Published online Jan 7, 2017. doi: 10.3748/wjg.v23.i1.48
Peer-review started: August 16, 2016
First decision: September 20, 2016
Revised: October 3, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: January 7, 2017
Processing time: 142 Days and 18.8 Hours
To develop a novel Helicobacter pylori (H. pylori) CagA antibody enzyme-linked immunosorbent assay (ELISA) suitable for detecting serum anti-CagA antibodies with high sensitivity.
Recombinant East Asian-type CagA protein was purified and immobilized for ELISA. Serum samples from 217 Vietnamese individuals (110 H. pylori-infected and 107 uninfected individuals) were applied. Conventional ELISA from Western-type CagA and our East Asian-type CagA ELISA were evaluated by comparing 38 subjects with the Western-type genotype and 72 subjects with the East Asian-type cagA genotype. Histological scores of the gastric mucosa were determined using the updated Sydney System to examine the relationship with anti-CagA antibody titers.
Recombinant 70-100 kDa fragments were immobilized on the ELISA plate. In ROC analysis, the area under the curve of our East Asian-type CagA ELISA was comparable to that of conventional CagA ELISA. The sensitivity of the two ELISAs differed depending on the cagA genotype. The sensitivity of East Asian-type CagA ELISA was higher for subjects infected with East Asian-type cagA H. pylori (P < 0.001), and the sensitivity of the conventional CagA ELISA tended to be higher for subjects infected with Western cagA H. pylori (P = 0.056). The titer of anti-CagA antibody tended to correlate with monocyte infiltration scores (r = 0.25, P = 0.058) and was inversely correlated with H. pylori density (r = -0.26, P = 0.043).
The novel ELISA is useful to detect anti-CagA antibodies in East Asian countries, and the titer may be a marker for predicting chronic gastritis.
Core tip: We developed a novel East Asian-type CagA enzyme-linked immunosorbent assay (ELISA) to determine whether this method could detect CagA seropositivity with greater sensitivity in East Asian countries than the conventional anti-CagA antibody ELISA, which utilizes Western-type CagA as the antigen. Our findings revealed that conventional CagA ELISA underestimated CagA seropositivity in East Asian countries and the novel CagA ELISA could detect anti-CagA antibodies with higher sensitivity. In addition, the anti-CagA antibody titer tended to correlate with chronic inflammation in the stomach. Therefore, the titer of East Asian CagA ELISA may be a useful marker for predicting chronic inflammation in the gastric mucosa.
- Citation: Matsuo Y, Kido Y, Akada J, Shiota S, Binh TT, Trang TTH, Dung HDQ, Tung PH, Tri TD, Thuan NPM, Tam LQ, Nam BC, Khien VV, Yamaoka Y. Novel CagA ELISA exhibits enhanced sensitivity of Helicobacter pylori CagA antibody. World J Gastroenterol 2017; 23(1): 48-59
- URL: https://www.wjgnet.com/1007-9327/full/v23/i1/48.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i1.48
Helicobacter pylori (H. pylori) is a gram-negative microaerophilic bacterium, which is etiologically associated with various diseases, such as gastritis, peptic ulcer, mucosa associated lymphoid tissue (MALT) lymphoma, and gastric cancer. Although over half of the world’s population is infected with H. pylori, the incidence of H. pylori-associated diseases varies geographically. These geographic differences in the incidence of gastric cancer can be explained, at least in part, by the presence of different types of H. pylori virulence factors, in particular cytotoxin-associated gene A (CagA), vacuolating cytotoxin A (VacA), and outer inflammatory protein A (OipA)[1].
CagA, the major virulence factor, is delivered into gastric epithelial cells via the type IV secretion system of H. pylori[2]. Structural variants of CagA have been shown to alter bacterial virulence. The C-terminus of CagA possesses a variable number of tyrosine phosphorylation sites, which are located within the Glu-Pro-Ile-Tyr-Ala (EPIYA) motif[3]. EPIYA segments can be classified into four types according to the amino acid sequence surrounding the EPIYA motif. H. pylori found in Western countries possess Western-type CagA, which contains EPIYA-A, EPIYA-B, and EPIYA-C segments. In contrast, H. pylori in East Asian countries possess East Asian-type CagA, which contains EPIYA-A, EPIYA-B, and EPIYA-D segments[4,5]. These EPIYA motifs can exhibit varying numbers and configurations in the C-terminal end of CagA variants[6]. The EPIYA-D segment has been reported to bind more strongly to the proto-oncogenic SH2-domain-containing tyrosine phosphatase (SHP2) than the EPIYA-C segment, leading to hyper-stimulation of Ras-Erk signaling[7,8]. Therefore, the East Asian-type CagA is associated with greater virulence than the Western-type CagA owing to the structural variance of CagA.
CagA is also a highly antigenic protein[9,10]. Comprehensive epidemiological studies have reported on the relationship between CagA seropositivity and clinical outcomes in Western and East Asian countries[11-17]; however, the results are controversial. Huang et al[18] used meta-analysis to analyze the relationship between CagA seropositivity and gastric cancer and concluded that infection with cagA-positive H. pylori further increased the risk of gastric cancer over that associated with cagA-negative H. pylori infection. Our previous meta-analysis also showed that CagA seropositivity was significantly associated with gastric cancer in East Asian countries[19]. However, the positive rate of CagA antibodies among H. pylori-infected Japanese individuals was relatively low (53.7% to 81.1%) although the majority of H. pylori strains in Japan possess an East Asian-type cagA gene[20,21]; the prevalence of cagA positive H. pylori was 95.0% to 95.5% in Vietnam[22,23] and 86.4% to 96.3% in Japan[24,25].
Therefore, we hypothesized that the commercially available CagA antibody enzyme-linked immunosorbent assay (ELISA), which uses Western-type CagA as the antigen, might underestimate serum CagA antibody levels in East Asian countries. In the present study we developed an East Asian-type CagA ELISA, which immobilizes East Asian-type recombinant CagA, and assessed the characteristics of two types of CagA based ELISA systems.
To examine differences in the performance of both types of CagA ELISA, we chose to use serum samples from Vietnamese individuals because cagA genotype prevalence is region-dependent in Vietnam. The predominant cagA genotype in the central region (Daklak province) is the Western-type cagA and in the northern region (Lao Cai province) is the East Asian-type cagA. Our results indicate that the accuracy of the two types of CagA ELISA is comparable for these Vietnamese samples. In addition, we examined the relationship between CagA antibody titer and the degree of inflammation in each individual.
The endoscopic survey was conducted in nine rural areas in the Daklak and Lao Cai provinces, Vietnam, from July 2012 and April 2013. We travelled to these areas twice and spent several days each visit recruiting the volunteers. Ethical approval was obtained from the Ethics Committees of Daklak Hospital and Lao Cai Hospital, Vietnam and the Oita University Faculty of Medicine, Japan. Written informed consent was obtained from all participants prior to the study.
Four biopsy specimens (three from the antrum and one from the upper posterior wall of the corpus) were obtained during endoscopy. The antrum specimens were used for the rapid urease test, H. pylori culturing, and histological examination. The corpus specimen was used for histological examination. Blood samples were collected from all participants immediately following endoscopy.
The rapid urease test, culturing test, histological tests confirmed by immunohistochemistry (IHC), and serum H. pylori antibody test were used to maximize the accuracy of the H. pylori infection diagnosis.
H. pylori was isolated using a standard culturing method[25]. The H. pylori total antibody titer in serum samples was measured by E-plate (Eiken Co. Ltd, Tokyo, Japan). CagA antibody titer in sera was measured using the CagA ELISA kit (Genesis Diagnostics Ltd, Ely, United Kingdom), which represented Western CagA ELISA in this study. Stomach biopsy specimens were also provided for histological testing as previously described[26].
In this study, H. pylori-infected status was defined as positive by H. pylori culturing. While, H. pylori-uninfected status was defined as all negative by H. pylori culturing, rapid urease test, serum H. pylori antibody, serum CagA antibody, and histopathological examination results.
Genomic DNA was extracted from cultured H. pylori using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany). The cagA genotypes (Western-type or East Asian-type) were determined by PCR based direct sequencing of the C-terminal region containing the EPIYA segments, as previously described[24]. The cagA genotype was determined by evaluating the amino acid sequences of the EPIYA segments using MEGA6 software[27].
The full length cagA gene was PCR amplified from the genomic DNA of a clinical H. pylori strain isolated from a Japanese volunteer with gastritis (Supplementary material). The 5′ terminus of the amplified fragment contained a SmaI recognition site and the 3′ terminus contained an Xho I recognition site. The 3.5-kb amplified cagA product was cloned into the SmaI and Xho I digested pGEX-6P-1 vector (GE Healthcare, Little Chalfont, United Kingdom) using T4 DNA ligase (TAKARA Inc) and the resulting plasmid, pGEX-CagA, was propagated in Escherichia coli DH5α competent cells (Merck Millipore, Darmstadt, Germany). pGEX-CagA was then transformed into the E. coli Rosetta Blue DE3 pLysS expression strain (Merck Millipore). These cells were then grown to an OD600 of 0.7 in Luria Bertani (LB) broth supplemented with ampicillin (100 μg/mL), chloramphenicol (40 μg/mL), and 0.2% (w/v) glucose at 37 °C. Expression of the glutathione sulfate-transferase (GST)-fused recombinant CagA (rCagA) protein was induced by the addition of 0.4 mM (final concentration) of isopropyl β-D-1-thiogalactopyranoside (IPTG) for 2 h at 30 °C; the cells were then harvested by centrifugation at 8000 ×g, 4 °C for 10 min.
The harvested E. coli cells were suspended in a sonication buffer. The E. coli lysate was sonicated and then centrifuge to remove the unlysed cells. The clarified supernatant was collected and the N-terminally tagged GST-rCagA was purified by GST-tag affinity chromatography, which utilizes the binding ability of GST to glutathione sepharose 4B resin (GE Healthcare). The resin was washed and the rCagA was eluted following the use of PreScission protease (GE Healthcare). The rCagA preparation buffer was exchanged to a phosphate buffer (pH 7.4) by ammonium sulfate precipitation. Each fraction was electrophoresed on sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and stained with Colloidal CBB stain (Bio-Rad). rCagA expression was confirmed by western blotting using anti-CagA Rabbit IgG (Austral Líneas Aéreas, Buenos Aires, Argentina) as the primary antibody and anti-Rabbit IgG conjugated alkaline phosphatase (Jackson Immuno Research Labs, West Grove PA, United States) as the secondary antibody.
East Asian-type rCagA (0.1 μg/well) in phosphate buffer was immobilized on Maxi-sorp 96-well plates (Thermo Fisher Scientific, Massachusetts, United States). Human serum samples were reacted with the rCagA immobilized plate. Anti-CagA rabbit IgG (1 mg/mL; Austral Líneas Aéreas) was reacted concurrently to obtain a standard curve for calculating the amount of human IgG. The plate was washed and then anti-human IgG conjugated horse radish peroxidase (anti-human IgG-HRP; Jackson Immuno Research Labs) and anti-rabbit IgG conjugated horse radish peroxidase (anti-rabbit IgG-HRP; Jackson Immuno Research Labs) were added to the plate. After washing the plate, ELISA peroxidase substrate 3,3’,5,5’-tetramethylbenzidine (TMB; NACALAI TESQUE, Kyoto, Japan) was used for coupling and then the absorbance at 450 nm was measured. The amount of CagA antibody was calculated from the standard curve using anti-CagA rabbit IgG/anti rabbit IgG-HRP. The detailed protocol is described in supplementary material.
Biopsy specimens were stained with hematoxylin and eosin. The grade of neutrophil infiltration, mononuclear cell infiltration, atrophy, and intestinal metaplasia were scored in each specimen based on the updated Sydney System (0, none; 1, mild; 2, moderate; and 3, severe)[28].
All statistical analyses was performed using EZR software[29]. Receiver Operating Characteristic (ROC) analysis was used to define the cut off value for a novel CagA antibody ELISA. Discrete variables were tested using the χ2 and McNemar’s test. Spearman rank coefficients (r) were determined to evaluate the association between CagA antibody levels and the histological score. P value < 0.05 was considered statistically significant. The statistical analysis of this study were reviewed by Kido Y, Akada J, Yamaoka Y.
To develop an East Asian CagA ELISA, we first cloned the cagA gene from a clinical H. pylori strain isolated from a Japanese gastritis patient and constructed a CagA expression vector. The EPIYA segments of cagA were confirmed as East Asian type cagA, ABD segments, by DNA sequencing (Gen Bank accession number LC158593).
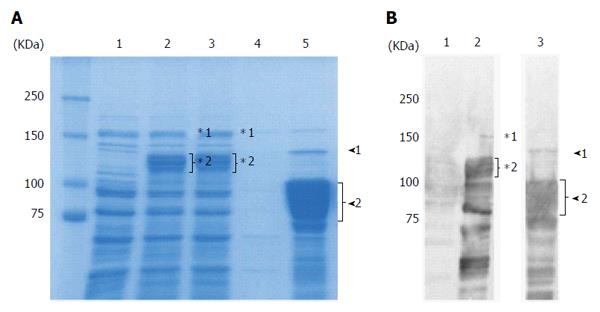
The full length East Asian-type rCagA was expressed in E. coli as a GST-tag fusion protein (deduced molecular size of 160 kDa). GST-rCagA was expressed to a high level in the soluble fraction of the E. coli lysate following induction (Figure 1A, lane 2 vs lane 1, uninduced). The GST-rCagA was bound to glutathione beads and rCagA was collected by cleaving the tag during the elution step (Figure 1A, lane 5). Western blotting using an anti-CagA antibody showed that the size of the eluted rCagA was 75-100 kDa (Figure 1B, lane 3); full length rCagA (135 kDa) was estimated to constitute < 1% of the total protein based on CBB stained SDS-PAGE (Figure 1A, lane 5). Hence, the full-length CagA was cleaved into 75-100-kDa fragments. We used this purified rCagA protein for the development of a novel East-Asian CagA ELISA.
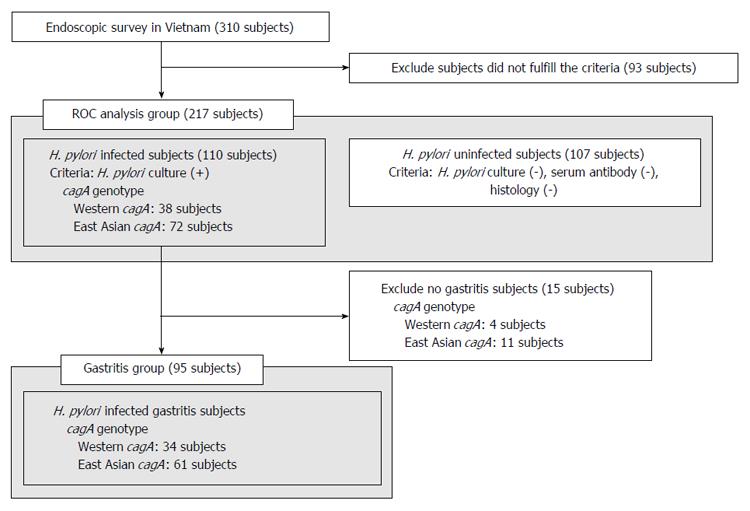
The subjects in this study included 310 subjects in Vietnam. As described at methods, H. pylori-positive status was defined. Our selection criteria to determine infected and uninfected samples presumably reduce pseudo-positive and pseudo-negative facilitating the evaluation of the a novel ELISA method; 110 H. pylori-infected subjects and 107 H. pylori-uninfected subjects (217 serum samples in total) were used for the initial experiment. Endoscopic diagnoses showed that most volunteers had only gastritis and that some had peptic ulcers and gastroesophageal reflux disease (GERD) (Table 1). PCR-based direct sequencing was used to determine the cagA genotype of the 110 H. pylori-infected volunteers; 38 were infected with Western-type cagA and 72 with East Asian-type cagA (Figure 2, ROC analysis group).
| H. pylori infection status and cagA genotype | Gastritis | Peptic ulcer | GERD | Total |
| H. pylori-infected | 95 | 12 | 3 | 110 |
| Western cagA | 34 | 4 | 0 | 38 |
| East Asian cagA | 61 | 8 | 3 | 72 |
| H. pylori-uninfected | 98 | 3 | 6 | 107 |
| Total | 193 | 15 | 9 | 217 |
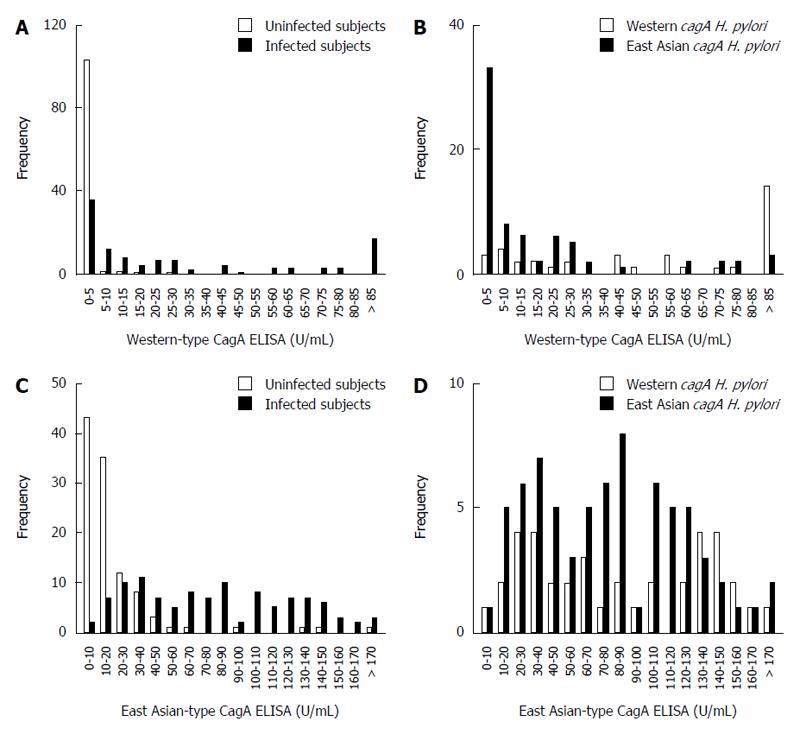
Next, we compared our East Asian-type CagA ELISA with a commercially available CagA ELISA, which utilizes Western-type CagA. The CagA antibody titer was measured for the 110 H. pylori-infected and 107 uninfected subjects (Table 2). Western-type CagA ELISA showed that 103 uninfected subject serum samples (96%) had a low titer (0-5 U/mL). The serum titers of infected subjects demonstrated two peaks; the titer of 36 subjects was within the 0-5 U/mL range, while the other 17 showed had titers > 85 U/mL (Figure 3A). The 36 subjects in the 0-5 U/mL range included 33 subjects infected with H. pylori with East Asian-type cagA and only 3 with Western-type cagA. The 17 subjects with a titer > 85 U/mL consisted of 14 subjects infected with H. pylori with Western-type cagA and only three with East-Asian cagA (Figure 3B). Additionally, the mean antibody titer against Western-type CagA (78.8 ± 71.6 U/mL) was significantly higher than that against East Asian-type CagA (22.6 ± 40.7 U/mL; P < 0.001). These data indicate that Western-type CagA ELISA reacts less efficiently with the CagA antibody present in individuals infected with H. pylori possessing East Asian-type CagA.
| H. pylori status and cagA genotype | H. pylori infection status | cagA genotype | ||
| Infected | Uninfected | Western | East Asian | |
| ROC analysis group | ||||
| n | 110 | 107 | 38 | 72 |
| Male | 63 (57%)1 | 46 (43%)1 | 17 (45%) | 46 (64%) |
| Age (yr) | ||||
| mean ± SD | 40.1 ± 12.9 | 38.5 ± 12.8 | 38.6 ± 10.8 | 41.0 ± 13.8 |
| Range | 18-76 | 19-78 | 18-69 | 21-76 |
| Gastritis subjects group | ||||
| n | 34 | 61 | ||
| Male | 19 (56%) | 38 (62%) | ||
| Age (yr) | ||||
| mean ± SD | 37.5 ± 8.5 | 39.3 ± 13.0 | ||
| Range | 22-60 | 20-70 | ||
In contrast, the East Asian-type CagA ELISA revealed that 101 (94%) uninfected volunteers had titers < 50 U/mL. The titers of the infected subjects ranged from 30-40 U/mL (Figure 3C). The mean ± SD CagA antibody titer was 85.9 ± 52.0 U/mL in subjects infected with Western-type cagA H. pylori and 79.1 ± 47.6 U/mL in subjects infected with East Asian-type cagA H. pylori; the differences were not statistically significant (P = 0.50; Figure 3D). These data show that the East Asian-type CagA ELISA reacted equally with CagA antibodies derived from subjects infected H. pylori with both types of CagA.
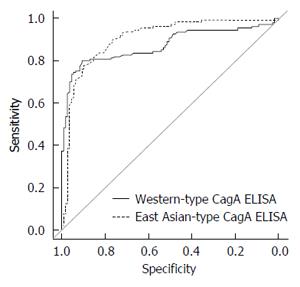
ROC analysis was used to evaluate the accuracy of East Asian-type CagA ELISA for all 217 subjects. The area under the curve (AUC) of the 110 H. pylori infected and 107 uninfected samples was nearly equal for both types of CagA ELISA: 0.91 for East Asian-type CagA ELISA and 0.87 for Western-type CagA ELISA (Table 3). These results indicate that the accuracy of East Asian-type CagA ELISA is comparable to that of Western-type CagA ELISA. Additionally, the positive cut off value of the East Asian-type CagA ELISA was determined as > 45 U/mL based on the ROC curve (Figure 4).
| Group name | n | Western-type CagA ELISA | East Asian-type CagA ELISA | ||||||
| cagA genotype | (cut off value: 6.25 U/mL) | (cut off value: 45.0 U/mL) | |||||||
| Sens. | Sp. | AUC | 95%CI | Sens. | Sp. | AUC | 95%CI | ||
| Western and East Asian | 110 | 62.7 | 97.2 | 0.87 | 0.82-0.92 | 70.9 | 93.5 | 0.91 | 0.86-0.95 |
| Western | 38 | 84.2 | 97.2 | 0.92 | 0.83-0.99 | 65.8 | 93.5 | 0.91 | 0.86-0.96 |
| East Asian | 72 | 51.41 | 97.2 | 0.85 | 0.79-0.91 | 73.61 | 93.5 | 0.90 | 0.85-0.95 |
The CagA antibody titers of all subjects were independently measured using the two types of CagA ELISA and plotted separately as the following four groups: H. pylori-uninfected group, H. pylori-infected group, subjects infected with Western-type cagA H. pylori group, and subjects infected with East Asian-type cagA H. pylori group. The cut off value for the East Asian-type CagA ELISA was determined to be 45 U/mL based on the ROC curve and that of Western-type CagA ELISA was used as 6.25 U/mL according to the manufacturer’s instruction.
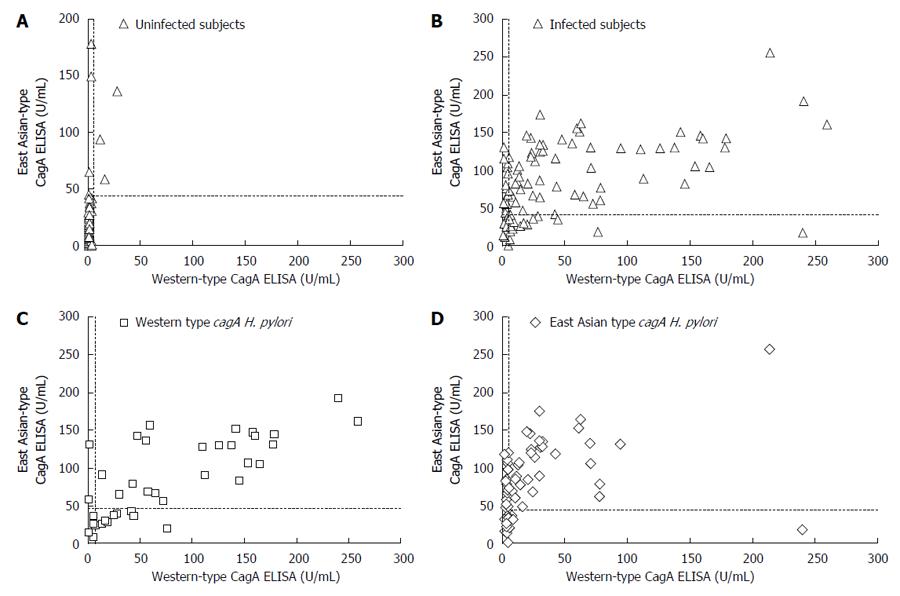
Of the 107 H. pylori-uninfected subjects, 104 subjects (97.2%) were negative by Western-type CagA ELISA and 100 were negative (93.5%) by East Asian-type CagA ELISA (Figure 5A). This indicates that the specificity of East Asian-type CagA ELISA is sufficiently high and similar to that of Western-type CagA ELISA. Of the 110 H. pylori infected subjects, 69 (62.7%) were identified as positive by Western-type CagA ELISA and 78 (70.9%) by East Asian-type CagA ELISA (Figure 5B). The accuracy of each of the ELISA results was further examined using the cagA genotype sub-group; of the 38 subjects infected with Western-type cagA H. pylori, 32 (84.2%) were identified as positive by Western-type CagA ELISA and 25 (65.8%) by East Asian-type CagA ELISA. In these 32 subjects, nine subjects were identified as positive by Western-type CagA ELISA, but negative by East Asian-type CagA ELISA (Figure 5C). Of the 72 subjects infected with East Asian-type cagA H. pylori, 53 (73.6%) were found to be positive by the East Asian-type CagA ELISA, and 37 (51.4%) were found to be positive by the Western-type CagA ELISA. Of these 53 subjects, 19 were identified as positive by the East Asian-type CagA ELISA, but negative by the Western-type CagA ELISA (Figure 5D).
Table 3 summarizes the sensitivity and specificity of both types of ELISA. The sensitivity of Western-type CagA ELISA tended to be higher than that of East Asian-type CagA ELISA for the sub-group of subjects infected with Western cagA H. pylori (P = 0.065; Table 3). Similarly, the sensitivity of East Asian-type CagA ELISA was higher than that of Western-type CagA ELISA for the sub-group of subjects infected with East Asian-type cagA H. pylori (P < 0.001; Table 3).
The relationship between lower CagA seropositivity and clinical outcomes in East Asian countries has not yet been clarified, even when almost all of the examined individuals have been infected with cagA-positive H. pylori. The novel East Asian-type CagA ELISA presented in this study had higher sensitivity for East Asian-type cagA H. pylori-infected subjects than Western-type CagA ELISA. Thus, East Asian-type CagA ELISA was used to investigate the relationship between the CagA antibody titer and histological scores of gastritis in subjects infected with either East Asian-type or Western-type cagA H. pylori.
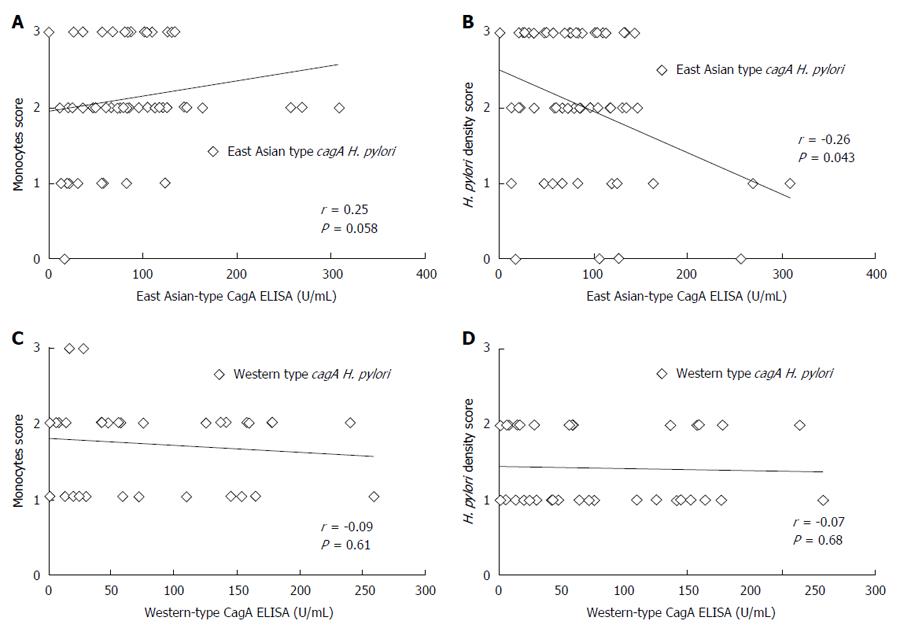
Ulcer and GERD subjects were excluded from the H. pylori infected subjects of ROC analysis group and all gastritis subjects were included in the gastritis group (Figure 2). A total of 95 volunteers, including 34 subjects infected with Western-type cagA H. pylori and 61 infected with East Asian-type cagA H. pylori, were used for this analysis (Table 2). The correlation between CagA antibody titer and histological score was examined using the Spearman rank coefficients test. Histological scores were evaluated using the updated Sydney System for the classification and grading of gastritis. The East-Asian CagA antibody titers of gastritis subjects infected with East Asian-type cagA H. pylori (measured by East Asian-type CagA ELISA) tended to correlate with the monocyte infiltration at the stomach antrum scores (r = 0.25, P = 0.058; Figure 6A). The monocyte score included monocytes as well as lymphocytes and plasma cells. Furthermore, the East-Asian CagA antibody titers were inversely correlated with H. pylori density in the antrum (r = -0.26, P = 0.043; Figure 6B). In contrast, no correlation was apparent for Western-CagA antibody titers (measured by Western-type CagA ELISA) among gastritis subjects infected with Western-type cagA H. pylori (Figure 6C and D).
In this study, we developed a novel East-Asian CagA antibody ELISA and compared it with commercial Western-type CagA ELISA using subjects infected with either East Asian-type or Western-type cagA H. pylori. The sensitivity of the East Asian-type CagA ELISA was higher than that of the Western-type CagA ELISA for the subgroup of individuals infected with East Asian-type cagA H. pylori, while the sensitivity of the Western-type CagA ELISA tended to be higher than that of the East Asian-type CagA ELISA for the subgroup of individuals infected with Western-type cagA H. pylori. These results indicate that Western-type CagA ELISA might not be suitable for application in East Asian countries and that East Asian-type CagA ELISA could be useful for detecting the East Asian-type CagA antibody with high sensitivity.
During the development of the East Asian-type CagA ELISA, we noted that full length rCagA seemed to be unstable and was cleaved into 75-100 kDa fragments. This instability of CagA has been previously demonstrated in both in vivo and in vitro studies[30-32]. CagA was cleaved into 100 kDa and 35 kDa fragments in vivo, with the N-terminus of CagA present in 100 kDa fragment. Although the C-terminus of CagA was cleaved during protein purification, our novel East Asian-type CagA ELISA still exhibited greater sensitivity for East Asian-type CagA. This suggests that the 75-100 kDa East Asian-type CagA fragment still contains East Asian-type specific epitopes.
The CagA antibody epitope seems to depend on cagA genotype. Klimovich et al[33] reported that the majority of H. pylori-positive serum samples reacted with rCagAfr.2 antigen, which was located in the middle section of the Western-type CagA fragment. Furthermore, the middle section of East Asian-type CagA has been found to be highly antigenic in serum samples from H. pylori-positive Japanese children[34]. Of the subjects infected with East Asian-type cagA H. pylori in the present study, 19 were identified as positive by the East Asian-type CagA ELISA, but negative by the Western-type CagA ELISA. In addition, the anti-CagA antibody titer of the CagA ELISA was independent of CagA EPIYA variant motifs, although the number of minor variant EPIYA motifs was very small. These results suggest that high-antigenicity regions specific to East Asian CagA are present in regions without EPIYA motifs. Moreover, the CagA antibody epitope may depend on the cagA genotype. Therefore, the novel East Asian-type CagA ELISA should be used in order to increase the sensitivity of CagA seropositivity detection in East Asian countries.
East Asian-type CagA antibody titer positively correlated with monocyte infiltration and negatively with H. pylori density in the antrum. These results are consistent with the fact that the atrophic mucosa cannot be colonized easily by H. pylori and that anti-H. pylori antibody titer decreases in a time-dependent manner[35-38]. Furthermore, the half-life of the CagA antibody is greater than that of the anti-H. pylori antibody[39-41]. These findings are consistent with the negative correlation between CagA antibody titer and H. pylori density in the stomach, which reflects the progression of atrophy at the stomach mucosa. Previous studies have demonstrated the increased antigenicity of East Asian-type CagA. Miura et al[42] showed that transgenic mice expressing East Asian-type CagA developed tumors more frequently than those expressing Western-type CagA; and Satomi et al[43] reported that patients infected with H. pylori carrying the East Asian-type CagA were associated with severe gastric atrophy and gastric cancer in Japan. Therefore, the correlation between anti-CagA antibody titer and histological score in this study may be a reflection of severe inflammation derived from the virulence of CagA.
In conclusion, the novel East Asian-type CagA ELISA developed in this study should be used concomitantly with Western-type CagA ELISA in order to increase the sensitivity of CagA seropositivity detection in East Asian countries. Moreover, subjects with higher CagA antibody titers could be classified into higher risk population to cause gastric cancer. The findings gained of this study may help us to further understand the potential marker of predicting H. pylori pathogenicity. Recently, it was reported that human genetic factor determines the antigen epitope and reduced the risk of severe gastric disease[44]. To fully appreciate the immunity to CagA and its interaction with the human genetic factor will need to reveal the relationship CagA antibody and clinical outcomes. In addition, further epidemiological studies are required to confirm the accuracy of East-Asian CagA ELISA and to determine a more reliable cut off value for the global use of this method.
We wish to thank Ms. Miyuki Matsuda, Ms. Ayaka Takahashi, and Ms. Yoko Kudo for their excellent technical assistance.
The cagA genotype is known to be responsible for the pathogenicity of Helicobacter pylori (H. pylori) as well as the geographic differences associated with the incidence of gastric cancer. The cagA genotype was classified according to the amino acid sequence of the surrounding EPIYA motifs. Most H. pylori strains isolated in East Asian countries possess the cagA gene; the predominant cagA genotype is the East Asian-type, which causes more severe inflammation than the Western-type. However, the relationship between CagA seropositivity and clinical outcomes is controversial.
Many researchers have reported the relationships between CagA antibody titers and clinical outcomes using conventional CagA ELISA, which was immobilized Western-type CagA. However, the positive rate of CagA antibodies among H. pylori-infected Japanese individuals was relatively low, although the majority of H. pylori strains in Japan possess an East Asian-type cagA gene. Therefore, conventional CagA ELISA may underestimate the seropositivity of anti-CagA antibody detection. Further studies are needed to support this hypothesis.
In present study, the authors developed a novel East Asian-type CagA ELISA to detect anti-CagA antibodies with higher sensitivity in East Asian countries and then demonstrated that conventional CagA ELISA underestimated the seropositivity of anti-CagA antibody detection for subjects infected with East Asian cagA H. pylori. CagA has sequence variations in different populations around the world, and CagA ELISAs specific for a geographic location may improve diagnoses.
The novel East Asian-type CagA ELISA developed in this study could be used concomitantly with the Western-type CagA ELISA in order to increase the sensitivity of CagA seropositivity detection in East Asian countries. This may be useful in South Asian countries in which H. pylori harbor East Asian-type or Western-type CagA. Moreover, the titer of East Asian CagA ELISA was correlated with the activity of chronic inflammation in the gastric mucosa. Thus, individuals with higher anti-CagA antibody titers could be classified as having a higher risk of gastric cancer.
Receiver operating characteristic (ROC) curve is used to evaluate the discrimination ability of the diagnostic tool for the target disease. The accuracy of the tool is evaluated from the area under the curve, and the cut-off value is determined based on the ROC curve. The sensitivity and specificity of the tool depend on the cut-off value.
These researchers determined the presence and the levels of anti-CagA antibodies in two groups of patients, one infected by H. pylori strains with East Asian-type CagA, the other one with Western type CagA. The gold standard for the H. pylori infectious status was endoscopy with rapid urease test, biopsy culture, histological tests confirmed by immunohistochemistry and detection of serum antibodies to whole H. pylori antigens. They observed that ELISA using East Asian-type CagA had greater sensitivity with patients infected by strains expressing East Asian CagA; in addition, the levels of anti-CagA antibodies in these patients tended to correlate with histologic chronic inflammation score. This study is important, since it partly solve a main problem of H. pylori and CagA serology, sensitivity. The manuscript is written in good English and is very clear.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ananthakrishnan N, Figura N, Garcia-Olmo D S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Posselt G, Backert S, Wessler S. The functional interplay of Helicobacter pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun Signal. 2013;11:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011;278:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Mueller D, Tegtmeyer N, Brandt S, Yamaoka Y, De Poire E, Sgouras D, Wessler S, Torres J, Smolka A, Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J Clin Invest. 2012;122:1553-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 5. | Hatakeyama M. Anthropological and clinical implications for the structural diversity of the Helicobacter pylori CagA oncoprotein. Cancer Sci. 2011;102:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 291] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Matozaki T, Murata Y, Saito Y, Okazawa H, Ohnishi H. Protein tyrosine phosphatase SHP-2: a proto-oncogene product that promotes Ras activation. Cancer Sci. 2009;100:1786-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [PubMed] |
| 10. | Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799-1809. [PubMed] |
| 11. | Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111-2115. [PubMed] |
| 12. | Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777-1780. [PubMed] |
| 13. | Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [PubMed] |
| 15. | Zhao Z, Li Y, Liu S, Fu W. Serum Helicobacter pylori CagA antibody may not be used as a tumor marker for diagnosing gastric cancer in east Asian countries. Tumour Biol. 2014;35:12217-12224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 16. | Yahav J, Fradkin A, Weisselberg B, Diver-Haver A, Shmuely H, Jonas A. Relevance of CagA positivity to clinical course of Helicobacter pylori infection in children. J Clin Microbiol. 2000;38:3534-3537. [PubMed] |
| 17. | Kato S, Sugiyama T, Kudo M, Ohnuma K, Ozawa K, Iinuma K, Asaka M, Blaser MJ. CagA antibodies in Japanese children with nodular gastritis or peptic ulcer disease. J Clin Microbiol. 2000;38:68-70. [PubMed] |
| 18. | Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636-1644. [PubMed] |
| 19. | Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225-228. [PubMed] |
| 21. | Yamaoka Y, Kodama T, Kashima K, Graham DY. Antibody against Helicobacter pylori CagA and VacA and the risk for gastric cancer. J Clin Pathol. 1999;52:215-218. [PubMed] |
| 22. | Nguyen TL, Uchida T, Tsukamoto Y, Trinh DT, Ta L, Mai BH, Le SH, Thai KD, Ho DD, Hoang HH. Helicobacter pylori infection and gastroduodenal diseases in Vietnam: a cross-sectional, hospital-based study. BMC Gastroenterol. 2010;10:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Truong BX, Mai VT, Tanaka H, Ly le T, Thong TM, Hai HH, Van Long D, Furumatsu K, Yoshida M, Kutsumi H. Diverse characteristics of the CagA gene of Helicobacter pylori strains collected from patients from southern vietnam with gastric cancer and peptic ulcer. J Clin Microbiol. 2009;47:4021-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Matsunari O, Shiota S, Suzuki R, Watada M, Kinjo N, Murakami K, Fujioka T, Kinjo F, Yamaoka Y. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012;50:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3:241-253. [PubMed] |
| 26. | Uchida T, Miftahussurur M, Pittayanon R, Vilaichone RK, Wisedopas N, Ratanachu-Ek T, Kishida T, Moriyama M, Yamaoka Y, Mahachai V. Helicobacter pylori Infection in Thailand: A Nationwide Study of the CagA Phenotype. PLoS One. 2015;10:e0136775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30102] [Cited by in RCA: 27920] [Article Influence: 2326.7] [Reference Citation Analysis (0)] |
| 28. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 29. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13175] [Article Influence: 1097.9] [Reference Citation Analysis (0)] |
| 30. | Moese S, Selbach M, Zimny-Arndt U, Jungblut PR, Meyer TF, Backert S. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: processing or breakage? Proteomics. 2001;1:618-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Jiménez-Soto LF, Kutter S, Sewald X, Ertl C, Weiss E, Kapp U, Rohde M, Pirch T, Jung K, Retta SF. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog. 2009;5:e1000684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Backert S, Moese S, Selbach M, Brinkmann V, Meyer TF. Phosphorylation of tyrosine 972 of the Helicobacter pylori CagA protein is essential for induction of a scattering phenotype in gastric epithelial cells. Mol Microbiol. 2001;42:631-644. [PubMed] |
| 33. | Klimovich AV, Samoylovich MP, Gryazeva IV, Terekhina LA, Suvorov AN, Klimovich VB. Development of immunoreagents for diagnostics of CagA-positive Helicobacter pylori infections. Helicobacter. 2010;15:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Akada J, Okuda M, Hiramoto N, Kitagawa T, Zhang X, Kamei S, Ito A, Nakamura M, Uchida T, Hiwatani T. Proteomic characterization of Helicobacter pylori CagA antigen recognized by child serum antibodies and its epitope mapping by peptide array. PLoS One. 2014;9:e104611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O’Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 36. | Maeda S, Yoshida H, Ogura K, Yamaji Y, Ikenoue T, Mitsushima T, Tagawa H, Kawaguchi R, Mori K, Mafune Ki, Kawabe T, Shiratori Y, Omata M. Assessment of gastric carcinoma risk associated with Helicobacter pylori may vary depending on the antigen used: CagA specific enzyme-linked immunoadsorbent assay (ELISA) versus commercially available H. pylori ELISAs. Cancer. 2000;88:1530-1535. [PubMed] |
| 37. | Kreuning J, Lindeman J, Biemond I, Lamers CB. Relation between IgG and IgA antibody titres against Helicobacter pylori in serum and severity of gastritis in asymptomatic subjects. J Clin Pathol. 1994;47:227-231. [PubMed] |
| 38. | Zagari RM, Pozzato P, Martuzzi C, Fuccio L, Martinelli G, Roda E, Bazzoli F. 13C-urea breath test to assess Helicobacter pylori bacterial load. Helicobacter. 2005;10:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Yanaoka K, Oka M, Mukoubayashi C, Yoshimura N, Enomoto S, Iguchi M, Magari H, Utsunomiya H, Tamai H, Arii K. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Kokkola A, Kosunen TU, Puolakkainen P, Sipponen P, Harkonen M, Laxen F, Virtamo J, Haapiainen R, Rautelin H. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619-624. [PubMed] |
| 41. | Karnes WE, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, Walsh JH. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167-174. [PubMed] |
| 42. | Miura M, Ohnishi N, Tanaka S, Yanagiya K, Hatakeyama M. Differential oncogenic potential of geographically distinct Helicobacter pylori CagA isoforms in mice. Int J Cancer. 2009;125:2497-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Satomi S, Yamakawa A, Matsunaga S, Masaki R, Inagaki T, Okuda T, Suto H, Ito Y, Yamazaki Y, Kuriyama M. Relationship between the diversity of the cagA gene of Helicobacter pylori and gastric cancer in Okinawa, Japan. J Gastroenterol. 2006;41:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Chen L, Li B, Yang WC, He JL, Li NY, Hu J, He YF, Yu S, Zhao Z, Luo P. A dominant CD4(+) T-cell response to Helicobacter pylori reduces risk for gastric disease in humans. Gastroenterology. 2013;144:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |