Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1857
Peer-review started: November 8, 2016
First decision: December 19, 2016
Revised: January 4, 2017
Accepted: February 7, 2017
Article in press: February 8, 2017
Published online: March 14, 2017
Processing time: 128 Days and 13.3 Hours
To determine whether hospital characteristics predict cirrhosis mortality and how much variation in mortality is attributable to hospital differences.
We used data from the 2005-2011 Nationwide Inpatient Sample and the American Hospital Association Annual survey to identify hospitalizations for decompensated cirrhosis and corresponding facility characteristics. We created hospital-specific risk and reliability-adjusted odds ratios for cirrhosis mortality, and evaluated patient and facility differences based on hospital performance quintiles. We used hierarchical regression models to determine the effect of these factors on mortality.
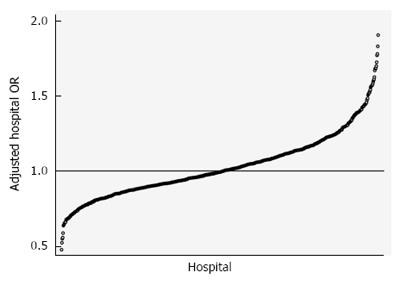
Seventy-two thousand seven hundred and thirty-three cirrhosis admissions were evaluated in 805 hospitals. Hospital mean cirrhosis annual case volume was 90.4 (range 25-828). Overall hospital cirrhosis mortality rate was 8.00%. Hospital-adjusted odds ratios (aOR) for mortality ranged from 0.48 to 1.89. Patient characteristics varied significantly by hospital aOR for mortality. Length of stay averaged 6.0 ± 1.6 days, and varied significantly by hospital performance (P < 0.001). Facility level predictors of risk-adjusted mortality were higher Medicaid case-mix (OR = 1.00, P = 0.029) and LPN staffing (OR = 1.02, P = 0.015). Higher cirrhosis volume (OR = 0.99, P = 0.025) and liver transplant program status (OR = 0.83, P = 0.026) were significantly associated with survival. After adjusting for patient differences, era, and clustering effects, 15.3% of variation between hospitals was attributable to differences in facility characteristics.
Hospital characteristics account for a significant proportion of variation in cirrhosis mortality. These findings have several implications for patients, providers, and health care delivery in liver disease care and inpatient health care design.
Core tip: Cirrhosis mortality varies across hospitals, but it is not well-understood what differences between hospitals contribute to this variation. In our study, using administrative data on cirrhosis discharges and a national dataset on hospital structural characteristics, we found that several hospital factors including payer-mix and staffing patterns were associated with risk-adjusted mortality, but hospital experience with cirrhosis and presence of a liver transplant program were associated with survival. Structural factors are vital components to cirrhosis care delivery, and account for a significant proportion of the variation in cirrhosis mortality observed between hospitals. Future research should focus on other areas of variation, including differences in processes of cirrhosis care.
- Citation: Mathur AK, Chakrabarti AK, Mellinger JL, Volk ML, Day R, Singer AL, Hewitt WR, Reddy KS, Moss AA. Hospital resource intensity and cirrhosis mortality in United States. World J Gastroenterol 2017; 23(10): 1857-1865
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1857
Cirrhosis is the final common pathway for chronic liver disease, which predisposes patients to life-threatening complications. These complications - including ascites, spontaneous bacterial peritonitis, hepatic hydrothorax, variceal hemorrhage, hepatic encephalopathy, and hepatocellular carcinoma - require significant inpatient resources for appropriate and timely management to ensure good clinical outcomes[1]. Inpatient admissions for cirrhosis represent a critical juncture in the con tinuum of care of liver disease, because hospitals differ in their ability to care for these patients. We have previously demonstrated that hospitals differ 14-fold in mortality rates for cirrhosis[1], but the mechanism behind these differences is relatively unknown.
A major impediment to improving mortality in cirrhosis is a lack of national-level clinical registry data, which obligates providers to utilizing inferential studies from a variety of data sources to change practices[1]. Conceptually, variation in cirrhosis mortality between hospitals, using the Donabedian model of health care quality, are attributable to differences in: (1) patient characteristics and severity of disease; (2) the structure of health care facilities; and (3) processes of cirrhosis care[1]. Two hospitals can only have different clinical outcomes if they differ in these factors. Processes of care are the way in which care is delivered in a particular context and how prevailing structural resources are used for patients. These are of critical importance, but cirrhosis quality measures are not tracked nationally[1]. However, facility resources create the context for these processes, and are easily measured. For inpatient cirrhosis care, these resources include hospital bed capacity, staffing patterns of physicians and nurses, teaching status, endoscopic and imaging services, critical care and transplant personnel, and other specialized services.
In this study, we aimed to further evaluate the mechanism behind hospital variation in cirrhosis mortality by evaluating structural differences while controlling for patient differences. Specifically, we aimed to identify specific factors that were different between hospitals based on their risk-adjusted performance, whether these factors were associated with survival, and to what extent did they explain variation in cirrhosis mortality across hospitals. This approach would therefore allow us to “partition the total variance” in cirrhosis outcomes among patient differences, which are largely immutable, and modifiable factors such as hospital resource intensity, and, by exclusion, processes of care.
We captured admissions from the Nationwide Inpatient Sample (NIS) from the years 2005-2011. The NIS is the largest publicly available inpatient care database in the United States. It is sponsored by the Agency for Healthcare Research and Quality (AHRQ) through the Healthcare Cost and Utilization Project (HCUP). It contains clinical and resource utilization information for over 7 million admissions per year from a stratified sample of 20% of discharges from US acute care hospitals. Facility characteristics were derived from the American Hospital Association (AHA) Annual Survey. This survey contains data on approximately 6500 hospitals nationwide with over 1000 elements covering hospital facilities, services, organization, and personnel. We merged NIS and AHA data using a common hospital identifier for each cirrhosis admission in each year.
We utilized admissions from NIS that contained an AHA Annual Survey identifier and had corresponding data in the AHA Survey. We captured cirrhotic admissions as previously described[1,2]. Briefly, we included admissions with a primary or secondary diagnosis of cirrhosis (alcoholic, non-alcoholic, or biliary) or a complication of cirrhosis (ascites, hepatic encephalopathy, hepatorenal syndrome, portal hypertension, or variceal bleed). We excluded admissions to hospitals with a cirrhotic volume less than 25 patients annually as well as admissions missing data on sex, insurance type, hospital bed size, or had inconsistent or undefined liver transplant program status across the study period[1,2].
We first created a risk and reliability-adjusted logistic regression model for mortality for any given hospital compared to an average hospital. We did this by first creating a hierarchical logistic regression model where patient covariates and year of admission were treated as fixed effects (level 1 variables) and hospital (level 2 variables) was treated as a random effect. Patient covariates included age, sex, race/ethnicity, cause of cirrhosis including hepatitis C virus positivity, alcoholic liver disease, or other, and presence of cirrhotic complications including ascites, variceal bleed, hepatic encephalopathy, portal hypertension, hepatorenal syndrome, liver transplant, hepatocellular carcinoma, requirement of paracentesis or esophagogastroduodenoscopy. Further adjustment for clinical co-morbidities was included in the model using the all patient refined-diagnosed related group (APR-DRG) risk of mortality[2,3].
Using this model, we then used the empirical Bayes technique to estimate a risk and reliability adjusted odds ratio for mortality via the random effects estimate[4,5]. Of note, this method is considered to be a relatively conservative one, as it “shrinks” variation towards the mean. This estimate incorporated the above fixed effects for risk adjustment and the hospital cirrhosis volume to adjust for reliability of the mortality measurement. We then partitioned hospital into quintiles based on the adjusted OR for mortality (quintile 1 included highest performing hospitals (lowest adjusted OR’s for mortality) and quintile 5 included lowest performing (highest adjusted OR’s for mortality).
We subsequently examined whether patient covariates and key hospital characteristics varied across quintiles. Hospital characteristics representing several structural domains including organizational structure, personnel, hospital facilities and services, and financial performance were selected based on previous literature[6] and included liver transplant program status, cirrhosis volume, the number of ICU beds, teaching status (number of resident full-time equivalents), and payer mix (the number of yearly Medicaid days), clinical staffing (licensed practical nurses (LPNs), and physicians per adjusted facility patient days). These structural variables were based on previous studies on the role of hospital structure on clinical outcomes[6], but were further expanded to include clinical resources specifically relevant to the management of cirrhotic patients.
We designed the statistical models to conceptually understand what factors account for differences in the mortality rate (risk and reliability-adjusted) between hospitals. Specifically, we assumed that variance in mortality was attributable to (1) clinical differences between patients; (2) hospital differences (further delineated by differences in structural variables); and (3) unmeasured factors (differences in unmeasured processes of care, other factors, and random error. We created a cumulative hierarchical logistic regression model that added these structural variables sequentially to calculate the fraction of inter-hospital variance in adjusted mortality explained by each structural variable. The baseline model adjusted for clinical differences between patients, and we evaluated the change in the calculated variance of the model with the sequential addition of specific structural characteristics. These were selected from the factors significantly different across mortality quintiles on univariate analysis. This approach assumed that structural factors contribute to variation in mortality between hospitals, and adjusting for them would attenuate the measured variance, i.e., more of the differences between hospitals would be explained by the model as more significant structural factors were included.
These data were publicly available administrative data and were exempt from Institutional Review Board approval at the University of Michigan. All statistical analyses were performed using Stata 13 (Stata Corp, College Station, TX, United States). Statistical significance was considered at the P = 0.05 level.
Figure 1 demonstrates the range of risk and reliability-adjusted odds ratios (aOR) for in-hospital mortality, or hospital performance in cirrhosis. The demographic characteristics of the study cohort are included in Table 1 and stratified by aOR quintiles. The study cohort included 72733 hospital admissions from 2005-2011 that were divided amongst 805 hospitals. 29.6% of hospitalizations occurred in the lowest-mortality 20% of hospitals, and 20.6% of hospitalizations occurred in the highest-mortality 20%. Patient demographic distributions varied significantly across quintiles, including age, sex, race, and payer mix. The mean age of the cohort was 57.2 years and was nearly 65% male. White patients had a higher proportion of admissions in the lowest-mortality hospitals, and minority race patients had higher relative proportions in the highest-mortality hospitals. Insurance status also varied significantly - the lowest performing hospitals had the highest relative proportions of government-paid admissions and the lowest proportions of privately insured patients. Differences in causes of cirrhosis were statistically significant, but absolute differences were quite small from a clinical standpoint. From a case mix perspective, the lowest performing hospitals had a greater proportion of the relatively lower risk patients (APR-DRG minor and moderate), whereas the highest performing hospitals had a greater proportion of the highest mortality risk patients (APR-DRG major and extreme). Within quintiles, the mean crude mortality rate ranged from 5.1%-12.3%.
| Q1 (highest performance) | Q2 | Q3 | Q4 | Q5 (lowest performance) | Overall | P value | |
| Patient characteristics | |||||||
| Patients | 21524 | 11117 | 12427 | 12648 | 15017 | 72733 | - |
| Demographics | |||||||
| Age | |||||||
| Mean age (yr) | 56.8 | 57.5 | 57.6 | 57.3 | 57.4 | 57.2 | < 0.00001 |
| Sex | |||||||
| Female (%) | 36.4 | 36.7 | 35.9 | 35.0 | 35.3 | 35.9 | 0.01381 |
| Race | |||||||
| White (%) | 59.9 | 55.6 | 54.8 | 51.7 | 52.6 | 55.4 | < 0.00001 |
| Black (%) | 5.8 | 7.7 | 5.6 | 5.7 | 9.0 | 6.7 | |
| Hispanic (%) | 15.2 | 20.2 | 21.5 | 17.4 | 20.3 | 18.5 | |
| Asian or Pacific Islander (%) | 1.6 | 1.8 | 2.3 | 1.3 | 2.2 | 1.8 | |
| Native American (%) | 1.6 | 0.5 | 0.8 | 1.1 | 0.9 | 1.1 | |
| Other (%) | 1.6 | 2.0 | 2.6 | 3.3 | 3.1 | 2.4 | |
| Missing (%) | 14.4 | 12.1 | 12.4 | 19.6 | 12.0 | 14.1 | |
| Insurance | |||||||
| Medicare (%) | 36.1 | 37.2 | 38.0 | 36.9 | 37.3 | 37.0 | < 0.00001 |
| Medicaid (%) | 22.9 | 24.5 | 24.8 | 23.8 | 26.5 | 24.4 | |
| Private Insurance (%) | 27.4 | 26.5 | 25.2 | 25.3 | 22.5 | 25.5 | |
| Self-Pay (%) | 7.5 | 7.4 | 7.3 | 8.4 | 7.8 | 7.7 | |
| No Charge (%) | 1.0 | 0.4 | 0.6 | 0.5 | 1.5 | 0.9 | |
| Other (%) | 5.0 | 4.0 | 4.0 | 5.0 | 4.3 | 4.6 | |
| Cause of cirrhosis1 | |||||||
| Alcoholic liver disease (%) | 61.0 | 62.5 | 61.0 | 60.8 | 60.3 | 61.1 | 0.00880 |
| Alcoholic cirrhosis (%) | 60.9 | 62.4 | 60.9 | 60.6 | 60.2 | 60.9 | 0.00589 |
| Non-alcoholic cirrhosis (%) | 38.0 | 36.9 | 38.0 | 38.6 | 39.0 | 38.1 | 0.00762 |
| Biliary cirrhosis (%) | 2.3 | 1.5 | 2.0 | 1.7 | 1.6 | 1.9 | < 0.00001 |
| HCV positive (%) | 25.3 | 23.0 | 22.1 | 25.1 | 26.4 | 24.6 | < 0.00001 |
| Unspecified liver disease or cirrhosis (%) | 2.7 | 2.0 | 2.0 | 2.0 | 1.8 | 2.2 | < 0.00001 |
| A1AT, Cu, Fe disease (%) | 0.89 | 0.65 | 0.64 | 0.74 | 0.68 | 0.74 | 0.04067 |
| Complications of Cirrhosis | |||||||
| Ascites (%) | 31.8 | 27.5 | 30.2 | 30.3 | 26.9 | 29.6 | < 0.00001 |
| Variceal bleed (%) | 9.6 | 10.3 | 9.6 | 9.3 | 9.4 | 9.6 | 0.05605 |
| Hepatic Encephalopathy (%) | 53.7 | 55.2 | 52.5 | 54.1 | 55.7 | 54.2 | < 0.00001 |
| Portal HTN (%) | 44.3 | 41.1 | 44.9 | 42.7 | 41.5 | 43.0 | < 0.00001 |
| Hepatorenal syndrome (%) | 9.3 | 7.8 | 8.2 | 8.3 | 8.9 | 8.6 | 0.00003 |
| Hepatocellular carcinoma (%) | 3.9 | 2.7 | 3.1 | 3.1 | 3.2 | 3.3 | < 0.00001 |
| Procedures | |||||||
| Liver transplant | 2.7 | 0.6 | 1.0 | 1.1 | 2.4 | 1.7 | < 0.00001 |
| Esophagogastroduodenoscopy | 18.1 | 19.0 | 20.4 | 19.3 | 18.3 | 18.9 | < 0.00001 |
| Paracentesis | 32.9 | 29.7 | 30.0 | 29.7 | 29.2 | 30.6 | < 0.00001 |
| Mortality characteristics | |||||||
| APR-DRG Risk of Mortality | |||||||
| Minor risk of mortality (%) | 5.9 | 7.8 | 6.8 | 6.5 | 9.1 | 7.1 | < 0.00001 |
| Moderate risk of mortality (%) | 31.3 | 34.1 | 34.4 | 33.2 | 34.0 | 33.2 | |
| Major risk of mortality (%) | 41.0 | 39.4 | 39.4 | 39.5 | 37.0 | 39.4 | |
| Extreme risk of mortality (%) | 21.8 | 18.7 | 19.4 | 20.7 | 19.9 | 20.3 | |
| Mortality | |||||||
| Expired during admission (%) | 5.1 | 6.0 | 7.5 | 9.8 | 12.3 | 7.9 | < 0.00001 |
| Hospital characteristic | |||||||
| Number of hospitals | 161 | 161 | 161 | 161 | 161 | 805 | - |
| Region | |||||||
| Northeast hospital region (%) | 19.3 | 25.5 | 27.3 | 24.8 | 34.2 | 26.2 | 0.06961 |
| Midwest hospital region (%) | 18.6 | 13.7 | 17.4 | 13.0 | 9.3 | 14.4 | |
| South hospital region (%) | 29.2 | 32.3 | 19.9 | 29.2 | 26.7 | 27.5 | |
| West hospital region (%) | 32.9 | 28.6 | 35.4 | 32.9 | 29.8 | 31.9 | |
| Bed capacity | |||||||
| Small bedsize (%) | 23.0 | 41.0 | 36.6 | 33.5 | 32.3 | 33.3 | 0.05554 |
| Medium bedsize (%) | 37.9 | 31.7 | 32.9 | 34.2 | 29.8 | 33.3 | |
| Large bedsize (%) | 39.1 | 27.3 | 30.4 | 32.3 | 37.9 | 33.4 | |
| Hospital ICU beds (mean) | 24.3 | 19.1 | 20.1 | 19.9 | 20.8 | 20.8 | 0.04608 |
| Patient casemix | |||||||
| Hospital Cirrhosis Annual Volume (mean) | 133.7 | 69.0 | 77.2 | 78.6 | 93.3 | 90.4 | < 0.00001 |
| Medicaid Days (mean) | 18139 | 15339 | 18158 | 18699 | 22662 | 18599 | 0.18260 |
| Admission characteristics | |||||||
| Length of stay (days) (mean) | 5.9 | 5.7 | 5.9 | 6.2 | 6.4 | 6.0 | 0.00019 |
| Total charges ($ mean)2 | 43391 | 36593 | 40500 | 43389 | 40340 | 40877 | 0.19458 |
| Expired during admission (%) | 4.0 | 5.3 | 7.3 | 10.1 | 13.2 | 8.0 | < 0.00001 |
| Staffing characteristics | |||||||
| Full-time LPN’s3 (mean) | 1.65 | 2.18 | 2.18 | 1.94 | 2.34 | 2.06 | 0.03218 |
| Full-time MD’s3 (mean) | 2.74 | 2.02 | 2.55 | 1.88 | 1.8 | 2.2 | 0.27122 |
| Teaching Status | |||||||
| Teaching hospital4 (%) | 42.9 | 32.9 | 38.5 | 39.8 | 41.6 | 39.1 | 0.40386 |
| Resident FTE’s (mean) | 79.2 | 40.7 | 57.7 | 59.0 | 65.7 | 60.5 | 0.33158 |
| Specialty Services | |||||||
| Liver transplant hospital (%) | 15.5 | 5.0 | 5.0 | 5.6 | 4.3 | 7.1 | 0.00020 |
Facility characteristics differed by hospital performance quintile in cirrhosis on univariate analysis (Table 1). Cirrhosis volume had a U-shaped distribution when distributed across mortality quintiles - the highest and lowest performing quintiles had an average of 20.8%-73.2% more cirrhosis admissions compared to the average performing hospitals (Table 1). The lowest quintile had nearly 25% greater Medicaid case-mix than the other groups. There were no significant differences in total bed capacity, but ICU bed capacity was roughly 20% larger in the highest quintile compared to the other groups. Staffing patterns were significantly different as well. Nurse staffing with LPNs was 40% greater in low performing hospitals compared to the highest quintile, however physician staffing was similar. 15.5% of the highest performing hospitals were liver transplant centers, which was three-fold greater than average or low performing hospitals. While not statistically significant, the geographic make-up of each performance quintile was also asymmetric. The highest performing group had more Western and Midwestern hospitals, and less Northeastern and Southern hospitals. This distribution was reversed in the lowest performing group - Northeastern hospitals made up more than 34% of this group.
In a multivariable risk-adjusted model evaluating predictors of cirrhosis mortality, both patient and facility factors were significant (Table 2). At the patient level, individual cirrhosis complications and co-morbidities were significant predictors of mortality - clinical presentation of hemorrhage, documented portal hypertension, hepatitis C status, alcoholic liver disease, and APR-DRG risk score were significant predictors of mortality (all, P≤ 0.005). Invasive diagnostic and therapeutic procedures including endoscopy, paracentesis, and liver transplantation were significantly protective (all, P < 0.01). There was also a significant era effect, as each successive year was independently associated with lower mortality (all, P < 0.001). At the facility level, each additional cirrhosis admission was associated with 0.05% lower odds of mortality. Liver transplant centers had 17% lower odds of mortality non-transplant facilities across all cirrhosis admissions. Medicaid payer-mix and more LPN nurse staffing were also associated with higher mortality.
| Characteristic | OR | 95%CI | P value |
| Patient covariates | |||
| Age | 1.00 | 0.97-1.01 | 0.592 |
| Female | 1.05 | 0.93-1.06 | 0.753 |
| Ascites | 1.79 | 0.96-1.15 | 0.262 |
| Variceal hemorrhage | 1.26 | 1.64-1.95 | < 0.001 |
| Hepatic encephalopathy | 0.63 | 1.18-1.34 | < 0.001 |
| Portal hypertension | 1.16 | 0.59-0.68 | < 0.001 |
| Hepatorenal syndrome | 0.20 | 1.07-1.25 | < 0.001 |
| Received liver transplant | 0.83 | 0.15-0.26 | < 0.001 |
| HCV positive | 1.43 | 1.33-1.54 | < 0.001 |
| Alcoholic liver disease | 1.23 | 1.33-1.54 | < 0.001 |
| Paracentesis during admission | 0.76 | 0.71-0.82 | 0.006 |
| EGD during admission | 0.81 | 0.75-0.88 | < 0.001 |
| Co-morbidity burden (APR DRG risk of mortality) | |||
| Minor | Reference | ||
| Moderate | 1.54 | 1.14-2.07 | 0.005 |
| Major | 6.02 | 4.55-7.96 | < 0.001 |
| Extreme | 58.8 | 44.5-77.8 | < 0.001 |
| Era (year of admission) | |||
| 2005 | Reference | ||
| 2006 | 0.89 | 0.79-1.00 | 0.053 |
| 2007 | 0.79 | 0.70-0.89 | < 0.001 |
| 2008 | 0.75 | 0.66-0.85 | < 0.001 |
| 2009 | 0.68 | 0.60-0.78 | < 0.001 |
| 2010 | 0.51 | 0.56-0.59 | < 0.001 |
| 2011 | 0.49 | 0.42-0.56 | < 0.001 |
| Hospital resources (hospital structural factors) | |||
| Hospital cirrhosis volume | 1.01 | 1.00-1.01 | 0.025 |
| Hospital medicaid days | 1.01 | 1.00-1.01 | 0.029 |
| Hospital liver transplant program | 0.83 | 0.71-0.98 | 0.026 |
| Full-time LPN’s | 1.02 | 1.00-1.04 | 0.015 |
| Full-time MD’s | 0.99 | 0.98-1.00 | 0.140 |
| Resident FTE’s | 1.00 | 1.00-1.00 | 0.115 |
| ICU beds | 1.00 | 1.00-1.00 | 0.446 |
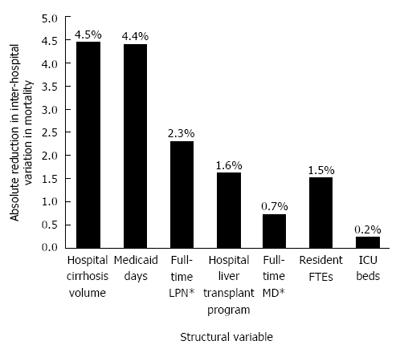
To better understand the magnitude of between-hospital variation in cirrhosis mortality further, we evaluated how individual facility characteristics cumulatively affected measured mortality (Figure 2). With the cumulative effect of all structural variables added into the model, overall, 15.3% of the total variation is related to these parameters. The remaining variation remains unmeasured.
Recent data suggest that cirrhosis mortality differs widely across hospitals in the United States. This alarming phenomenon naturally lends itself to questions about what factors within hospitals contribute to this variation. For decades, variation in health care quality has been tied, in part, to differences in structure responsible for health care delivery[7]. In this analysis, we sought to better understand to what extent hospital facility characteristics affect the outcomes of patients admitted with cirrhosis to United States hospitals. Our study demonstrates that several hospital structural domains - case mix, payer mix, staffing patterns, and the presence of specialty liver transplant service line - are predictors of inpatient cirrhosis outcomes, account for a sizable fraction (15%) of the overall variation in mortality.
Recent data have demonstrated that cirrhosis mortality for hospitalized patients has improved over time[8,9], which we also observed in our analysis. While inpatient care in cirrhosis seems to be improving over time overall, nearly 40% of patients with life-threatening cirrhotic complications were admitted to hospitals with higher than expected risk-adjusted mortality rates. Even after adjusting for this era effect, our findings add to a growing body of work that show the importance of specific hospital resources in the care of patients with liver disease, surgical diseases, and malignancy[6,9-20]. Hospitals with higher than expected risk-adjusted cirrhosis mortality have less resources than those performing better. Resource intensity remains a predictor of survival which is likely driven by the development of care processes based on the local care environment within hospitals.
The specific hospital resources associated with cirrhosis mortality were interesting and speak to a structural footprint for quality cirrhosis care. High cirrhosis volume was significant, and the volume-outcome relationship has long been established in the health services research literature[15,21,22]. In the current study, higher Medicaid payer-mix and higher utilization of LPNs (vs RNs) were responsible for a significant portion of the total variation attributable to structural differences. These metrics may be proxies for financial difficulties in resource-strapped facilities that may not have services available that complicated cirrhosis patients require. The presence of a liver transplant program was significant in reducing mortality, but establishing these programs may be a significant hurdle for hospitals and may not be warranted based on current estimates of liver disease mortality nationally. Interestingly, ICU bed capacity, teaching status, and physician staffing were important but not as relevant as the aforementioned factors. This may be related to the near ubiquity of these resources across the population of hospitals in this study. From a clinical perspective, providers understand the ideal structural footprint of a hospital managing advanced cirrhosis- multi-specialty physician staffing, quality nursing, intensive care beds, radiological and endoscopy capacity, and many other services. This notion and some of the findings of this study naturally imply that further capital investments by hospitals may improve clinical outcomes in cirrhosis.
However, boosting resource intensity everywhere is neither the entire solution nor is it financially realistic[16,17,23]. The residual variation in cirrhosis mortality across hospitals remained vast even after accounting for structural differences, implying that unmeasured sources of variation likely account for major differences in clinical performance. By exclusion, based on our conceptual model, this residual variation can be attributable to processes of care and random error. Processes of cirrhosis care were not directly evaluated in this study. Kanwal et al[16,18,19] have outlined usable process of care measures for cirrhosis, but there neither is a national impetus to adopt these, nor is there a platform to track this data on a population level. Based on our findings, it is possible that differences in adherence to quality practices could account for a significant degree of variation. However, since nearly 85% of the variation was attributable to processes of care, care innovation in average and low performing hospitals may demonstrate immediate benefits. Recent initiatives to develop cirrhosis quality improvement programs by the sharing of regional or national data between hospitals holds significant promise, particularly for hospitals with higher than expected mortality.
These findings also have implications for developing care processes beyond the local hospital environment. The hospital characteristic that had the largest effect size in favor of survival was the presence of a liver transplant program. In fact, the beneficial effect of a liver transplant program was observed in non-transplant cases within that facility. In the interest of population health management, our findings imply that liver transplant programs should partner with providers in resource-poor institutions in order to improve care. The development of robust care networks between hospitals may, for example, help expedite transfers of critically ill patients who require liver transplant evaluation, optimize care for those patients who may not be liver transplant candidates, and improve quality of care in cirrhosis in general. There are obvious incentives for creating these networks for both resource-intense and resource-poor institutions, and has been alluded to in centralizing care for other conditions including cancer[24-26].
Based on our study, resource intensity seems to be responsible for some of this variation, but the totality of differences between hospitals was not completely adjusted for in the analysis. The limitations of this study are related to the inherent nature of the data source - administrative hospital discharge data - which do not adequately capture hospital-specific processes of care in cirrhosis, which are poorly defined in general[16,18,19]. Unmeasured structural variables, unmeasured processes of care, and unmeasured clinical granularity at the patient level may have affected the estimate of mortality risk during a given cirrhosis hospitalization, and so these results have to considered in that context. The statistical approach and study design cannot be used to determine causality, and population-based conclusions can lead to incorrect inferences when applied to individual patient treatments.
Given these limitations, the contribution of resource intensity to hospital variation in cirrhosis mortality is an important consideration for patients, providers, and payers despite secular improvements in inpatient mortality in general. Quality improvement within hospitals and collaboration between centers hold the most promise to address these differences over time.
Hospitals vary significantly in cirrhosis mortality which can be attributable to multiple factors, which can be considered in the context of structural factors, processes of care, and patient differences.
Hospital structural characteristics are a marker of hospital resources and affect outcomes. The effect of hospital resource differences on cirrhosis mortality has never been studied in a population context.
The authors were able to merge the Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample data on hospital discharges with the American Hospital Association Annual Survey data on hospital structural resources to create a hierarchical logistic regression model to better understand hospital-level predictors of risk-adjusted mortality and how much hospital factors contribute to the overall variation in cirrhosis mortality between hospitals.
This analysis indicates that specific hospital resources are associated with mortality in cirrhosis, including staffing patterns and a shift in payer-mix toward public payers. However, hospital cirrhosis volume and liver transplant program status were independently associated with survival. These findings should help design cirrhosis care protocols and encourage care-networking between hospitals that have vast resource differences.
It is interesting to explore the relationship between the hospital characteristics and mortality. This article entitled “Hospital resource intensity and cirrhosis mortality in United States” by Mathur et al showed that “Hospital characteristics account for a significant proportion of variation in cirrhosis mortality”. This paper provides readers new insight of the health care and management in patients with cirrhosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Giorgio A, Kreisel W, Zheng SJ S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Nguyen GC, Segev DL, Thuluvath PJ. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin Gastroenterol Hepatol. 2007;5:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among United States hospitals in inpatient mortality for cirrhosis. Clin Gastroenterol Hepatol. 2015;13:577-584; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Iezzoni LI, Ash AS, Shwartz M, Daley J, Hughes JS, Mackiernan YD. Judging hospitals by severity-adjusted mortality rates: the influence of the severity-adjustment method. Am J Public Health. 1996;86:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. College Station, Tex: Stata Press Publication 2012; . |
| 5. | Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. Hoboken: John Wiley & Sons, Inc 2013; . [DOI] [Full Text] |
| 6. | Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44:Suppl: 166-206. [PubMed] |
| 8. | Schmidt ML, Barritt AS, Orman ES, Hayashi PH. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology. 2015;148:967-977.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Kanwal F. Decreasing mortality in patients hospitalized with cirrhosis. Gastroenterology. 2015;148:897-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 343] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 11. | Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 12. | Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Birkmeyer JD, Sun Y, Goldfaden A, Birkmeyer NJ, Stukel TA. Volume and process of care in high-risk cancer surgery. Cancer. 2006;106:2476-2481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg. 2003;238:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Eng J Med. 2002;346:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3695] [Cited by in RCA: 3773] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 16. | Kanwal F, Volk M, Singal A, Angeli P, Talwalkar J. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Kanwal F. Coordinating care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2013;11:859-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, Li J, El-Serag HB. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 19. | Kanwal F, Kramer J, Asch SM, El-Serag H, Spiegel BM, Edmundowicz S, Sanyal AJ, Dominitz JA, McQuaid KR, Martin P. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Mathur A, Luberice K, Ross S, Choung E, Rosemurgy A. Pancreaticoduodenectomy at High-volume Centers: Surgeon Volume Goes Beyond the Leapfrog Criteria. Ann Surg. 2015;262:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Finlayson EV, Birkmeyer JD. Effects of hospital volume on life expectancy after selected cancer operations in older adults: a decision analysis. J Am Coll Surg. 2003;196:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 344] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol. 2013;11:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 24. | Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27:4671-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 25. | DeMeester S. Centralization of esophageal cancer surgery: the right thing to do is seldom easy. Ann Surg Oncol. 2009;16:1760-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Greenberg CC, Ashley SW, Schrag D. Centralization of cancer surgery: what does it mean for surgical training? J Clin Oncol. 2009;27:4637-4639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |