Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2308
- This article has been corrected.
- See: World J Gastroenterol. May 21, 2025; 31(19): 108304
Peer-review started: January 10, 2017
First decision: February 23, 2017
Revised: February 27, 2017
Accepted: March 6, 2017
Article in press: March 6, 2017
Published online: April 7, 2017
Processing time: 86 Days and 19.7 Hours
To investigate the mechanism of chaperone-mediated autophagy (CMA)-induced resistance to irradiation-triggered apoptosis through regulation of the p53 protein in hepatocellular carcinoma (HCC).
Firstly, we detected expression of lysosome-associated membrane protein 2a (Lamp-2a), which is the key protein of CMA, by western blot in HepG2 and SMMC7721 cells after irradiation. We further used shRNA Lamp-2a HCC cells to verify the radioresistance induced by CMA. Next, we detected the HMGB1 and p53 expression after irradiation by western blot, and we further used RNA interference and ethyl pyruvate (EP), as a HMGB1 inhibitor, to observe changes of p53 expression. Finally, an immunoprecipitation assay was conducted to explore the interaction between Lamp-2a and HMGB1, and the data were analyzed.
We found the expression of Lamp-2a was increased on irradiation while apoptosis decreased in HepG2 and SMMC7721 cells. The apoptosis was increased markedly in the shRNA Lamp-2a HepG2 and SMMC7721 cells as detected by western blot and colony formation assay. Next, we found p53 expression was gradually reduced on irradiation but obviously increased in shRNA Lamp-2a cells. Furthermore, p53 increased the cell apoptosis on irradiation in Hep3B (p53-/-) cells. Finally, p53 levels were regulated by HMGB1 as measured through RNA interference and the EP treatment. HMGB1 was able to combine with Lamp-2a as seen by immunoprecipitation assay and was degraded via the CMA pathway. The decreased HMGB1 inhibited p53 expression induced by irradiation and further reduced the apoptosis in HCC cells.
CMA pathway activation appears to down-regulate the susceptibility of HCC to irradiation by degrading HMGB1 with further impact on p53 expression. These findings have clinical relevance for radiotherapy of HCC.
Core tip: The activation of chaperone-mediated autophagy plays an important role in reducing hepatocellular carcinoma (HCC) cell apoptosis in response to irradiation through degraded HMGB1 protein which reduces p53 expression. The discovery of this mechanism will be beneficial for inhibiting radioresistance of HCC and has a promising value in clinical treatment strategy.
- Citation: Wu JH, Guo JP, Shi J, Wang H, Li LL, Guo B, Liu DX, Cao Q, Yuan ZY. CMA down-regulates p53 expression through degradation of HMGB1 protein to inhibit irradiation-triggered apoptosis in hepatocellular carcinoma. World J Gastroenterol 2017; 23(13): 2308-2317
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2308
Hepatocellular carcinoma (HCC) is a rapidly progressive fatal malignancy, with an increasing incidence of HCC-related morbidity demonstrated at present[1]. Radiotherapy is regarded as a therapeutic modality and has achieved efficacious tumor control as well as the lengthening of patients’ lives in advanced disease[2,3]. However, HCC has commonly been regarded as a radioresistant tumor for a long time[4], because varieties of cellular processes are activated in radiotherapy involving essential proteins which make a difference in the curative effects[5]. Recent research has found that autophagy is not only fundamental for the cellular response to stress[6], but also strongly links with cancer resistance in therapy[7]. Chaperone-mediated autophagy (CMA) is a selective form of autophagy and mainly recognizes and degrades KFERQ sequence motifs[8]. There is a growing interest to investigate CMA function as well as the related mechanism in tumor therapy. It has been shown that CMA activation significantly reduced therapeutic efficacy in tumor therapy[9]. Nevertheless, the related specific mechanism remains to be elucidated.
p53 is functional when cell damage such as irradiation occurs in the DNA resulting in the inducing of growth arrest in G1 or G2 phases of the cell cycle or leading to cell apoptosis, thus protecting cells from uncontrolled proliferation and inhibiting tumor development[10,11]. Generally, the loss of p53 function is responsible for increased tumor resistance. It has been reported that CMA can regulate p53 protein expression, but not degrade p53 protein, to influence the Bcl-2 and Bax levels in cytoplasm[12]. Therefore, there must be another molecule to regulate the p53 expression.
High-mobility group box 1 (HMGB1), a chromatin associated nuclear protein and extracellular damage associated molecular pattern molecule (DAMP), is a protein that has complicated functions in cancer. Reports have shown that HMGB1 expresses highly in varieties of cancers including HCC[13], lung[14], gastric[15,16] etc., which means HMGB1 is closely related with tumor development, infiltration and metastases. Besides, HMGB1 can bind many proteins involved in autophagy including Beclin1, Atg5[17,18]. Some studies showed that p53 and HMGB1 could form a complex that regulates the cytoplasmic localization of the binding protein and impacts on cell autophagy as well as apoptosis[19]. This means that HMGB1 could impact the p53 protein expression and then regulate the anti-apoptotic function of p53. Hence, this paper will focus on CMA-induced radioresistance as well as the connection among CMA activation, p53 and HMGB1 protein expression, and ascertain the mechanism of CMA-induced radioresistance in HCC cells through degrading HMGB1 and further regulating p53 expression.
Antibodies targeted to Bcl-2, Caspase 3(cleaved), HMGB1, p53 (rabbit), p21, and Tubulin were obtained from Cell Signaling Technology (United States). Anti-p53 Ab (mouse) was obtained from Santa Cruz Biotechnology, Inc. (United States). Antibodies targeted to Lamp-2a were obtained from Abcam (United States). Ethyl pyruvate (EP) (CAS: 617-35-6) was obtained from SIGMA (United States). Protein A/G sepharose beads were obtained from SIGMA (United States). Lamp2a shRNA (sh-Lamp2a)-expressing lentivirus (target sequence 5 -GCAGTGCAGATGACGACAA-3) and a nonsilencing sequence-expressing lentivirus (sh-NC) (5-TTCTCCGAACGTGTCACGTTTC-3) were supplied commercially by GenePharma Co. Ltd. (Suzhou, China).
The human SMMC7721 (wt p53), HepG2 (wt p53), Hep3B (p53-/-) cell lines were cultured at 37 °C in a humidified atmosphere containing 5% CO2 in the following media: high-glucose DMEM (Gibco) supplemented with 10% heat-inactivated FBS, 100 units/mL penicillin and 100 mg/mL streptomycin.
Cells were cultured as described above and irradiated with a 6-MV X-ray linear accelerator (BJ6B/400 AFC system; Beijing, China) at a dose rate of 360 cGy/min; the total dosage was 0-10 Gy. The control cells were cultured with medium alone. At 48 h after the irradiation, the cells were used for further experiments.
About 2000 cells were planted to each well of a 6-well culture plate, and each group contained three wells. After incubation at 37 °C for 14 d the cells were washed twice with PBS and stained with Crystal Violet Staining Solution. The number of colonies containing 50 cells was counted under a microscope.
Short hairpin RNA (shRNA) expressing stable Lamp-2α transformants were generated by infecting the cells with lentiviral expressing specific shRNA (sh-Lamp2a-expressing lentivirus or sh-NC-expressing lentivirus). Western blots were performed to determine the knockdown efficiency.
Cells were cultured and transfected with purified, annealed and desalted double-stranded siRNA (40 μg/2 × 106 cells) using the Amaxa nucleofection system (kit V, program G-16). siRNA targeted against lHMGB1. (HMGB1 sense strand siRNA: UGUUACAGAGCGGAGAGAGUU, HMGB1 antisense strand siRNA: CUCUCUCCGCUCUGUAACAUU. Control sense strand siRNA: GAUGAUCUAAUGGC, Control antisense strand siRNA: GUCUCACUCGCUCUCUAUACU).
Cells were collected and lysed in whole-cell lysate (containing PMSF and a phosphatase inhibitor). Equal amounts of cell lysate were separated by SDS-PAGE and electrotransferred to polyvinylidene difluoride membranes. Proteins were detected using an ECL system (Pierce, Thermo, United States). Each experiment was repeated three times, and similar results were obtained.
The cell lysate (500 μg total protein) was combined with 30 μL of protein A/G sepharose beads and 2 μg of primary antibody followed by continuous rotation at 4 °C overnight. Beads were washed four times with immunoprecipitation (IP) buffer for 5 min at 2500 rpm and at 4 °C. Next, 1 × SDS buffer was added to the samples, which were then treated at 100 °C for 10 min. Immunoprecipitated samples were analyzed by Western blotting using anti-Lamp-2a, anti-HMGB1.
Statistical analysis was performed with SPSS 17.0 software. Graphs were analyzed using Image Lab system. The data are expressed as mean ± SD of the values from three independent determinations and statistical analysis was conducted using either the Student’s t-test or one-way analysis of variance in comparison with corresponding controls. Probability values less than 0.05 were considered statistically significant.
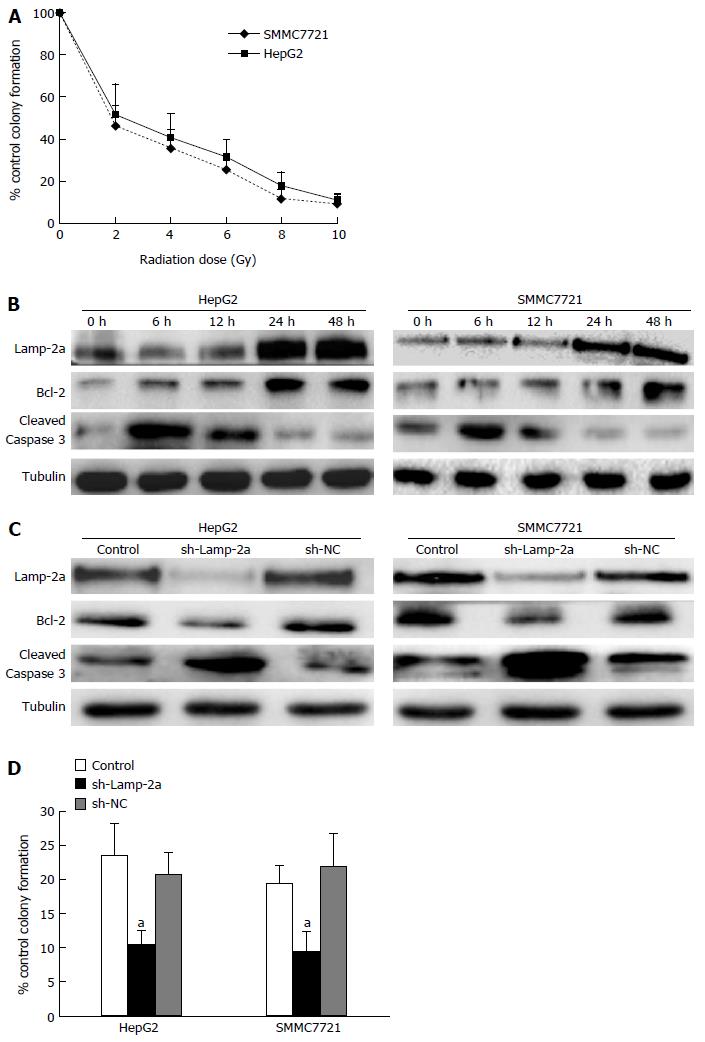
One of the major therapeutic mechanisms of irradiation is to induce target cell apoptosis[20]. In this study, the apoptosis of HepG2 and SMMC7721 after irradiation increased in a dose-dependent manner (Figure 1A). The apoptosis also took on a dynamical change: it increased at 6-12 h, and decreased at 24-48 h (Figure 1B). In consideration of the declining apoptosis induced by radiotherapy, we thought there must exist some factors to reduce the irradiation-induced apoptosis. Studies showing that CMA pathway activation plays a role in regulating cancer cell proliferation or apoptosis gave rise to our attention[21]. To determine whether CMA pathway activation impacts on the irradiation-induced cancer cell apoptosis, we firstly detected the activation of the CMA pathway. Lamp-2a, the key protein in the CMA pathway[22], was gradually increased on irradiation and peaked at 48 h (Figure 1B). Contrary to activation of the CMA pathway, the apoptosis levels decreased at 24-48 h. To confirm whether CMA activation has functions in down-regulating irradiation-induced apoptosis, we used sh-Lamp-2a cells to investigate the effects of CMA induced by irradiation in HCC cells. The results showed that the apoptosis increased obviously in sh-Lamp-2a cells (Figure 1C), and the clone formation assay results provided more evidence (Figure 1D). Taken together, from these results, we believe that the activated CMA pathway plays an important role in down-regulating the apoptosis in HCC cells on irradiation.
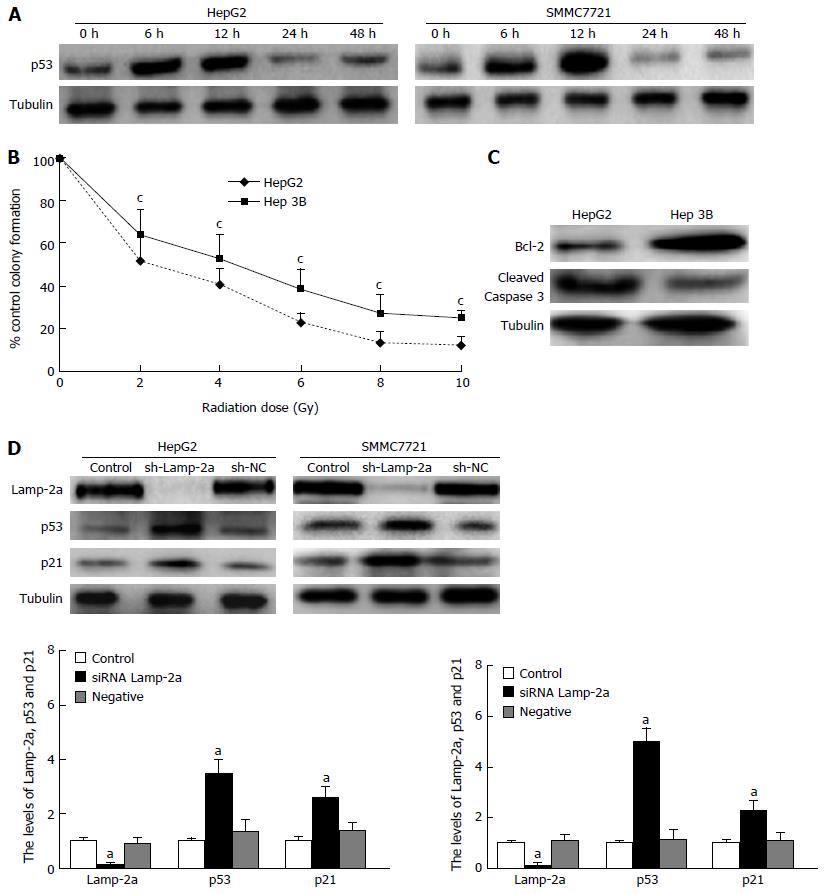
It is well known that p53, an important tumor suppressor, is able to impact on cell apoptosis through a variety of pathways. To find out the role of p53 in HCC cell irradiation, we firstly detected the p53 expression in irradiated HepG2 and SMMC7721 cells. The results showed p53 gradually increased in 6-12 h, and began to decrease in 24-48 h on irradiation (Figure 2A). Meanwhile, HepG2 and SMMC7721 cell apoptosis greatly reduced at 24-48 h after radiotherapy (Figure 1B). From the similar tendency between down-regulated apoptosis and decreased p53 expression on irradiation, we wondered whether the reduced p53 expression induced the down-regulated apoptosis on irradiation. In order to confirm this hypothesis, we detected the apoptosis and growth of HepG2, Hep3B (p53-/-) cells on irradiation. The results demonstrated that the susceptibility to irradiation of Hep3B (p53-/-) was lower than HepG2 (Figure 2B and C). Therefore, we confirmed p53 played key roles in radioresistance. As shown in Figure 1B and Figure 2A, we found the level of p53 protein was just the opposite of the increased CMA pathway activation. This result made us speculate whether there were somehow links between p53 reduction and CMA pathway activation. To confirm whether the reduced levels of p53 had some links with the CMA pathway activation, we carried out the following experiments. We constructed the sh-Lamp-2a HepG2 and sh-Lamp-2a SMMC7721 cells and treated them with irradiation. We found expressions of the p53 and its downstream effector protein p21 were both higher than those in wild type cells (Figure 2D). These results revealed that p53 expression was regulated by the CMA pathway.
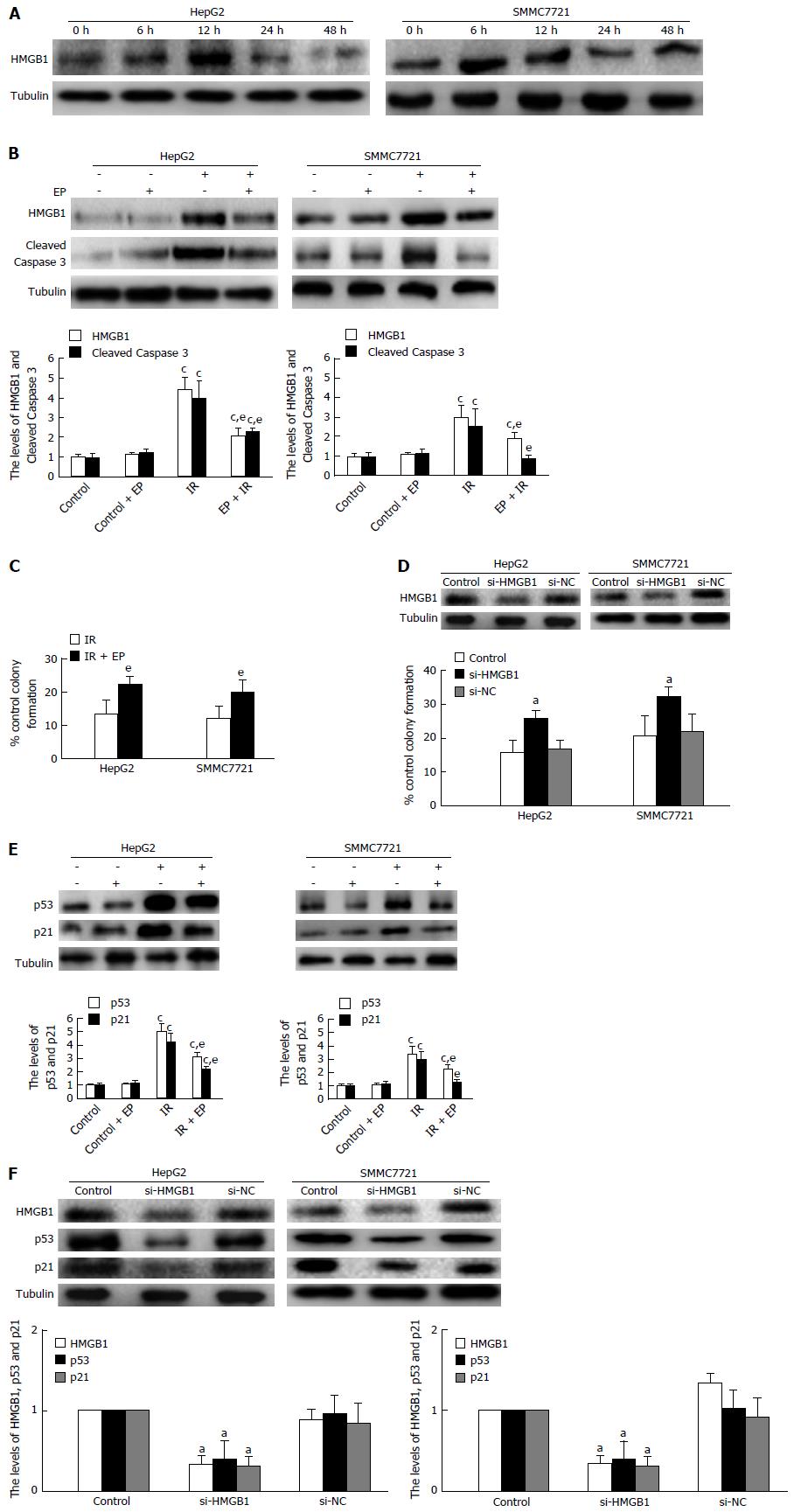
Previous studies showed that p53 was not degraded by the CMA pathway but rapidly degraded by the activity of the p53-targeting ubiquitin ligase MDM[23]. Nevertheless, we had confirmed that p53 expression was regulated by the CMA pathway activation from our results. So we thought there must be another protein which connected p53 reduction and CMA pathway activation on irradiation. It was reported that p53 could interact with HMGB1 in cells to regulate cell autophagy and apoptosis[24]. That led us to suspect that HMGB1 may be the key molecule to regulate the p53 reduction on irradiation. So we firstly detected HMGB1 expression in HepG2 and SMMC7721 cells treated with irradiation at 6 Gy. The results showed that HMGB1 increased at 6-12 h and decreased at 24-48 h on irradiation (Figure 3A). To further investigate whether HMGB1 plays a role in regulating apoptosis of HCC cells on irradiation, we performed the experiments described below. Firstly, we used EP, the specific inhibitor of HMGB1, to suppress HMGB1 expression and detected the apoptosis at 48 h after irradiation at 6 Gy. The result revealed that cell apoptosis significantly declined (Figure 3B); meanwhile the colony-forming efficiency was markedly increased (Figure 3C). Next, we further tested and verified the role of HMGB1 by RNA interference. In si-HMGB1 cells, cell colony-forming efficiency increased in contrast to that seen in HCC cells or si-NC cells (Figure 3D). The above data affirmed that HMGB1 was able to regulate HCC apoptosis on radiotherapy. When EP and siRNA of HMGB1 were applied again to detect the change in p53 expression, we found that the expression of p53 and p21 had declined distinctly (Figure 3E and F). Previous studies had shown that knock-out p53 could reduce the apoptosis on irradiation, so HMGB1 could influence HCC cell apoptosis through regulation of p53 expression on radiotherapy.
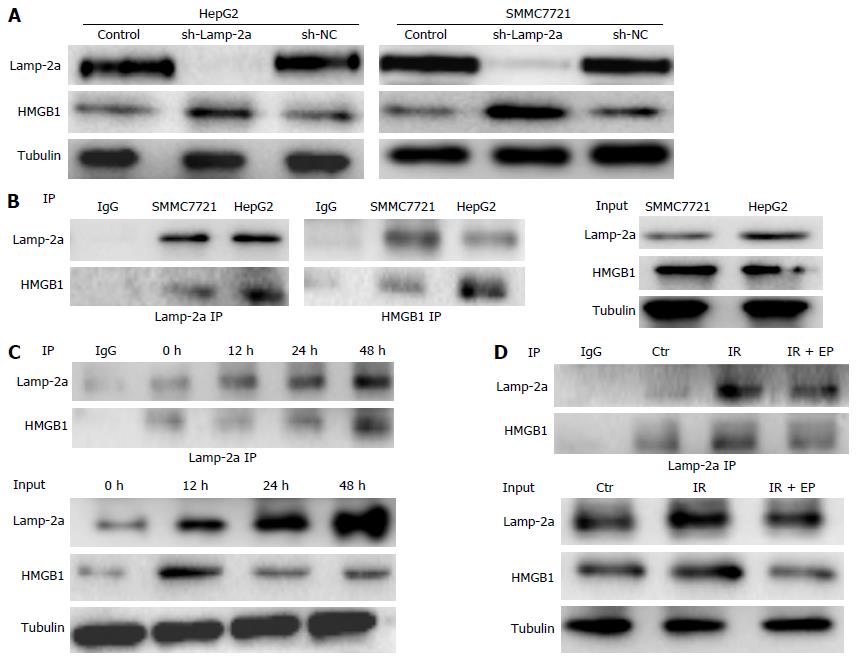
The above experiments confirmed CMA pathway activation could reduce p53 expression. What’s more, p53 was also regulated by HMGB1 on irradiation. So we speculated as to whether HMGB1 was degraded by CMA pathway activation and then reduced the p53 expression. To confirm our hypothesis, we first tested the HMGB1 levels in sh-Lamp-2a HepG2 and sh-Lamp-2a SMMC7721 cells. As Figure 4A shows, the HMGB1 increased obviously in sh-Lamp-2a cells on irradiation, which indicated CMA pathway activation has a function in regulating the HMGB1 expression. Through immunoprecipitation analysis, we found that HMGB1 could interact with Lamp-2a and formed a complex in HCC cells (Figure 4B). The HMGB1 combined with Lamp-2a increased gradually from 12 to 48 h on irradiation. Meanwhile, the HMGB1 in cytoplasm was decreased (Figure 4C). This meant that HMGB1 degraded by CMA resulted in low cytoplasm HMGB1 levels. Further, we continued to detect HMGB1 combined with Lamp-2a in the presence of ethyl pyruvate (EP, which was applied as an inhibitor of HMGB1) by immunoprecipitation assay. As results show in Figure 4D, the HMGB1 reduced markedly in the presence of EP; meanwhile HMGB1 combined with Lamp-2a also decreased. This provides just one more testament to the HMGB1 degradation through CMA pathway activation.
HCC is a common malignant tumor and radioresistance is a critical issue in clinical treatment[25]. In radiotherapy, induction of apoptosis is the major therapeutic mechanism. Once the apoptosis induced by irradiation declines, the tumor appears to have comparatively low sensitivity to irradiation. Recently, CMA has been gaining more and more attention with regard to tumor progression and therapy because of its function in regulation of apoptosis. In our study, we found the CMA pathway was activated gradually in radiotherapy of HCC cells, especially in the late stage of irradiation. Inhibition of CMA pathway activation could sensitize tumor cells to undergo apoptosis, implying CMA could protect hepatocarcinoma cells from radioactive harm, and induce cell production of radioresistance. Besides the CMA pathway, there are varieties of cellular processes that determine the success of treatment, including p53 protein[26]. p53 is a transcription factor associated with more than 50% of human tumors and a key molecule involved in response to cell apoptosis in radiotherapy[27-29]. In our study, apoptosis was much lower on irradiation in Hep3B cells (p53-/-) than in HepG2 cells. This made us convinced that on irradiation, p53 plays an important role in apoptosis regulation. Since p53 protein and CMA pathway activation had important functions in HCC cells irradiation, we thought there maybe existed an interaction between CMA pathway activation and p53 protein expression. In sh-Lamp-2a HepG2 and SMMC7721 cells, p53 protein and the downstream p21 protein both increased sharply. From this, we confirmed CMA pathway activation did regulate p53 expression on irradiation. However, Vakifahmetoglu-Norberg[12] mentioned that p53 was not directly degraded through the CMA pathway; so there must be another way to regulate the p53 expression.
It is well known that many proteins in cells can interact with p53 protein and further influence cell progression and apoptosis. The interactions are important for its activity and function. Studies have shown that p53 interacts with HMGB1 proteins in cells and further regulates the cell proliferation and migration[30-32]. In our study, we found that HMGB1 was significantly expressed in irradiated HCC cells but markedly reduced in the late stage on irradiation. At the same time, the HCC cell apoptosis also reduced in line with the HMGB1 reduction. When HepG2 and SMMC7721 had HMGB1 expression blocked with EP or RNA interference, p53 and its downstream molecule p21 were obviously decreased. Combined with previous p53 regulation of apoptosis results, we confirmed that in the late stage of irradiation, HMGB1 plays a vital role in inducing apoptosis through regulation of p53 expression. Although some studies have revealed that the CMA pathway could impact the HMGB1 expression, the mechanism has been unclear. In our study, we confirmed that HMGB1 could interact with Lamp-2α and form a complex through immunoprecipitation assay. This means that HMGB1 induced by irradiation maybe contains KFERQ-like sequence motifs which might permit Hsc70 to recognize and in turn promote the degradation of HMGB1 in a lysosome-dependent manner.
To summarize the above, CMA pathway activation could down-regulate HCC cell susceptibility to apoptosis on irradiation through degradation of HMGB1 protein which reduced p53 expression. These findings suggest that CMA plays a critical role in the irradiation treatment of HCC and that it has a promising value in clinical treatment strategy.
The activation of chaperone-mediated autophagy (CMA) plays an important role in reducing hepatocellular carcinoma (HCC) cell susceptibility to apoptosis during irradiation. In this paper, we mainly explore the possible mechanism of radioresistance of HCC induced by the CMA pathway and the findings will be beneficial to clinical treatment strategy.
HCC is a common malignant tumor and radioresistance is critical in clinical treatment. Recent research has found that autophagy is not only fundamental for the cellular response to stress, but also strongly links with cancer resistance in therapy. There is a growing interest to investigate CMA function as well as the related mechanism in tumor therapy.
This paper mainly focuses on CMA-induced radioresistance as well as the connection among CMA, p53 and HMGB1 proteins, and ascertains the mechanism of CMA-induced radioresistance in HCC cells through degradation of HMGB1 and further regulating p53 expression.
This study revealed that CMA plays a critical role in the reduction of apoptosis of HCC cells in irradiation treatment. The discovery of this mechanism will be beneficial to inhibiting radioresistance of HCC and has a promising value in clinical treatment strategy.
It is well-known that p53 is one of the important molecules for anticancer mechanism. The authors showed the CMA activation was associated with reduced p53 and apoptosis. They showed that CMA (not MDM) could decrease p53 protein through the reduction of HMGB1. This paper is generally well-written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Enomoto H S- Editor: Ma YJ L- Editor: Logan S E- Editor: Zhang FF
| 1. | Steel JL, Nadeau K, Olek M, Carr BI. Preliminary results of an individually tailored psychosocial intervention for patients with advanced hepatobiliary carcinoma. J Psychosoc Oncol. 2007;25:19-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Kalogeridi MA, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, Kouloulias V. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015;7:101-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (2)] |
| 3. | Kuo YC, Chiu YM, Shih WP, Yu HW, Chen CW, Wong PF, Lin WC, Hwang JJ. Volumetric intensity-modulated Arc (RapidArc) therapy for primary hepatocellular carcinoma: comparison with intensity-modulated radiotherapy and 3-D conformal radiotherapy. Radiat Oncol. 2011;6:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Liu CY, Chang HS, Chen IS, Chen CJ, Hsu ML, Fu SL, Chen YJ. Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat Oncol. 2011;6:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Gomes AR, Abrantes AM, Brito AF, Laranjo M, Casalta-Lopes JE, Gonçalves AC, Sarmento-Ribeiro AB, Botelho MF, Tralhão JG. Influence of P53 on the radiotherapy response of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:257-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829-4840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 478] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 635] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 9. | Saha T. LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy. 2012;8:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 798] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 11. | Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1427] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 12. | Vakifahmetoglu-Norberg H, Kim M, Xia HG, Iwanicki MP, Ofengeim D, Coloff JL, Pan L, Ince TA, Kroemer G, Brugge JS. Chaperone-mediated autophagy degrades mutant p53. Genes Dev. 2013;27:1718-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem. 2010;337:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Wang C, Fei G, Liu Z, Li Q, Xu Z, Ren T. HMGB1 was a pivotal synergistic effecor for CpG oligonucleotide to enhance the progression of human lung cancer cells. Cancer Biol Ther. 2012;13:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Kou YB, Zhu JS, Chen WX, Li S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-κB pathway in vitro and in vivo. Int J Oncol. 2014;44:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Song B, Song WG, Li ZJ, Xu ZF, Wang XW, Wang CX, Liu J. Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells. Cell Biochem Funct. 2012;30:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Gao XS, Zhao J, Xiong W, Zhang M, Li HZ, Zhou DM, Jin X, Zhang DS. Differential gene expression profiles of DNA repair genes in esophageal cancer cells after X-ray irradiation. Chin J Cancer. 2010;29:865-872. [PubMed] |
| 18. | Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ, Li L. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Sun AM, Li CG, Han YQ, Liu QL, Xia Q, Yuan YW. X-ray irradiation promotes apoptosis of hippocampal neurons through up-regulation of Cdk5 and p25. Cancer Cell Int. 2013;13:47. [PubMed] |
| 21. | Xie W, Zhang L, Jiao H, Guan L, Zha J, Li X, Wu M, Wang Z, Han J, You H. Chaperone-mediated autophagy prevents apoptosis by degrading BBC3/PUMA. Autophagy. 2015;11:1623-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Napolitano G, Johnson JL, He J, Rocca CJ, Monfregola J, Pestonjamasp K, Cherqui S, Catz SD. Impairment of chaperone-mediated autophagy leads to selective lysosomal degradation defects in the lysosomal storage disease cystinosis. EMBO Mol Med. 2015;7:158-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296-299. [PubMed] |
| 24. | Rowell JP, Simpson KL, Stott K, Watson M, Thomas JO. HMGB1-facilitated p53 DNA binding occurs via HMG-Box/p53 transactivation domain interaction, regulated by the acidic tail. Structure. 2012;20:2014-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Zheng L, Ding Y, Li Q, Wang R, Liu T, Sun Q, Yang H, Peng S, Wang W. MiR-20a Induces Cell Radioresistance by Activating the PTEN/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Takahashi A. Different inducibility of radiation- or heat-induced p53-dependent apoptosis after acute or chronic irradiation in human cultured squamous cell carcinoma cells. Int J Radiat Biol. 2001;77:215-224. [PubMed] |
| 27. | Brito AF, Abrantes AM, Ribeiro M, Oliveira R, Casalta-Lopes J, Gonçalves AC, Sarmento-Ribeiro AB, Tralhão JG, Botelho MF. Fluorine-18 Fluorodeoxyglucose Uptake in Hepatocellular Carcinoma: Correlation with Glucose Transporters and p53 Expression. J Clin Exp Hepatol. 2015;5:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Yan HX, Wu HP, Zhang HL, Ashton C, Tong C, Wu H, Qian QJ, Wang HY, Ying QL. p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release. J Hepatol. 2013;59:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Lee YR, Park SY. P53 expression in hepatocellular carcinoma: influence on the radiotherapeutic response of the hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:230-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201:613-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 31. | Stros M, Muselíková-Polanská E, Pospísilová S, Strauss F. High-affinity binding of tumor-suppressor protein p53 and HMGB1 to hemicatenated DNA loops. Biochemistry. 2004;43:7215-7225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Chen Y, Lin C, Liu Y, Jiang Y. HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2. Tumour Biol. 2016;37:4399-4408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |