell carcinoma (SRC), or mucinous adenocarcinoma (MAC) according to the JGCA system[21]. In addition, all cancer lesions were classified into intestinal and non-intestinal tumors according to Lauren classification. Diffuse, mixed, and non-classifiable types were classified as the non-intestinal type[13].
After curative surgical resection, all patients underwent clinical follow-up consisted of blood tests, contrast-enhanced CT, and gastroduodenoscopy every 6-8 mo for the first 3 years after the operation and every 10-12 mo thereafter. When abnormal finding was found during follow-up, additional diagnostic studies and/or pathological studies were performed to determine cancer recurrence.
All patients were instructed to fast for at least 6 h before PET/CT scans. Before injecting FDG, blood glucose level was < 150.0 mg/dL in every patient. FDG was intravenously administered at a dose of 4.07 MBq/kg 60 min before the PET/CT scan. FDG PET/CT images were acquired from the skull base to the proximal thigh using a Biograph mCT 128 scanner (Siemens Healthcare, Knoxville, TN, United States). In cases with no symptoms of gastric obstruction, patients were instructed to drink at least 500 mL of water before PET/CT scanning. CT scan was initially performed at 100 mA and 120 kVp without contrast enhancement. Afterwards, PET scan was performed in 1.5 min per one bed position. PET images were reconstructed with an iterative algorithm using True X and time-of-flight reconstruction with attenuation correction.
All PET/CT images were retrospectively assessed by two nuclear medicine physicians. At first, FDG uptake of gastric cancer lesions was visually and quantitatively evaluated. Gastric lesions that showed focally increased FDG uptake exceeding the uptake of the surrounding gastric wall and corresponding to cancer lesions on contrast-enhanced CT images and gastroduodenoscopy were considered as positive FDG uptake lesions. In contrast, cancer lesions without focally increased FDG uptake or with diffusely increased FDG uptake being unable to differentiate from physiological uptake of surrounding gastric wall were considered as negative FDG uptake lesions. For patients with positive FDG uptake on gastric cancer lesions, a spheroid-shaped volume of interest (VOI) was drawn over the tumor lesion and the maximum standardized uptake value (SUV) of gastric cancer lesion (Tmax) was measured. SUV was calculated as [decay-corrected activity (kBq)/tissue volume (mL)]/[injected FDG activity (kBq)/body mass (g)]. Afterwards, FDG uptake of BM was measured for each patient. A spheroid-shaped VOI was drawn over the vertebral body of at least six vertebrae of thoracic and lumbar spines (mostly T10-T12 spine and L3-5 spine, unless they showed compression fracture, severe osteoarthritic changes, or post-operative change for spinal disease). It has been reported that the mean SUV using 75% cut-off value of the maximum SUV shows substantial agreement between observers[17,22]. Therefore, mean SUV of the vertebral body was measured using an automatic isocontour set at 75% of the maximum SUV within each VOI. The mean value of mean SUV of vertebral body of vertebrae was defined as BM SUV. Mean SUV of the normal liver was measured by drawing 2 cm-sized spheroid-shaped VOI in the right lobe of the liver, and BM SUV-to-normal liver uptake ratio (BLR) was calculated for each patient.
Using results of white blood cell counts, NLR and PLR were calculated for each patient. To evaluate the relationship bewteen FDG uptake of BM and hematologic parameters, inflammatory markers, and Tmax, Spearman’s rank correlation coefficients were calculated for BM SUV and BLR with regard to white blood cell count, hemoglobin level, NLR, PLR, serum albumin and CRP level, and Tmax. To assess differences in BM SUV and BLR according to the pathology of primary tumor, T stage, status of lymph node, FDG uptake of primary tumor, and occurrence of recurrence, one way analysis of variance and Student t-test were performed. To assess the predictive values of variables for RFS and OS, univariate and multivariate analyses were performed using a Cox proportional hazards regression model. Survival time was defined as the time from operation to the date of detection of cancer recurrence (for RFS) or death (for OS). Continuous variables in the model were dichotomized according to specific cut-off values determined by the maximally selected χ2 method. Hazard ratios with Wald 95%CI were provided for survival analyses. For T stage and BLR, survival curves were estimated with the Kaplan-Meier method and compared with the log-rank test. Recurrence rates according to the combination of T stage and BLR were compared by χ2 test. All statistical analyses were performed with R 2.13.0 software (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS ver. 20.0 for Windows software (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant.
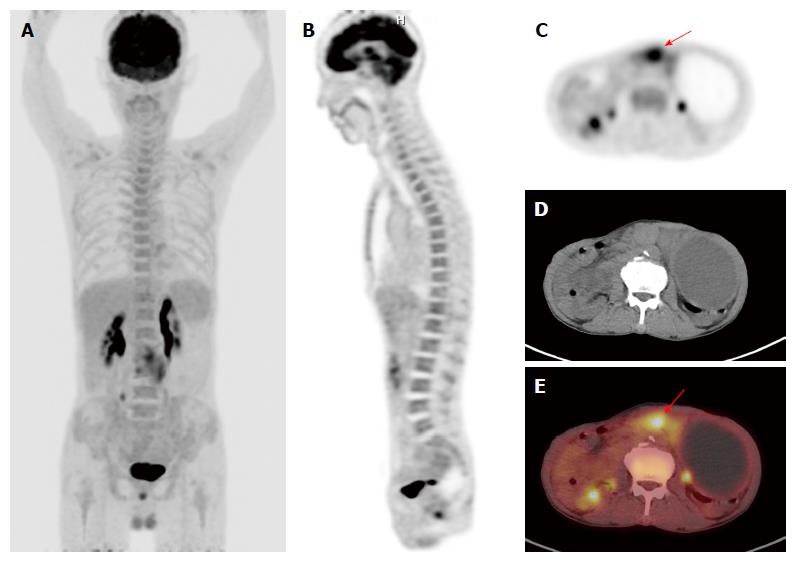
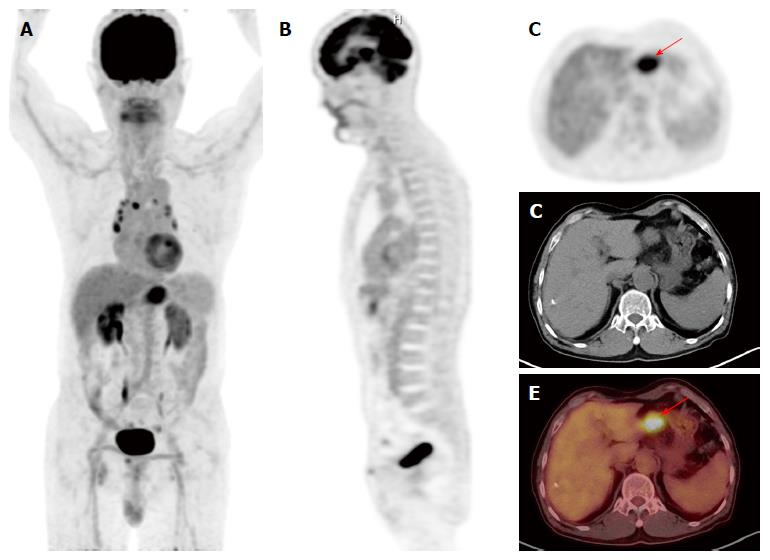
The characteristics of enrolled patients are summarized in Table 1. Of the 309 patients, 183 patients (59.2%) had early gastric cancer (T1 tumors regardless of lymph node status) and 126 patients (40.8%) had advanced gastric cancer (T2-T4 tumors). On PET/CT, positive FDG uptake of gastric cancer was observed in 156 patients (50.5%) and Tmax was measured in these 156 patients. Among the 183 patients with early gastric cancer, positive FDG uptake was observed in 64 patients (35.0%), and among the 126 patients with advanced gastric cancer, 92 patients (73.0%) showed positive FDG uptake. Of all patients, BM SUV in 26 patients (8.4%) was higher than the mean SUV of normal liver (Figures 1 and 2).
| Characteristics | n (%) | Median | Range | |
| Age (yr) | 60 | 29-82 | ||
| Sex | Male | 77 (24.9) | ||
| Female | 232 (75.1) | |||
| Tumor location | Upper | 11 (3.6) | ||
| Middle | 134 (43.4) | |||
| Lower | 158 (51.1) | |||
| Multiple | 6 (1.9) | |||
| Operation type | Subtotal gastrectomy | 139 (45.0) | ||
| Total gastrectomy | 170 (55.0) | |||
| Pathology | PAC/TAC | 200 (64.7) | ||
| PDAC | 74 (23.9) | |||
| SRC/MAC | 35 (11.3) | |||
| Lauren classification | Intestinal | 137 (44.3) | ||
| Non-intestinal | 172 (55.7) | |||
| T stage | T1 | 183 (59.2) | ||
| T2 | 57 (18.4) | |||
| T3 | 41 (13.3) | |||
| T4 | 28 (9.1) | |||
| Lymph node metastasis | Absence | 212 (68.6) | ||
| Presence | 97 (31.4) | |||
| Tumor size (cm) | 3.0 | 0.5-17.0 | ||
| Tumor involvement of resection margin | Negative | 302 (97.7) | ||
| Positive | 7 (2.3) | |||
| Adjuvant chemotherapy | No | 220 (71.2) | ||
| Yes | 89 (28.8) | |||
| White blood cell (× 1012 cells/L) | 6.87 | 3.09-20.13 | ||
| Hemoglobin (g/dL) | 13.6 | 4.1-17.3 | ||
| NLR | 1.96 | 0.13-22.80 | ||
| PLR | 126.05 | 2.93-557.13 | ||
| Albumin (g/dL) | 4.3 | 2.5-5.4 | ||
| CRP (mg/dL) | 1.61 | 0.0-115.05 | ||
| FDG uptake | Negative | 153 (49.5) | ||
| Positive | 156 (50.5) | |||
| Tmax1 | 4.71 | 2.62-37.80 | ||
| BM SUV | 1.45 | 0.55-2.66 | ||
| BLR | 0.70 | 0.28-1.35 | ||
During clinical follow-up, 38 patients (12.3%) experienced cancer recurrence and 18 patients (5.8%) died. The median duration of clinical follow-up was 33.8 mo (range, 2.6-67.5 mo). Of the 38 patients with recurrence, 16 patients (42.1%) experienced distant lymph node and organ metastases while 14 patients (36.8%) experienced peritoneal recurrence. Locoregional recurrence was observed in the remaining 9 patients (21.1%).
To reveal clinical factors that might affect FDG uptake of BM, relationships of FDG uptake of BM with various tumor factors, hematologic parameters, and serum inflammatory markers were assessed. Both BM SUV and BLR were significantly correlated with hemoglobin level (P = 0.039 for BM SUV; P = 0.002 for BLR), NLR (P = 0.033 for BM SUV; P = 0.001 for BLR), and PLR (P = 0.005 for BM SUV; P < 0.001 for BLR; Table 2). BLR was negatively correlated with serum albumin level (P = 0.003). Patients with advanced gastric cancer had higher BM SUV (P = 0.042) and BLR (P = 0.003) than those with early gastric cancer. Patients with recurrence also had higher values of BM SUV (P = 0.001) and BLR (P < 0.001) than those with no recurrence (Table 3). Results for the relationship between tumor and BM FDG uptake revealed that patients with positive FDG uptake had higher BM SUV (P = 0.007) and BLR (P = 0.006) than those with negative FDG uptake. In 156 patients with positive FDG uptake, Tmax showed significant association with BLR (P = 0.002; Tables 2 and 3).
| White blood cells | Hemoglobin | NLR | PLR | Albumin | CRP | Tmax1 | |
| BM SUV | r = 0.039 | r = -0.117 | r = 0.121 | r = 0.158 | r = -0.041 | r = 0.100 | r = 0.093 |
| P = 0.600 | P = 0.039 | P = 0.033 | P = 0.005 | P = 0.474 | P = 0.079 | P = 0.104 | |
| BLR | r = 0.033 | r = -0.172 | r = 0.224 | r = 0.250 | r = -0.168 | r = 0.094 | r = 0.212 |
| P = 0.563 | P = 0.002 | P = 0.001 | P < 0.001 | P = 0.003 | P = 0.100 | P = 0.002 |
| BM SUV | P value | BLR | P value | ||
| Pathology | PAC/TAC | 1.49 ± 0.34 | 0.143 | 0.73 ± 0.17 | 0.081 |
| PDAC | 1.54 ± 0.37 | 0.75 ± 0.16 | |||
| SRC/MAC | 1.41 ± 0.32 | 0.68 ± 0.16 | |||
| T stage | T1 | 1.46 ± 0.35 | 0.042 | 0.71 ± 0.16 | 0.003 |
| T2-T4 | 1.54 ± 0.33 | 0.78 ± 0.17 | |||
| Lymph node metastasis | Absence | 1.47 ± 0.34 | 0.123 | 0.72 ± 0.16 | 0.010 |
| Presence | 1.54 ± 0.36 | 0.77 ± 0.17 | |||
| FDG uptake | Negative | 1.46 ± 0.30 | 0.007 | 0.71 ± 0.14 | 0.006 |
| Positive | 1.52 ± 0.38 | 0.75 ± 0.18 | |||
| Recurrence | No | 1.47 ± 0.34 | 0.001 | 0.72 ± 0.16 | <0.001 |
| Yes | 1.68 ± 0.36 | 0.85 ± 0.16 |
The prognostic values of clinical factors and FDG PET/CT parameters for predicting RFS and OS on univariate analysis are shown in Table 4. The optimal cut-off values determined by the maximal χ2 method for age, tumor size, white blood cell count, hemoglobin, NLR, PLR, albumin, CRP, Tmax, BM SUV, and BLR were 68 years, 3.0 cm, 9.5 ×1012 cells/L, 12.0 g/dL, 2.10, 210.0, 3.9 g/dL, 20.0 mg/dL, 6.0, 1.50, and 0.72, respectively. Operation type and adjuvant treatment were excluded from the survival analysis, because they were determined by other tumor factors and were not considered as independent factors. On univariate analysis, T stage, lymph node metastases, tumor size, tumor involvement of surgical resection margin, hemoglobin level, PLR, serum albumin level, positive FDG uptake, Tmax, and BLR were significantly associated with both RFS and OS (P < 0.05). Meanwhile, age, tumor location, white blood cell count, NLR, serum CRP level, and BM SUV were significant prognostic factors only for RFS (P < 0.05).
| Variables | RFS | OS | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| Age (≤ 68 yr vs > 68 yr) | 0.025 | 2.07 (1.10-3.91) | 0.058 | 2.46 (0.97-6.23) |
| Sex (female vs male) | 0.658 | 1.19 (0.56-2.53) | 0.078 | 2.38 (0.91-6.22) |
| Tumor location | ||||
| Body | 1.00 | 1.00 | ||
| Fundus | 0.788 | 0.76 (0.10-5.72) | 0.961 | 0.85 (0.18-6.62) |
| Antrum | 0.869 | 0.94 (0.48-1.85) | 0.591 | 1.30 (0.50-3.35) |
| Multiple | 0.009 | 5.15 (1.50-17.72) | 0.973 | 1.10 (0.15-7.54) |
| Pathology | ||||
| PCA/TAC | 1.00 | 1.00 | ||
| PDAC | 0.591 | 1.22 (0.59-2.53) | 0.643 | 0.76 (0.24-2.41) |
| SRC/MAC | 0.182 | 1.86 (0.75-4.64) | 0.457 | 1.62 (0.45-5.83) |
| Lauren classification (intestinal vs non-intestinal) | 0.368 | 1.35 (0.70-2.62) | > 0.999 | 1.00 (0.39-2.53) |
| T stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2 | 0.005 | 4.97 (1.62-15.21) | 0.300 | 2.83 (0.40-20.24) |
| T3 | < 0.001 | 9.78 (3.34-28.64) | 0.008 | 9.86 (1.80-53.93) |
| T4 | < 0.001 | 34.10 (12.24-94.94) | < 0.001 | 52.24 (11.13-245.23) |
| Lymph node metastasis (absence vs presence) | < 0.001 | 11.14 (5.08-24.44) | < 0.001 | 13.62 (3.93-47.22) |
| Tumor size (≤ 3.0 cm vs > 3.0 cm) | < 0.001 | 5.50 (2.30-13.19) | 0.006 | 17.13 (2.27-128.96) |
| Tumor involvement of resection margin (negative vs positive) | 0.013 | 4.48 (1.38-14.60) | 0.018 | 5.87 (1.35-25.56) |
| White blood cell (≤ 9.5 × 1012 cells/L vs > 9.5 × 1012 cells/L) | 0.006 | 2.73 (1.32-5.62) | 0.910 | 0.92 (0.21-3.99) |
| Hemoglobin (≤ 12.0 g/dL vs > 12.0 g/dL) | < 0.001 | 0.25 (0.13-0.48) | < 0.001 | 0.12 (0.05-0.31) |
| NLR (≤ 2.10 vs > 2.10) | 0.002 | 4.04 (1.96-8.33) | 0.117 | 2.13 (0.83-5.51) |
| PLR (≤ 210.0 vs > 210.0) | < 0.001 | 4.03 (2.03-7.99) | 0.005 | 4.12 (1.55-10.98) |
| Albumin (≤ 3.9 g/dL vs > 3.9 g/dL) | 0.001 | 0.27 (0.14-0.52) | 0.003 | 0.23 (0.09-0.60) |
| CRP (≤ 20.0 mg/dL vs > 20.0 mg/dL) | 0.013 | 2.49 (1.21-5.14) | 0.306 | 1.79 (0.59-5.49) |
| FDG uptake (negative vs positive) | < 0.001 | 7.82 (3.05-20.07) | 0.004 | 19.68 (2.61-148.27) |
| Tmax (≤ 6.0 vs > 6.0)1 | < 0.001 | 5.72 (3.01-10.86) | 0.002 | 5.35 (1.88-15.21) |
| BM SUV (≤ 1.50 vs > 1.50) | 0.016 | 2.27 (1.16-4.45) | 0.196 | 1.87 (0.72-4.84) |
| BLR (≤ 0.72 vs > 0.72) | < 0.001 | 8.25 (3.22-21.15) | 0.003 | 20.69 (2.75-155.64) |
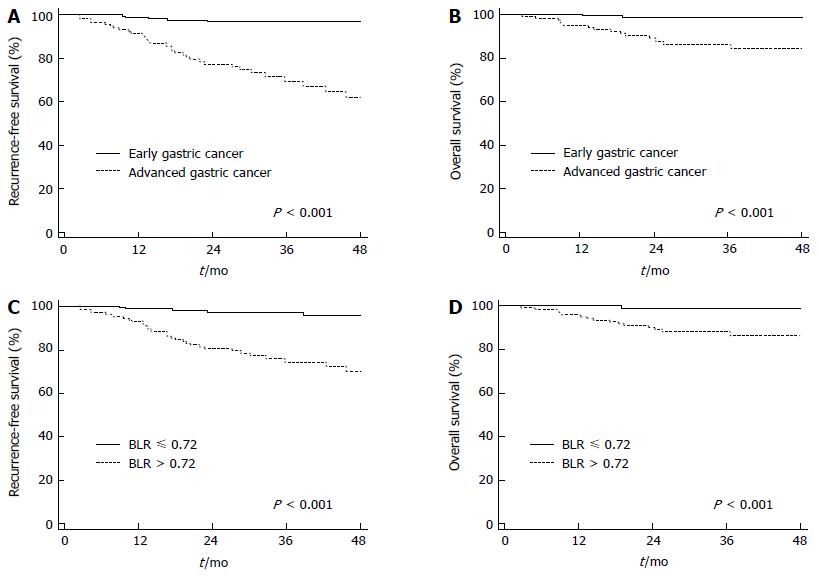
Of the variables, those with a p-value of less than 0.05 in univariate analysis were selected for multivariate analysis. On multivariate analysis, T4 stage, lymph node metastasis, and BLR were found to be independent prognostic factors for both PFS and OS (P < 0.05). Multiple tumors and tumor involvement of the resection margin were only significantly associated with PFS and hemoglobin level was only significantly associated with OS (P < 0.05; Table 5). In contrast, positive FDG uptake failed to show significance in predicting RFS or OS in multivariate analysis (P = 0.058 for RFS and P = 0.197 for OS). On Kaplan-Meier analysis, patients with early gastric cancer showed higher rates of 2-year RFS (97.0% vs 77.4%; P < 0.001; Figure 3A) and OS (98.8% vs 89.0%; P < 0.001; Figure 3B) than those with advanced gastric cancer. Patients with low BLR also showed higher rates of 2-year RFS (97.2% vs 80.5%; P < 0.001; Figure 3C) and OS (99.2% vs 89.1%; P < 0.001; Figure 3D), which is similar to those in patients with early gastric cancer, than those with high BLR.
| Variables | Model with all patients | Model with patients with positive FDG uptake | ||
| P value | HR (95%CI) | P value | HR (95%CI) | |
| RFS | ||||
| Age | 0.461 | 1.38 (0.58-3.28) | 0.228 | 1.69 (0.72-3.96) |
| Location | ||||
| Body | 1.00 | 1.00 | ||
| Fundus | 0.543 | 0.50 (0.05-4.78) | 0.634 | 0.58 (0.06-5.32) |
| Antrum | 0.276 | 0.64 (0.29-1.42) | 0.695 | 0.86 (0.41-1.82) |
| Multiple | 0.037 | 6.06 (1.36-26.92) | 0.034 | 5.19 (1.13-23.76) |
| T stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2 | 0.342 | 1.81 (0.53-6.19) | 0.250 | 2.05 (0.60-6.99) |
| T3 | 0.200 | 2.31 (0.64-8.29) | 0.096 | 3.01 (0.82-10.97) |
| T4 | 0.020 | 4.99 (1.29-19.38) | 0.014 | 5.50 (1.42-21.31) |
| Lymph node metastasis | 0.037 | 2.96 (1.07-8.21) | 0.004 | 4.50 (1.61-12.54) |
| Tumor size | 0.661 | 1.27 (0.43-3.75) | 0.594 | 1.34 (0.46-3.95) |
| Tumor involvement of resection margin | 0.035 | 4.32 (1.11-16.81) | 0.023 | 4.94 (1.24-19.62) |
| White blood cell | 0.150 | 1.96 (0.78-4.92) | 0.053 | 2.37 (0.99-5.67) |
| Hemoglobin | 0.652 | 1.24 (0.49-3.11) | 0.955 | 0.97 (0.38-2.50) |
| NLR | 0.611 | 1.25 (0.53-2.90) | 0.607 | 1.24 (0.54-2.86) |
| PLR | 0.563 | 1.30 (0.53-3.18) | 0.197 | 1.78 (0.74-4.27) |
| Albumin | 0.060 | 0.42 (0.17-1.04) | 0.021 | 0.34 (0.14-0.85) |
| CRP | 0.173 | 1.88 (0.76-4.68) | 0.181 | 1.85 (0.75-4.55) |
| FDG uptake | 0.058 | 2.72 (0.97-7.65) | ||
| Tmax | 0.215 | 1.33 (0.70-2.39) | ||
| BM SUV | 0.945 | 0.94 (0.38-2.33) | 0.597 | 0.80 (0.34-1.86) |
| BLR | 0.001 | 6.42 (2.07-19.84) | 0.005 | 7.67 (2.44-24.12) |
| OS | ||||
| T stage | ||||
| T1 | 1.00 | 1.00 | ||
| T2 | 0.994 | 1.01 (0.13-8.41) | 0.965 | 1.93 (0.29-13.05) |
| T3 | 0.537 | 1.78 (0.28-11.15) | 0.500 | 1.93 (0.29-13.05) |
| T4 | 0.002 | 5.33 (1.88-15.14) | < 0.001 | 6.15 (2.18-17.34) |
| Lymph node metastasis | 0.016 | 5.08 (1.36-18.94) | 0.092 | 3.40 (0.82-14.11) |
| Tumor size | 0.243 | 3.62 (0.42-31.35) | 0.242 | 3.77 (0.41-34.80) |
| Tumor involvement of resection margin | 0.194 | 3.16 (0.56-17.93) | 0.209 | 3.13 (0.53-18.54) |
| Hemoglobin | 0.026 | 0.32 (0.11-0.87) | 0.463 | 0.46 (0.14-1.57) |
| PLR | 0.741 | 0.82 (0.25-2.66) | 0.896 | 0.92 (0.29-2.99) |
| Albumin | 0.759 | 0.84 (0.27-2.61) | 0.675 | 0.78 (0.24-2.52) |
| FDG uptake | 0.197 | 4.29 (0.47-39.12) | ||
| Tmax | 0.059 | 2.89 (0.96-8.72) | ||
| BLR | 0.025 | 10.39 (1.34-80.33) | 0.022 | 10.87 (1.42-83.31) |
The combination of T stage and BLR could further enhance their predictive value for RFS. For early gastric cancer patients, there was no significant difference in recurrence rate between patients with BLR ≤ 0.72 (1.8%, 2 out of 114 patients) and those with BLR > 0.72 (4.1%, 3 out of 74 patients; P = 0.340). However, for advanced gastric cancer patients, patients with BLR > 0.72 (44.1%, 30 out of 68 patients) had significantly higher recurrence rate than those with BLR ≤ 0.72 (5.7%, 3 out of 53 patients; P < 0.001).
We further analyzed the prognostic value of variables in 156 patients with positive FDG uptake to compare the prognostic value of FDG uptake of BM and Tmax (Table 5). For patients with positive FDG uptake, multiple tumors, T4 stage, lymph node metastasis, tumor involvement of resection margin, serum albumin level, and BLR were significantly associated with RFS (P < 0.05), and T4 stage and BLR were significantly associated with OS in multivariate analysis (P < 0.05). However, Tmax failed to show significance on multivariate analysis for RFS (P = 0.215) and OS (P = 0.059).
On FDG PET/CT, BM in normal healthy subjects usually shows mild degree of FDG uptake. In contrast, previous studies have revealed that patients with various inflammatory diseases and cancers could have increased FDG uptake of BM and that FDG uptake of BM is significantly associated with white blood cell and neutrophil counts and CRP level[18,23]. Furthermore, in patients with lung cancer, FDG uptake of BM is significantly higher than that in those with benign lung nodules and is associated with white blood cell counts, NLR, PLR, and serum levels of albumin and CRP[17,24,25]. These results suggest that FDG uptake of BM can reflect the degree of BM activation due to systemic inflammatory response to malignancy[17,18,25]. The results of the present study also showed significant correlation of BM SUV and BLR with NLR and PLR. BLR showed greater statistical significance and higher correlation coefficients with serum inflammatory markers compared to BM SUV, in agreement with the results of previous studies showing that BLR could reduce the inter-individual variation of FDG uptake of BM[17,18,25]. In addition, patients with advanced gastric cancer, recurrence, and positive FDG uptake of primary cancer had higher FDG uptake than those with early gastric cancer, no recurrence, and negative FDG uptake, respectively, indicating that gastric cancer patients with advanced stage and aggressive features might have higher degrees of systemic inflammatory response.
FDG uptake of BM of patients used in the study also had significant negative correlation with blood hemoglobin level. Previous studies have demonstrated controversial results for the relationship between FDG uptake of BM and hemoglobin level in various kinds of malignancy[17,18,22,23,25]. In gastric cancer, significant portion of patients had anemia and hemoglobin level is a significant prognostic factor for survival[26]. The results of the present study suggest that, in gastric cancer patients, red marrow hyperplasia due to low hemoglobin level can affect FDG uptake of BM.
Recently, inflammation has been recognized as one of the hallmarks of cancer. It can stimulate cancer development, proliferation, and metastasis[8,27]. Neutrophils can remodel the extracellular matrix by releasing inflammatory cytokines and promote tumor angiogenesis and metastasis, and platelet is believed to contribute to the survival of cancer cells in the circulation and formation of metastatic niches[28,29]. Meanwhile, lymphocytes can function as anti-tumor immune cells[7]. Therefore, pre-operative NLR and PLR can be used as prognostic factors in gastric cancer patients with surgical resection and changes in NLR and PLR following chemotherapy are useful for predicting clinical outcomes of unresectable gastric cancer patients[9,30,31]. Considering the significant relationship of BM FDG uptake with NLR and PLR, we hypothesized that FDG uptake of BM might also be useful for predicting prognosis of gastric cancer patients with curative surgical resection. Results of our study demonstrated that BLR was an independent prognostic factor for predicting both RFS and OS, along with other known prognostic clinical factors including T4 stage, lymph node metastasis, tumor involvement of resection margin, and hemoglobin level[13,26,32]. It has been shown that FDG uptake BM has significant association with survival in patients with head and neck cancer and lung cancer[17,22,25,33]. The present study also corroborates this association in patients with gastric cancer. Furthermore, BLR remained as an independent prognostic factor even in patients with positive FDG uptake when compared to the prognostic value with Tmax. The prognostic value of BLR was further enhanced when it was combined with T stage. The subgroup of patients who had advanced gastric cancer and high BLR showed the highest recurrence rate of 44.7%, while other patient subgroups had recurrence rate of less than 6.0%. Both tumor factors and systemic inflammatory response seemed to play important roles in cancer recurrence.
On multivariate survival model with tumor factors (tumor location, T stage, lymph node metastasis, tumor size, and tumor involvement of resection margin) and PET/CT parameters of primary tumor (positive FDG uptake and Tmax), positive FDG uptake of primary tumor and Tmax were found to be independent prognostic factors for RFS and OS (data not shown). However, after including serum inflammatory markers, BM SUV, and BLR in the multivariate survival model, positive FDG uptake and Tmax of primary tumor failed to show statistical significance. They only showed borderline significance for FDG uptake in predicting RFS and for Tmax in predicting OS. Given that FDG uptake of malignant lesion is partly affected by intra-tumoral inflammatory cells and the density of tumor infiltrative immune cells is associated with serum inflammatory markers such as NLR[34-37], the prognostic value of FDG uptake of primary tumor might be influenced, at least in part, by systemic inflammatory response.
The present study has some limitations. First, the study was a retrospective single-center study with a relatively small number of patients. Further studies are needed to validate the results of this study. Second, because we only enrolled patients who underwent curative surgical resection, a significant proportion of patients had early gastric cancer, resulting in overall good prognosis. Third, further examinations including BM aspiration and serum cytokine levels are required to identify the mechanism of FDG uptake of BM in gastric cancer patients. Lastly, although we followed the method of measuring BM FDG uptake used in previous studies[17,22], a recent study has revealed that the correlation of BM FDG uptake with hematological parameters can vary among skeletal regions[38]. Further research is needed to develop a method of BM FDG uptake measurement that can accurately reflect BM metabolism.
In conclusion, BLR was an independent prognostic factor for predicting RFS and OS after curative surgical resection in gastric cancer patients. Patients with low BLR had better survival than those with high BLR. In addition, BLR was significantly associated with hemoglobin level, NLR, PLR, and serum albumin level. Moreover, patients with advanced gastric cancer and positive FDG uptake of primary tumor had higher FDG uptake of BM than those with early gastric cancer and negative FDG uptake. BLR can provide information on the degree of systemic inflammatory response and the prognosis of gastric cancer after surgical resection.
F-18 fluorodexoyglucose (FDG) positron emission tomography/computed tomography (PET/CT) has shown clinical usefulness in various kinds of malignancy. In normal subjects, bone marrow (BM) shows only mild degree of FDG uptake. By contrast, increased FDG uptake of BM is observed in some patients with malignancy. FDG uptake of BM in patients with malignancy is significantly associated with serum inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and C-reactive protein. Because systemic inflammatory response has an important role in cancer progression and serum inflammatory markers are shown to be significant prognostic factors in various malignant tumors, FDG uptake of BM can have prognostic value for predicting clinical outcomes of gastric cancer patients.
FDG uptake of BM was determined to be significant prognostic factor for patients with head and neck cancer and lung cancer. However, to the best of our knowledge, the clinical implication of FDG uptake of BM in gastric cancer patients has not been reported.
In gastric cancer patients with surgical resection, mean FDG uptake of bone marrow (BM SUV) and BM-to-liver uptake ratio (BLR) were significantly associated with hemoglobin level and serum inflammatory markers including NLR, PLR, and albumin. Patients with advanced gastric cancer, positive FDG uptake, and recurrence showed higher values of BM SUV and BLR than those with early gastric cancer, negative FDG uptake, and no recurrence. BLR showed greater statistical significance and higher correlation coefficients with serum inflammatory markers compared to BM SUV. On survival analysis, BLR was an independent prognostic factor for predicting recurrence-free survival and overall survival after curative surgical resection in addition to T4 stage and lymph node metastasis.
This study suggested that FDG uptake of BM on PET/CT could be used to assess the degree of systemic inflammatory response and predict the clinical outcome of gastric cancer patients who underwent curative surgical resection.
The authors have analyzed the relationship between FDG uptake of bone marrow on PET/CT and clinical factors and assessed the prognostic value of FDG uptake of BM in gastric carcinoma. They provided interesting results and submitted a well-written manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Park WS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8971] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 2. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 3. | Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel). 2013;5:48-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Kang WM, Meng QB, Yu JC, Ma ZQ, Li ZT. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol. 2015;21:5934-5940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 5. | Lee JW, Jo K, Cho A, Noh SH, Lee JD, Yun M. Relationship Between 18F-FDG Uptake on PET and Recurrence Patterns After Curative Surgical Resection in Patients with Advanced Gastric Cancer. J Nucl Med. 2015;56:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Sun X, Wang J, Liu J, Chen S, Liu X. Albumin concentrations plus neutrophil lymphocyte ratios for predicting overall survival after curative resection for gastric cancer. Onco Targets Ther. 2016;9:4661-4669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8182] [Article Influence: 545.5] [Reference Citation Analysis (0)] |
| 8. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47141] [Article Influence: 3367.2] [Reference Citation Analysis (5)] |
| 9. | Yu L, Lv CY, Yuan AH, Chen W, Wu AW. Significance of the preoperative neutrophil-to-lymphocyte ratio in the prognosis of patients with gastric cancer. World J Gastroenterol. 2015;21:6280-6286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Gu X, Gao XS, Cui M, Xie M, Peng C, Bai Y, Guo W, Han L, Gu X, Xiong W. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget. 2016;7:49878-49887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Woo Y, Hyung WJ, Obama K, Kim HI, Pak KH, Son T, Noh SH. Elevated high-sensitivity C-reactive protein, a marker of advanced stage gastric cancer and postgastrectomy disease recurrence. J Surg Oncol. 2012;105:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Lee JW, Lee SM, Lee MS, Shin HC. Role of 18F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging. 2012;39:1425-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kim HW, Won KS, Song BI, Kang YN. Correlation of Primary Tumor FDG Uptake with Histopathologic Features of Advanced Gastric Cancer. Nucl Med Mol Imaging. 2015;49:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, Ozugur S, Gumus M. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients’ treatment decision making? Eur J Nucl Med Mol Imaging. 2011;38:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Lee JW, Lee SM, Son MW, Lee MS. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging. 2016;43:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Lee JW, Na JO, Kang DY, Lee SY, Lee SM. Prognostic Significance of FDG Uptake of Bone Marrow on PET/CT in Patients With Non-Small-Cell Lung Cancer After Curative Surgical Resection. Clin Lung Cancer. 2017;18:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Inoue K, Goto R, Okada K, Kinomura S, Fukuda H. A bone marrow F-18 FDG uptake exceeding the liver uptake may indicate bone marrow hyperactivity. Ann Nucl Med. 2009;23:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, Kwee TC. Variety in bone marrow 18F-FDG uptake in Hodgkin lymphoma patients without lymphomatous bone marrow involvement: does it have an explanation? Nucl Med Commun. 2016;37:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 21. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 22. | Prévost S, Boucher L, Larivée P, Boileau R, Bénard F. Bone marrow hypermetabolism on 18F-FDG PET as a survival prognostic factor in non-small cell lung cancer. J Nucl Med. 2006;47:559-565. [PubMed] |
| 23. | Murata Y, Kubota K, Yukihiro M, Ito K, Watanabe H, Shibuya H. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: measurements by PET/CT. Nucl Med Biol. 2006;33:999-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Bural GG, Torigian DA, Chen W, Houseni M, Basu S, Alavi A. Increased 18F-FDG uptake within the reticuloendothelial system in patients with active lung cancer on PET imaging may indicate activation of the systemic immune response. Hell J Nucl Med. 2010;13:23-25. [PubMed] |
| 25. | Lee JW, Seo KH, Kim ES, Lee SM. The role of (18)F-fluorodeoxyglucose uptake of bone marrow on PET/CT in predicting clinical outcomes in non-small cell lung cancer patients treated with chemoradiotherapy. Eur Radiol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH. Pretreatment anemia is associated with poorer survival in patients with stage I and II gastric cancer. J Surg Oncol. 2005;91:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1502] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 28. | Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol. 2014;110:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Wang F, Liu ZY, Xia YY, Zhou C, Shen XM, Li XL, Han SG, Zheng Y, Mao ZQ, Gong FR. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol Lett. 2015;10:3411-3418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Postlewait LM, Squires MH, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR. The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: A multi-institutional study of the US Gastric Cancer Collaborative. J Surg Oncol. 2015;112:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Cicone F, Loose D, Deron P, Vermeersch H, Signore A, Van de Vyvere F, Scopinaro F, Van de Wiele C. Prognostic value of FDG uptake by the bone marrow in squamous cell carcinoma of the head and neck. Nucl Med Commun. 2008;29:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972-1980. [PubMed] |
| 35. | Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells important? J Nucl Med. 1995;36:1854-1861. [PubMed] |
| 36. | Dirican N, Karakaya YA, Günes S, Daloglu FT, Dirican A. Association of Intratumoral Tumor Infiltrating Lymphocytes and Neutrophil-to- Lymphocyte Ratio Are an Independent Prognostic Factor in Non-Small Cell Lung Cancer. Clin Respir J. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Ma C, Wang M, Hou H, Cui L, Jiang C, Sun J, Qu X. Prognostic significance of immune cells in the tumor microenvironment and peripheral blood of gallbladder carcinoma patients. Clin Transl Oncol. 2017;19:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Yagi M, Froelich J, Arentsen L, Shanley R, Ghebre R, Yee D, Hui S. Longitudinal FDG-PET Revealed Regional Functional Heterogeneity of Bone Marrow, Site-Dependent Response to Treatment and Correlation with Hematological Parameters. J Cancer. 2015;6:531-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |