Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1843
Peer-review started: October 11, 2016
First decision: November 21, 2016
Revised: December 18, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: March 14, 2017
Processing time: 155 Days and 20.9 Hours
To compare the efficacy and safety of a hook knife (HO) with a hybrid knife (HK) during endoscopic submucosal tunnel dissection (ESTD) procedure.
Between August 2012 and December 2015, the ESTD procedure was performed for 83 upper GI submucosal lesions, which originated from the muscularis propria layer identified by upper endoscopy and endoscopic ultrasonography. Of these, 34 lesions were treated by a HO, whereas 49 lesions were treated by a HK. Data regarding age, gender, presenting symptoms, tumor location and size, procedure time, complications, en bloc resection rate and others were analyzed and compared between the two groups.
There were no significant differences in the age, gender, presenting symptoms and tumor location between the two groups. ESTD was successfully completed in all the patients, and no case was converted to laparoscopy. The mean procedure time was significantly shorter in the HK group than in the HO group (41.3 ± 20.3 min vs 57.2 ± 28.0 min, P = 0.004). The mean frequency of device exchange was 1.4 ± 0.6 in the HK group and significantly less than 3.3 ± 0.6 in the HO group (P < 0.001). The differences in tumor size and histopathological diagnoses were not significant between the two groups (P = 0.813, P = 0.363, respectively). Both groups had an equal en bloc resection rate and complete resection rate. Additionally, the complication rate was similar between the two groups (P = 0.901). During the follow-up, no recurrence occurred in either group.
We demonstrate for the first time that HO and HK do not differ in efficacy or safety, but HK reduces the frequency of device exchange and procedure time.
Core tip: Settings for the endoscopic submucosal tunnel dissection (ESTD) procedure have not been standardized, and no studies have directly compared ESTD devices in humans. We compared the performance between hook knife (HO) and hybrid knife (HK) in the ESTD procedure for upper gastrointestinal submucosal tumors. Our study demonstrated for the first time that HO and HK do not differ in terms of efficacy or complication rates during ESTD procedure, but HK can significantly reduce the frequency of device exchange and procedure time.
- Citation: Zhou JQ, Tang XW, Ren YT, Wei ZJ, Huang SL, Gao QP, Zhang XF, Yang JF, Gong W, Jiang B. Endoscopic submucosal tunnel dissection of upper gastrointestinal submucosal tumors: A comparative study of hook knife vs hybrid knife. World J Gastroenterol 2017; 23(10): 1843-1850
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1843.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1843
Although most submucosal tumors (SMTs) are benign, some of them have malignant potential. Resection of SMTs would be helpful in diagnosis and may be curative[1-3]. To date, several therapies have been described to resect SMTs, including open and laparoscopic surgery[4], endoscopic submucosal dissection (ESD)[5-7], endoscopic full-thickness resection (EFR), etc.[8,9]. Surgical approaches are more invasive with a longer hospital stay of patients and have greater cost compared with endoscopic methods. However, ESD and EFR also have their limitations, and they can be associated with major complications such as perforation, which may be difficult to close and even require surgery.
More recently, endoscopic submucosal tunnel dissection (ESTD) has been a novel treatment for upper gastrointestinal (GI) SMTs originating from the muscularis propria (MP). Since Inoue et al[10] first reported the usefulness of ESTD for SMTs in humans, a number of authors have reported its efficacy and safety. According to a review by Kobara et al[11], the complete resection rate of ESTD for upper GI SMTs ranged from 85.7% to 100%, and the complication rate was 0%-16.7%. With regard to the endoscopic devices used in ESTD procedure, there has been wide variability among different centers. A hook knife (HO), a hybrid knife (HK) and an insulated-tip knife were reported in different studies[12-14]. For the two endoscopic devices: HO and HK, no study has assessed and compared their clinical outcomes in the ESTD procedure. Hence, this study was conducted to evaluate the clinical efficacy and safety of HK compared with HO for ESTD.
Between August 2012 and December 2015, 83 consecutive patients with upper GI SMTs were treated with ESTD at the Department of Gastroenterology, Nanfang Hospital, Southern Medical University, China. The data were prospectively collected and retrospectively reviewed. All the procedures were performed by one experienced endoscopist (Gong W). After the operator performed 15 cases (10 cases were performed by a HO and 5 cases by a HK), the procedure time became stable and the complication rate was low. Therefore, the first 15 cases were not included in our analysis to eliminate the effect of the learning curve. Patients were divided into the following two groups based on the type of endoscopic devices used in ESTD: the hook knife (HO group, n = 34) and the hybrid knife (HK group, n = 49). Indications for the ESTD procedure were SMTs originating from the MP layer identified by upper endoscopy and endoscopic ultrasonography (EUS), lesions smaller than 4 cm, and patients who can tolerate general anesthesia with tracheal intubation. Data regarding age, gender, presenting symptoms, tumor location and size, procedure time, complications, en bloc resection rate and others were analyzed and compared between the two groups. Written informed consent was obtained from all patients before the procedure.
A forward-viewing endoscope (GIFQ240Z; Olympus, Tokyo, Japan) was used with a transparent distal cap attachment (MH-588; Olympus). A hook knife (KD-620L; Olympus) and an injection needle (NM-4L-1, Olympus), or a hybrid knife (ERBE, Tübingen, Germany) were used to dissect the submucosal layer and the tumors. A pair of coagulating forceps (Coagrasper, FD-410L; Olympus) was used to coagulate large vessels prior to dissection and for hemostasis. A carbon dioxide (CO2) insufflator (UCR; Olympus) was used. For electrosurgery, a VIO 200 D electrogenerator (ERBE) was used, and for final closure of the mucosal entry site, hemostatic clips (EZ-CLIP, HX-110QR, Olympus; or Resolution, M00522610; Boston Scientific, Boston, United States) were applied. All the ESTD procedures were performed by a single operator (Gong W).
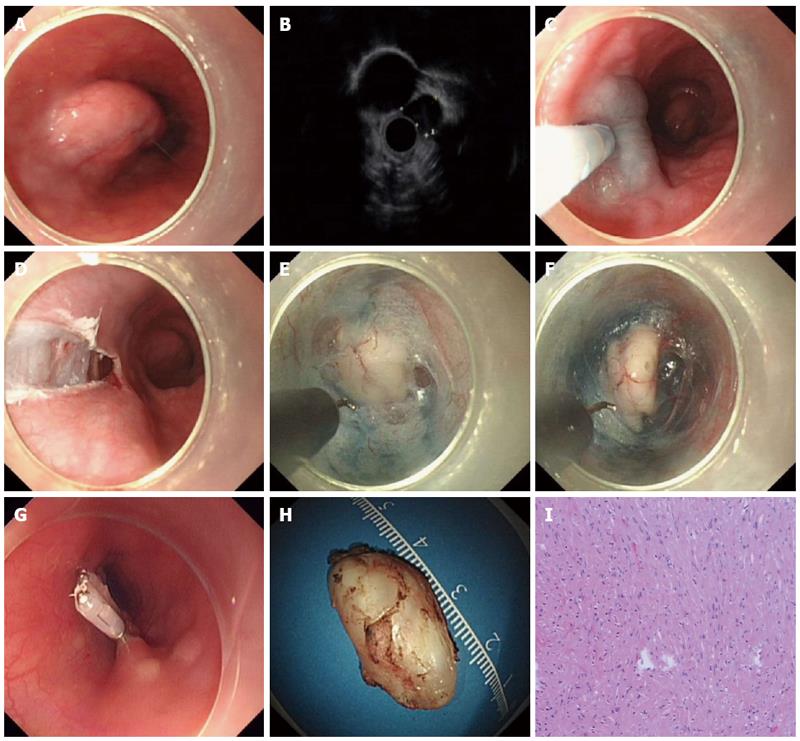
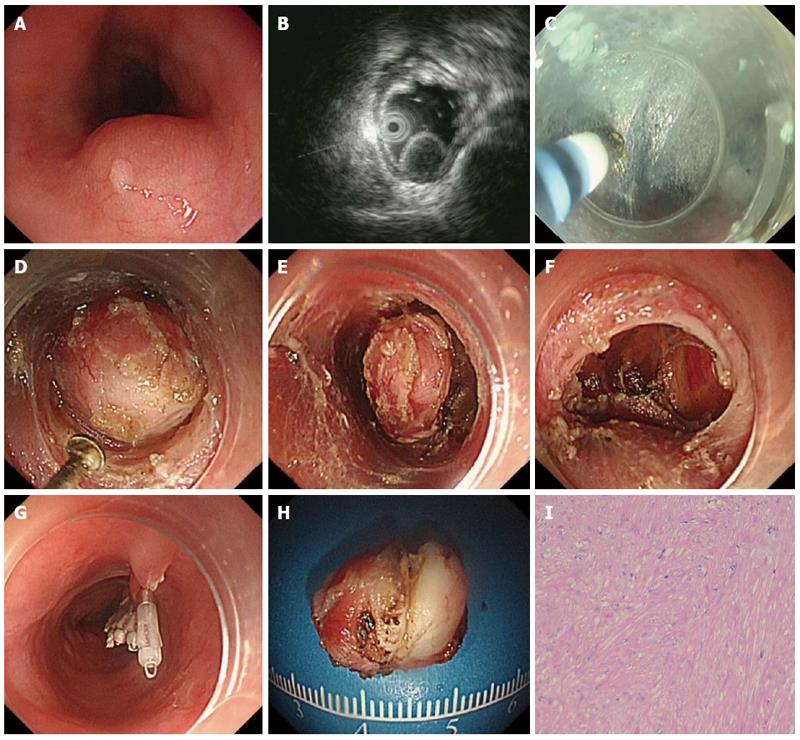
The patients underwent the procedures under general anesthesia with endotracheal intubation. CO2 was used during the procedure. The standard steps of the procedure were described previously (Figures 1 and 2)[15].
The hook knife was developed by Oyama et al[16], and the tip of the knife is bent at a right angle for marking and cutting mucosa as well as the submucosal fibers. The length of the hook is 1.3 mm and the length of the arm is 4.5 mm. The knife is hosted within an outer sheath.
The hybrid knife has combined capabilities of radiofrequency application and needle-less waterjet injection in a single instrument[17,18]. The outer diameter is 2.1 mm and the lumen is 120 μm. There are two types of hybrid knife: I-type and T-type. In our study, only the T-type was used.
Resected specimens were retrieved and immediately fixed in a 10% buffered formalin solution. Hematoxylin and eosin and immunohistochemical staining (CD34, CD117, actin, S-100, desmin, vimentin, and Ki67) were carried out.
All patients were scheduled for a follow-up visit at 3 and 6 months and 1 year after ESTD for upper endoscopy and EUS. If there is no residual tumor, follow-up is performed annually.
The procedure time was defined as from the moment of submucosal injection to closure of mucosal entry after removal of the lesion. En bloc resection was defined as resection of the target tumor in one piece. Complete resection was defined as follows: the capsule of the tumor was intact and the basal and lateral margins were free of tumor cells. Complications were defined as bleeding, perforation or subcutaneous emphysema. Bleeding was defined as an oozing or spurting bleeding observed and requiring the use of coagulating forceps or endoclips. Perforation was defined as having occurred when a hole was easily recognizable by endoscopy during ESTD, or when free air was detected on a plain radiograph taken after ESTD.
Continuous variables are presented as the mean ± SD. Categorical variables were expressed as numbers and percentages. Statistical differences were analyzed with Student’s t-test, χ2 test or Fisher’s exact test. Values of P < 0.05 were considered statistically significant. Data were analyzed using commercially available statistical software packages SPSS version 19.0 (SPSS Inc., Chicago, IL, United States).
Eighty-three patients with a total of 83 lesions were enrolled in this study. A summary of patient baseline characteristics is shown in Table 1. There were no significant differences in age, gender, presenting symptoms and tumor location between the two groups.
| HO group (n = 34) | HK group (n = 49) | P value | |
| Gender, female | 11 (32.4) | 22 (44.9) | 0.251 |
| Age, years (range) | 50.1 ± 9.4 (27-66) | 46.9 ± 12.9 (19-68) | 0.195 |
| Presenting symptoms | |||
| Asymptomatic | 11 (32.4) | 19 (38.8) | |
| Epigastric discomfort | 10 (29.4) | 14 (28.6) | |
| Chest discomfort | 8 (23.5) | 11 (22.4) | |
| Dysphagia | 3 (8.8) | 3 (6.1) | |
| Regurgitation | 2 (5.9) | 2 (4.1) | 0.965 |
| Tumor location | |||
| Esophagus | 23 (67.6) | 34 (69.4) | |
| Cardia | 7 (20.6) | 10 (20.4) | |
| Stomach | 4 (11.8) | 5 (10.2) | 0.973 |
ESTD was successfully completed in all the patients and no case was converted to laparoscopy. The clinicopathological details of patients and lesions are shown in Table 2. The mean size of the resected specimens was 19.7 ± 7.2 mm in the HO group and 19.3 ± 7.5 mm in the HK group. This difference was not significant (P = 0.813). The number of tumors larger than 20 mm was 8 (23.5%) in HO group and 13 (26.5%) in HK group (P = 0.757). With regard to histopathological results, there were 27 (79.4%) leiomyomas, 6 (17.6%) GISTs and 1 (2.9%) lipoma in the HO group, and there were 42 (85.7%) leiomyomas and 7 (14.3%) GISTs in the HK group. The proportion of tumor pathology was not significantly different between the two groups (P = 0.363).
| HO group (n = 34) | HK group (n = 49) | P value | |
| Procedure time, min (range) | 57.2 ± 28.0(30-150) | 41.3 ± 20.3 (15-120) | 0.004b |
| Frequency of device exchange, n (range) | 3.3 ± 0.6 (2-5) | 1.4 ± 0.6 (1-3) | 0.000b |
| Frequency of coagulation forceps use, n (range) | 0.2 ± 0.4 (0-1) | 0.2 ± 0.4 (0-1) | 0.625 |
| Tumor size, mm (range) | 19.7 ± 7.2 (10-40) | 19.3 ± 7.5 (8-40) | 0.813 |
| No. of tumors based on size (mm) | |||
| ≤ 20 | 26 (76.5) | 36 (73.5) | |
| > 20 | 8 (23.5) | 13 (26.5) | 0.757 |
| Histopathological diagnosis | |||
| Leiomyoma | 27 (79.4) | 42 (85.7) | |
| Gastrointestinal stromal tumor | 6 (17.6) | 7 (14.3) | |
| Lipoma | 1 (2.9) | 0 (0) | 0.363 |
| En bloc resection | 32 (94.1) | 49 (100) | 0.165 |
| Complete resection | 32 (94.1) | 49 (100) | 0.165 |
| Complications | |||
| Perforation | 2 (5.9) | 2 (4.1) | |
| Bleeding | 1 (2.9) | 0 (0) | |
| Subcutaneous emphysema | 1 (2.9) | 2 (4.1) | 0.568 |
| Hospital stay, days (range) | 5.6 ± 1.4 (3-10) | 5.8 ± 1.5 (3-10) | 0.501 |
| Mean follow-up time, months (range) | 27.2 ± 6.4 (18.9-41.4) | 25.5 ± 4.0 (20.6-39.7) | 0.175 |
| Recurrence rate, % | 0 | 0 | 1.000 |
The en bloc resection and complete resection were achieved in 32 out of 34 lesions (94.1%) in the HO group and in all 49 lesions (100%) in the HK group, which were similar in the two groups (P = 0.165). The mean duration of the hospital stay was 5.6 ± 1.4 d in the HO group and 5.8 ± 1.5 d in the HK group (P = 0.501). During the follow-up time, no recurrence occurred in either group.
The total procedure time in the HK group was significantly shorter than in the HO group (41.3 ± 20.3 min vs 57.2 ± 28.0 min, P = 0.004). The mean frequency of device exchange was 1.4 ± 0.6 in the HK group and 3.3 ± 0.6 in the HO group (P < 0.001), and the mean frequency of coagulation forceps use was similar between the two groups (P = 0.625).
Four perforations (two in the HO group and two in the HK group) occurred during ESTD and were closed by endoclips. Intraoperative bleeding occurred in 1 patient of the HO group and was successfully controlled by coagulating forceps. No other complications were recorded during the procedures. Three patients (one in the HO group and two in the HK group) developed subcutaneous emphysema after the procedure, all of which resolved spontaneously after the ESTD.
ESTD has been an emerging novel therapeutic option for upper GI SMTs originating from the MP layer. It has several advantages over other treatment modalities, such as ESD and EFR. First, through the submucosal tunnel, SMTs can be dissected and resected under direct endoscopic vision, and the bleeding spot can be detected immediately and managed successfully by hemostasis. Second, the ESTD procedure maintains the mucosa layer above the SMTs intact, thereby maintaining the integrity of the GI tract mucosa. Finally, the entry to the submucosal tunnel can be easily closed with several clips.
For the equipment used in the ESTD procedure, a variety of endoscopic knives were applied in different studies. Li et al[12] preferred to use a hook knife or hybrid knife during the procedure, Liu et al[13] and Wang et al[14] chose to use an insulated-tip knife or a hybrid knife. In our center, a hook knife or hybrid knife was mostly used in ESTD. Different endoscopic devices have various properties and advantages. Therefore, we conducted this study to compare clinical outcomes for upper GI ESTD with the use of HO or HK. Here, we demonstrated for the first time that HK had the advantage of a shorter operation time compared with HO, and the frequency of device exchange in the HK group was less than in the HO group. The complication rate, en bloc resection and complete resection rate were almost the same between the two groups. During the follow-up time, no recurrence occurred in the two groups.
Primarily used in the EMR procedure, HO allows for marking and cutting mucosa, submucosal fibers, and vessels, as well as for hemostasis of minor bleeding[16]. Because the direction of the hook knife can be controlled and held parallel with the muscularis propria layer, it has the advantage of preventing perforation during ESD[16]. However, it is notable that HO does not enable injection of dyed saline to stain submucosal fibers, and it requires a frequent exchange of devices, resulting in prolonged procedural time. Recently, HK was developed to simplify the endoscopic procedure. It is multifunctional with the abilities of submucosal injection, circumferential cutting, dissection of lesions, and coagulation of bleeding[18]. Therefore, the procedure can be performed by the same HK without the need of device exchange.
A few studies have demonstrated that HK can reduce the frequency of device exchange and the procedure time[19,20]. Zhou et al[19] compared the procedure time, efficacy, and safety of HK-assisted ESD with the conventional technique in patients with early gastric cancer (EGC) in a randomized controlled trial. They revealed that HK-assisted ESD was as effective and safe as conventional devices (the IT-2 knife, Dual Knife and Hook Knife)-assisted ESD for treating EGC, but the HK group was associated with a shorter procedure time and a lower frequency of device exchange compared with the conventional group[19]. Furthermore, Cai et al[20] also demonstrated that HK achieved shorter procedure time, fewer accessory exchanges, less frequent use of coagulation forceps to control bleeding, and a lower bleeding rate in comparison with a triangle knife. Our study found that HK shortened the operation time and required fewer device exchanges in the ESTD procedure, which was comparable to these ESD studies above.
ESTD consisted of 4 standard steps: mucosal incision, creation of the submucosal tunnel, dissection of the lesion and closure of mucosal entry. The step of submucosal tunnel creation was a major and integral part of the whole procedure, which is very time-consuming. In addition to HO, HK, or other endoknives, some novel devices or techniques were also described to facilitate submucosal tunnel creation. Sumiyama et al[21] reported using high-pressure carbon dioxide injection and balloon dissection to create a submucosal tunnel, and this was also called the mucosal flap safety valve (SEMF) technique. Khashab et al[22] used a novel gel with dissecting properties for facilitating submucosal tunneling during peroral endoscopic myotomy (POEM). The preliminary animal study demonstrated that the gel consistently resulted in efficient auto-tunneling without any complications. The authors suggested that this novel material may replace other endoknives to create submucosal tunnels and has the potential to revolutionize the submucosal tunnel technique and ESD. Moreover, Khashab et al[23] also showed that direct jet injection of dyed saline through the dedicated channel of the gastroscope could result in adequate, consistent and reliable staining of submucosal fibers and made submucosal dissection accurate and safe. However, the prerequisite of this method is availability of a gastroscope with a dedicated water jet channel, which may limit its further widespread use. Some chemical agent was also applied to facilitate submucosal tunnel creation. Kawahara et al[24] recently demonstrated that submucosal injections of mesna could soften tissues and facilitate POEM. In the porcine study, submucosal tunneling time was significantly shorter in the mesna group than in the saline group, regardless of the dissection method.
We acknowledge that there are some limitations in the current study. First, it is not a prospectively randomized controlled design. Therefore, the clinical data is not sufficient. The time required for submucosal tunnel creation and tumor dissection during the whole procedure is lacking, so we cannot evaluate in which step of ESTD the HK can reduce operation time. Second, our study only involved a single operator and institution. Finally, the follow-up time was not long enough, hence we cannot comment on long-term efficacy.
In conclusion, our results indicated that a hook knife and a hybrid knife do not differ in terms of efficacy or complication rates, but the hybrid knife can reduce the frequency of device exchange and procedure time. Further randomized controlled trials with large-volume cases are warranted to evaluate the efficacy and safety of these two devices.
We thanked the statistician Xiao-Fei Zhang from Beijing Tsinghua Changgung Hospital for the review and suggestion about our statistics analysis.
There have been several treatments available for upper gastrointestinal (GI) submucosal tumors (SMTs), which included open and laparoscopic surgery, endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFR) and so on. More recently, endoscopic submucosal tunnel resection (ESTD) has been emerging as a novel treatment for upper GI SMTs originating from the muscularis propria (MP). And a number of authors demonstrated the safety and efficacy of ESTD for upper gastrointestinal SMTs. However, settings for ESTD procedure have not been standardized, and no studies have directly compared different ESTD devices in humans.
ESTD has been an emerging novel therapeutic option for upper GI SMTs originating from the MP layer. It has several advantages over other treatment modalities (ESD and EFR), such as the completed resection, immediate hemostasis and easily closed entry. With regard to the endoscopic devices used in ESTD procedure, there has been wide variability among different centers. Hook knife (HO), hybrid knife (HK) and insulated-tip knife were reported in different studies.
Given settings for ESTD procedure have not been standardized, the authors of this study for the first time compared the application of the HO and the HK during the ESTD procedure. This study demonstrated that HO and HK do not differ in terms of the efficacy or complication rates, but HK can reduce frequency of device exchange and procedure time.
This study indicated the equal safety and efficacy of HO and HK in the ESTD procedure for upper GI SMTs, and also highlighted the advantage of HK with regard to device exchange times and procedure time. This finding may provide evidences about the setting choice to the endoscopist.
ESTD is an emerging endoscopic therapeutic modality. The standard ESTD procedure for GI SMTs includes four major steps: mucosal incision, creation of the submucosal tunnel, dissection of the lesion and closure of mucosal entry. ESTD procedure for GI SMTs has the advantages of high resection rate and the low complication rate.
The manuscript is of interest for a broad readership and the feasibility of ESTD procedures in order to remove upper GI submucosal lesions is highlighted clearly.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Reinehr R S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Wang CH
| 1. | Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33-54, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Ponsaing LG, Kiss K, Hansen MB. Classification of submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3311-3315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | von Rahden BH, Stein HJ, Feussner H, Siewert JR. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. Surg Endosc. 2004;18:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Park YS, Park SW, Kim TI, Song SY, Choi EH, Chung JB, Kang JK. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc. 2004;59:409-415. [PubMed] |
| 8. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Sun S, Jin Y, Chang G, Wang C, Li X, Wang Z. Endoscopic band ligation without electrosurgery: a new technique for excision of small upper-GI leiomyoma. Gastrointest Endosc. 2004;60:218-222. [PubMed] |
| 10. | Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, Maselli R, Kudo S. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 11. | Kobara H, Mori H, Rafiq K, Fujihara S, Nishiyama N, Ayaki M, Yachida T, Matsunaga T, Tani J, Miyoshi H. Submucosal tunneling techniques: current perspectives. Clin Exp Gastroenterol. 2014;7:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, Zhong YS, Yao LQ, Xu MD. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc. 2015;29:3640-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Wang H, Tan Y, Zhou Y, Wang Y, Li C, Zhou J, Duan T, Zhang J, Liu D. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol. 2015;27:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [PubMed] |
| 17. | Yahagi N, Neuhaus H, Schumacher B, Neugebauer A, Kaehler GF, Schenk M, Fischer K, Fujishiro M, Enderle MD. Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy. 2009;41:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Neuhaus H, Wirths K, Schenk M, Enderle MD, Schumacher B. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Zhou PH, Schumacher B, Yao LQ, Xu MD, Nordmann T, Cai MY, Charton JP, Vieth M, Neuhaus H. Conventional vs. waterjet-assisted endoscopic submucosal dissection in early gastric cancer: a randomized controlled trial. Endoscopy. 2014;46:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Cai MY, Zhou PH, Yao LQ, Xu MD, Zhong YS, Li QL, Chen WF, Hu JW, Cui Z, Zhu BQ. Peroral endoscopic myotomy for idiopathic achalasia: randomized comparison of water-jet assisted versus conventional dissection technique. Surg Endosc. 2014;28:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Sumiyama K, Tajiri H, Gostout CJ. Submucosal endoscopy with mucosal flap safety valve (SEMF) technique: a safe access method into the peritoneal cavity and mediastinum. Minim Invasive Ther Allied Technol. 2008;17:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Khashab MA, Sharaiha RZ, Saxena P, Law JK, Singh VK, Lennon AM, Shin EJ, Canto MI, Aguila G, Okolo PI. Novel technique of auto-tunneling during peroral endoscopic myotomy (with video). Gastrointest Endosc. 2013;77:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Khashab MA, Messallam AA, Saxena P, Kumbhari V, Ricourt E, Aguila G, Roland BC, Stein E, Nandwani M, Inoue H. Jet injection of dyed saline facilitates efficient peroral endoscopic myotomy. Endoscopy. 2014;46:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kawahara Y, Sumiyama K, Tajiri H. Chemically assisted peroral endoscopic myotomy with submucosal mesna injection in a porcine model. Minim Invasive Ther Allied Technol. 2015;24:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |