Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10189
Peer-review started: August 6, 2016
First decision: September 21, 2016
Revised: October 11, 2016
Accepted: November 15, 2016
Article in press: November 16, 2016
Published online: December 14, 2016
Processing time: 129 Days and 19.9 Hours
To evaluate outcomes associated with use of a saline coupled bipolar sealer during open partial liver resection.
This retrospective analysis utilized the United States Premier™ insurance claims database (2010-2014). Patients were selected with codes for liver malignancy and partial hepatectomy or lobectomy. Cases were defined by use the saline-coupled bipolar sealer; controls had no use. A Propensity Score algorithm was used to match one case to five controls. A deviation-based cost modeling (DBCM) approach provided an estimate of cost-effectiveness.
One hundred and forty-four cases and 720 controls were available for analysis. Patients in the case cohort received fewer transfusions vs controls (18.1% vs 29.4%, P = 0.007). In DBCM, more patients in the case cohort experienced “on-course” hospitalizations (53.5% vs 41.9%, P = 0.009). The cost calculation showed an average savings in total hospitalization costs of $1027 for cases vs controls. In multivariate analysis, cases had lower odds of receiving a transfusion (OR = 0.44, 95%CI: 0.27-0.71, P = 0.0008).
Use of a saline-coupled bipolar sealer was associated with a greater proportion of patients with an “on course” hospitalization.
Core tip: This study evaluated outcomes associated with use of a saline coupled bipolar sealer during open partial liver resection. Using US Premier insurance claims data, Cases with use of the saline-coupled bipolar sealer (SCBS) were propensity-score matched to controls with no use. A deviation-based cost modeling (DBCM) approach provided an estimate of cost-effectiveness. Results demonstrated that use of the SCBS in open partial liver resection for hepatic malignancy is associated with reduction in the need for transfusion, and is cost-effective in a DBCM analysis. This technology provides an alternative solution for bleeding control in partial liver resection compared to traditional methods.
- Citation: Nichols CI, Vose JG. Use of a saline-coupled bipolar sealer open liver resection for hepatic malignancy: Medical resource use and costs. World J Gastroenterol 2016; 22(46): 10189-10197
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10189
Liver resection remains the only curative treatment for primary and metastatic liver malignancy. However, despite advances in surgical technique over the past two decades, blood transfusions are still required in a proportion of patients undergoing liver resection (3.3%-59%), varying by the extent of the procedure and device combinations used[1-5]. Predictors of transfusion include factors related to the operative procedure (resection technique, extent of resection, tumor size, need for other major resections during the same hospitalization) as well as patient-specific characteristics (pre-operative hemoglobin and albumin levels, pre-operative biliary drainage, and diagnosis of a primary liver tumor, coronary artery disease, or cirrhosis)[6-9].
The most serious complication associated with transfusion, beyond simple transfusion-related reactions or immunomodulatory effects, is the increased risk of tumor recurrence[6,8-10]. In a meta-analysis of 22 studies evaluating the impact of perioperative allogenic blood transfusion on long term outcomes following hepatocellular carcinoma (HCC) resection, authors found the risk of tumor recurrence was significantly higher among patients with a transfusion at one (OR = 1.70, 95%CI: 1.38-1.10), three (OR = 1.22, 95%CI: 1.08-1.38), and five years (OR = 1.16, 95%CI: 1.08-1.24) post-resection compared with patients with no transfusion. This finding was confirmed in a Cochrane meta-analysis evaluating the risk of cancer recurrence following surgery for colorectal cancer among patients with vs without receipt of a transfusion[11]. These studies suggest that transfusion may result in immunosuppression in the early postoperative period, which could allow for the progression of residual carcinoma and influence survival[12].
Prior research has demonstrated the effects of surgical technique, peri-operative blood management protocols, and use of surgical technologies on the risk of transfusion[1-5]. Peri-operatively, studies have examined autologous blood donation, intravenous iron therapy, and strict transfusion protocols. Intraoperatively, other studies have examined the effects of clamping the hepatic artery and portal vein (i.e., Pringle’s Maneuver), topical hemostatic agents, and use of technologies such as the cavitron ultrasonic surgical aspirator (CUSA), saline-coupled bipolar sealer (SCBS), argon beam coagulation (ABC), harmonic scalpel, bipolar scissors, vessel sealers, cell saver systems, and hydrodissector[13]. The majority of these studies examined clinical outcomes alone, with few examining the total cost of the procedure or incremental costs associated with complications. Two prior high-quality cost studies applied a novel methodology, deviation-based cost modeling (DBCM), however the primary comparison was of open vs laparoscopic approach rather than specific surgical technologies utilized during the procedure[14,15].
Given that few studies to date summarize total direct hospitalization costs by choice of surgical technology during hepatic resection, we sought to examine the resource use and costs by technology choice. Specifically, in the present study we evaluated the clinical and economic outcomes associated with the SCBS during open partial liver resections, using real-world data from a nationally representative US claims database.
This retrospective database analysis reviewed recent healthcare insurance claims data from the Premier Perspective™ database (Premier Inc., Charlotte, NC, United States). Data were analyzed over the period 01/2010 to 06/2014. The database includes information on patient demographics, diagnosis and procedure codes, and cost information for over 2000 hospitals and 300 million patient encounters. This database is limited to the inpatient period, with no ability to track patients longitudinally in follow-up. The Premier database allows for tracking of total hospitalization cost information on a per-patient basis. However, the inherent tradeoff of working with retrospective claims data is the reliance on ICD-9 diagnosis and procedure codes to identify liver resections - with the codes providing no information on the specific number of segments, lobes, or tissue volume resected. Given this study used de-identified patient data, it was not subject to Institutional Review Board approval. The study dataset and full study tables are available from the corresponding author.
Patients aged 18 and older with records that included International Classification of Diseases (ICD-9-CM) or Current Procedural Technology (CPT) procedure codes for liver resection during a hospitalization episode (50.22 - partial hepatectomy or 50.3x - lobectomy), accompanied by a diagnosis code for primary malignant neoplasm of the liver (155.0x) or metastatic neoplasm of the liver (197.7x), were selected. Those with benign neoplasms (211.5x) were excluded to reduce the potential confounding effects of different liver pathology and bleeding risk. Total liver resection and transplant procedures were excluded. Operations using ablation procedures or laparoscopic approaches (as identified by ICD-9-CM codes and key terms in Premier Chargemaster records) were excluded due to the high cost of these procedures and to better isolate the effects of SCBS use. Open SCBS device use was identified by the hospital Chargemaster file; laparoscopic SCBS models were excluded.
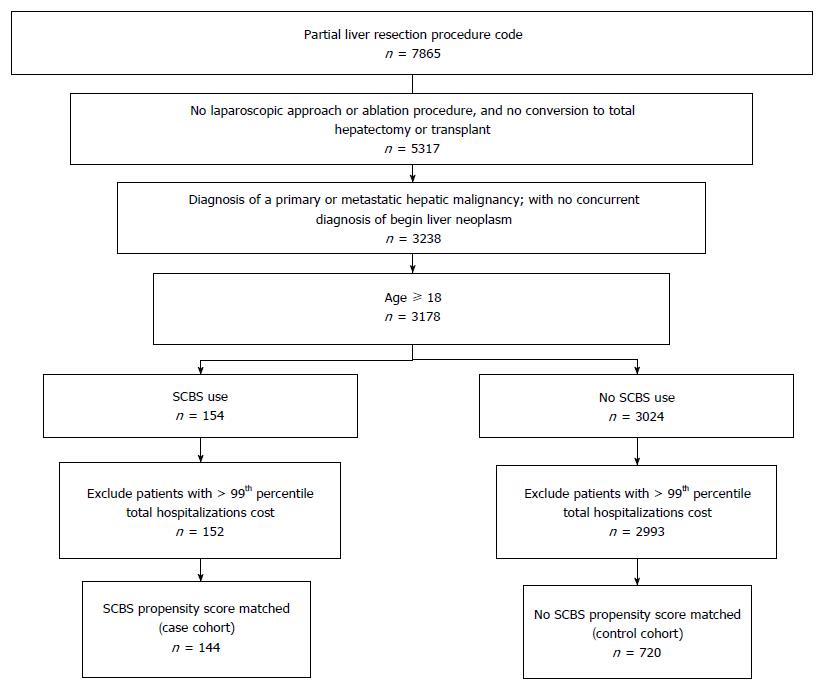
The “case” cohort was defined as any hospitalization episode meeting all inclusion criteria listed above, where the SCBS was used. The “control” cohort was defined as cases in which the SCBS was not used. Similar to prior cost analyses[16], patients in the top one percent of total hospitalization cost within each cohort were excluded from analysis in order to reduce the effects of extreme outliers (> $87262 among cases and > $153428 among controls). Figure 1 provides a summary of patient selection.
Study measures included patient demographic, clinical, hospital, and surgeon characteristics, transfusion procedures and other complications during index hospitalization, hospital length of stay (LOS) and costs. Comorbidity status was evaluated with diagnoses recorded during the one year prior to admission (baseline period) through the index hospitalization episode. The Charlson Comorbidity Index (CCI) score, a composite measure of physical health status commonly used in studies of medical claims and chronic disease[17,18] was calculated for each patient. For this study, malignancy, metastatic solid tumor, and mild or moderate liver disease were excluded from the CCI calculation as these were present for most patients.
In order to address selection bias and ensure demographic and hospitalization characteristics were similar across the case and control cohorts, a propensity-score matching algorithm was applied. Each case was matched to five controls based on age group, gender, race, region, primary payor, procedure type, indicating diagnosis, other comorbid liver-related conditions, CCI, surgeon specialty, and the proportion of surgeons with history of at least one liver procedure performed in the prior year. These matching covariates were chosen both based on significant differences observed in unmatched cohorts (P values < 0.05), and on the basis of clinical and demographic factors that may have impacted surgeon choice of technology use. Matching was applied using the nearest neighbor approach, with a caliper width of 0.10 of the standard deviation of the logit of the propensity score.
Transfusion procedures were identified by ICD-9 (V58.2, 99.00-99.04) or CPT codes (36430, P9010, P9011) or presence of the term “blood transfusion” in the hospital Chargemaster file. Topical hemostat use was identified by any mention of “hemostat” or “sealant” in the Chargemaster file under the “Medical Surgical Supplies” category.
A DBCM approach was employed to account for variation in resource use associated with different hospital LOS categories and severity of complications[14,15,19]. Vanounou et al[15,19] originally developed this approach in analyses evaluating the economic impact of pancreaticoduodenectomy procedures and a comparison of laparoscopic vs open liver resection. This methodology measures the frequency and severity of deviations from an “expected” postoperative course and calculates the economic consequences of hospitalizations that do not follow expected outcomes. The benefits of this approach are the incorporation of complications, LOS, and costs into one measure, providing a single outcomes-based metric that provides more information than simply clinical or cost data alone[14,15]. Data on LOS and complications were combined to create four deviation classes: on-course, minor, moderate, and severe. Definitions for each class are listed in Table 1. Once deviation groups were defined, a weighted average mean cost (WAMC) was calculated by multiplying the percentage of patients in each category by the mean cost of that category.
| Deviation | LOS | Complication group1 |
| On course | ≤ 50th percentile | No complication |
| Minor deviation | > 50th percentile | No complication |
| ≤ 50th | Minor complication, no moderate or major | |
| Moderate deviation | > 50th percentile | Minor complication, no moderate or major |
| Any LOS | Moderate no major | |
| Major deviation | Any LOS | Major |
Analyses were performed using the Instant Health Data Suite (Boston Health Economics, Inc., Boston, MA) and SAS software (Version 9.2, SAS Institute, Cary, NC, United States). All costs were inflation-adjusted to 2014 USD using the medical care component of the Consumer Price Index. Statistical significance testing was performed with the Chi-square (χ2) test for categorical variables (or Fisher’s Exact with cell frequencies < 10) and Wilcoxon-Mann-Whitney test for non-normal continuous variables. Predictors of topical hemostat use and transfusion, controlling for demographic and clinical characteristics, provider specialty and experience, and study cohort, were evaluated using logistic regression analysis.
Between January 2010 and June 2014, 152 cases and 2993 unmatched controls were available for analysis after applying all sample selection criteria, with procedures performed at 284 hospitals nationally. Following application of the propensity score algorithm 144 cases and 720 controls were available for matched analyses (Table 2). Post-match, differences between cohorts were removed, with all clinical characteristics statistically similar.
| Unmatched | P value | Matched | P value | |||
| SCBS | No SCBS | SCBS | No SCBS | |||
| n | 152 | 2993 | 144 | 720 | ||
| Age, mean (SD) | 62 (12.5) | 61.58 (12.1) | 0.683 | 61.49 (12.5) | 62.14 (12.1) | 0.568 |
| Age group1 | 0.960 | |||||
| 18 to 44 | 10 (6.6) | 262 (8.8) | 0.868 | 10 (6.9) | 49 (6.8) | |
| 45 to 54 | 29 (19.1) | 536 (17.9) | 28 (19.4) | 143 (19.9) | ||
| 55 to 64 | 44 (28.9) | 914 (30.5) | 44 (30.6) | 198 (27.5) | ||
| 65 to 74 | 46 (30.3) | 862 (28.8) | 41 (28.5) | 220 (30.6) | ||
| 75 plus | 23 (15.1) | 419 (14.0) | 21 (14.6) | 110 (15.3) | ||
| Race | 0.148 | 0.692 | ||||
| Black | 18 (11.8) | 368 (12.3) | 18 (12.5) | 87 (12.1) | ||
| Caucasian | 83 (54.6) | 1788 (59.7) | 76 (52.8) | 407 (56.5) | ||
| Hispanic | 0 (0) | 41 (1.4) | 0 (0) | 0 (0) | ||
| Other | 51 (33.6) | 796 (26.6) | 50 (34.7) | 226 (31.4) | ||
| Region | < 0.001 | 0.892 | ||||
| Midwest | 31 (20.4) | 304 (10.2) | 26 (18.1) | 112 (15.6) | ||
| Northeast | 52 (34.2) | 959 (32.0) | 49 (34.0) | 260 (36.1) | ||
| South | 41 (27.0) | 1321 (44.1) | 41 (28.5) | 206 (28.6) | ||
| West | 28 (18.4) | 409 (13.7) | 28 (19.4) | 142 (19.7) | ||
| Sex | ||||||
| Female | 71 (46.7) | 1315 (43.9) | 0.556 | 67 (46.5) | 308 (42.8) | 0.461 |
| Payor | 0.577 | 0.903 | ||||
| Commercial | 49 (32.2) | 1166 (39.0) | 49 (34.0) | 234 (32.5) | ||
| Medicare | 21 (13.8) | 362 (12.1) | 21 (14.6) | 111 (15.4) | ||
| Medicaid | 73 (48.0) | 1302 (43.5) | 65 (45.1) | 341 (47.4) | ||
| Other | 9 (5.9) | 163 (5.5) | 9 (6.3) | 34 (4.7) | ||
In matched analysis, patients in the case cohort had lower incidence of transfusions vs the control cohort, with an absolute risk reduction of 11.3% and relative risk reduction of 38.7% (18.1% vs 29.4%, P = 0.007). Additionally, patients in the case cohort had fewer cases of acute kidney failure occurring during the same hospitalization episode (3.5% vs 8.8%, P = 0.048). All other inpatient complications were statistically similar across cohorts, including infection, urinary tract infection, acute respiratory failure, pneumonia, deep vein thrombosis, hemorrhage or hematoma, wound disruption, and bile leak. One patient (0.694%) in the case cohort had evidence of bile leak vs eight patients in the control cohort (1.11%), however this difference was not significant (P = 1.00). No patients in the case cohort experienced acute liver failure, pulmonary embolism, or transfusion-related complications, while 1.8%, 1.0%, and 0.6% of control patients developed these complications during the inpatient visit (all P > 0.05).
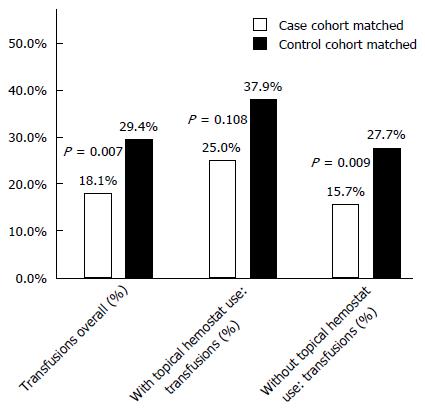
Overall, 25.0% of the case cohort showed evidence of topical hemostat use during the liver resection procedure, while 17.2% of the control cohort showed evidence of topical hemostat use (P = 0.038). Among patients with topical hemostat use, the incidence of transfusions was lower in the case cohort, however the difference was not statistically significant (25.0% vs 37.9%, P = 0.108), Figure 2. When a topical hemostat was not used, the case cohort had lower incidence of transfusion compared to the control cohort (15.7% vs 27.7%, P = 0.009).
Length of stay was shorter in the case cohort, however the difference was not statistically significant (7.38 d vs 8.18 d, P = 0.210; Table 3); the median LOS was six days for each cohort. A greater proportion of patients in the case cohort had an on-course hospitalization vs the control cohort (53.5% vs 41.9%, P = 0.013; Table 4). The proportion in other deviation classes was statistically similar across cohorts. Mean total hospitalization costs were greater among those with an on-course hospitalization in the case cohort vs controls ($18000 vs $16813, P = 0.029); costs in other deviation classes were not statistically different. Overall, accounting for the distribution of patients in each deviation class and mean cost by deviation class, the WAMC for the case cohort was $25503 vs $26530 for controls. This represents an average savings of $1027 in the total hospitalization cost per patient when the SCBS was used.
| Unmatched | P value | Matched | P value | |||
| SCBS | No SCBS | SCBS | No SCBS | |||
| n | 152 | 2993 | 144 | 720 | ||
| Procedure Type | ||||||
| Partial hepatectomy | 99 (65.1) | 2061 (68.9) | 0.308 | 98 (68.1) | 478 (66.4) | 0.772 |
| Lobectomy | 53 (34.9) | 964 (32.2) | 0.552 | 46 (31.9) | 251 (34.9) | 0.564 |
| Indicating diagnosis | ||||||
| Primary hepatobiliary malignancy | 63 (41.5) | 850 (28.4) | 0.001 | 56 (38.9) | 300 (41.7) | 0.599 |
| Metastatic liver neoplasm | 89 (58.6) | 2143 (71.6) | 0.001 | 88 (61.1) | 420 (58.3) | 0.599 |
| Comorbid liver diagnoses | ||||||
| Alcoholic cirrhosis | 1 (0.66) | 33 (1.1) | 0.908 | 1 (0.69) | 4 (0.56) | 1.000 |
| Non-alcoholic cirrhosis | 23 (15.1) | 287 (9.6) | 0.036 | 20 (13.9) | 100 (13.9) | 1.000 |
| Hepatitis A | 0 (0) | 6 (0.2) | 1.000 | 0 (0) | 0 (0) | N/A |
| Hepatitis B | 18 (11.8) | 193 (6.4) | 0.015 | 16 (11.1) | 96 (13.3) | 0.556 |
| Hepatitis C | 17 (11.2) | 271 (9.1) | 0.457 | 17 (11.8) | 71 (9.9) | 0.580 |
| Charlson score group1 | 0.518 | 0.704 | ||||
| 0 | 76 (50) | 1637 (54.7) | 74 (51.4) | 370 (51.4) | ||
| 1 | 45 (29.6) | 816 (27.3) | 43 (29.9) | 196 (27.2) | ||
| ≥ 2 | 31 (20.4) | 540 (18.0) | 27 (18.8) | 154 (21.4) | ||
| Provider specialty | ||||||
| Surgical oncology | 59 (38.8) | 531 (17.7) | < 0.001 | 56 (38.9) | 285 (39.6) | 0.950 |
| General surgery | 79 (52.0) | 1993 (66.6) | < 0.001 | 74 (51.4) | 369 (51.3) | 1.000 |
| Other | 14 (9.2) | 469 (15.7) | 0.041 | 14 (9.7) | 66 (9.2) | 0.958 |
| Surgeon experience | ||||||
| ≥ 1 liver procedure in prior year | 125 (82.2) | 1991 (66.6) | < 0.001 | 117 (81.3) | 595 (82.6) | 0.780 |
| Characteristic | SCBS | No SCBS | P value |
| Length of stay (LOS), d | |||
| mean (SD) | 7.38 (5.18) | 8.18 (7.27) | 0.210 |
| 25th percentile | 4 | 4 | |
| Median | 6 | 6 | |
| 75th percentile | 8 | 8 | |
| Deviation mix, n (%) | |||
| On course | 77 (53.5) | 302 (41.9) | 0.013 |
| Minor deviation | 30 (20.8) | 187 (26.0) | 0.208 |
| Moderate deviation | 28 (19.4) | 150 (20.8) | 0.821 |
| Major deviation | 9 (6.3) | 81 (11.3) | 0.074 |
| mean (SD) total hospital cost | |||
| On course | 18000 (5746) | 16813 (6588) | 0.029 |
| Minor deviation | 28379 (14863) | 25452 (11186) | 0.454 |
| Moderate deviation | 36558 (17777) | 35261 (19390) | 0.571 |
| Major deviation | 45717 (19045) | 49082 (35303) | 0.568 |
| WAMC total hospitalization cost | $25503 | $26530 | |
| WAMC difference | $1027 |
In logistic regression analysis of predictors of topical hemostat use, patients residing in the South were at greater odds of topical hemostat use compared to those in the Northeast, while patients with the surgery performed by a surgical oncologist were at lower odds of hemostat use compared to general surgeons (Table 5). Patients in the case cohort were at higher odds of topical hemostat use vs controls (OR = 2.56, 95%CI: 1.70-3.86, P < 0.001).
| Parameter | Predictors of topical hemostat use | Predictors of transfusion | ||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | |
| Age group (vs 18 to 44) | ||||||
| 75 plus | 1.03 | 0.50-2.12 | 0.421 | 4.55 | 1.95-10.59 | < 0.0001 |
| Race (vs caucasian) | ||||||
| Black | 1.21 | 0.75-1.97 | 0.275 | 1.97 | 1.21-3.19 | 0.017 |
| United States geographic region (vs northeast) | ||||||
| South | 3.67 | 2.38-5.65 | 0.0004 | 1.87 | 1.19-2.96 | 0.001 |
| Partial hepatectomy (vs lobectomy) | 1.25 | 0.91-1.73 | 0.175 | 1.62 | 1.16-2.27 | 0.005 |
| Primary malignancy (vs metastatic) | 0.80 | 0.53-1.20 | 0.281 | 1.54 | 1.00-2.38 | 0.050 |
| Provider specialty (vs general surgery) | ||||||
| Surgical oncology | 0.30 | 0.20-0.46 | < 0.0001 | 0.65 | 0.42-1.01 | 0.005 |
| Other specialty | 0.68 | 0.40-1.16 | 0.402 | 1.48 | 0.85-2.57 | 0.023 |
| Case cohort (vs matched controls) | 2.56 | 1.70-3.86 | < 0.0001 | 0.44 | 0.27-0.71 | 0.0008 |
| Topical hemostat use | N/A | N/A | N/A | 1.87 | 1.33-2.64 | 0.0004 |
In a regression evaluating predictors of a transfusion during the hospitalization (Table 5), patients aged 75 or older (vs ages 18 to 44), Black race (vs Caucasian), and patients residing in the South (vs Northeast), and patients operated on by an other surgical specialist (vs general surgeons) were at higher odds of receiving a transfusion. Patients undergoing a lobectomy (vs partial hepatectomy) were at higher odds, as were patients whose diagnosis was a primary malignancy (vs metastatic). Controlling for topical hemostat use, patients in the case cohort were at lower odds of transfusion vs controls (OR = 0.44, 95%CI: 0.27-0.71, P = 0.0008)
This retrospective database analysis evaluated the use of the SCBS in open partial liver resection for hepatic malignancy. After matching, patients treated with the SCBS had a lower incidence of transfusions (18.1% vs 29.4%, P = 0.007). Controlling for topical hemostat use, the reduction of transfusion incidence in univariate analysis was confirmed in multivariate analysis, with SCBS use associated with a lower odds of transfusion vs no use (OR = 0.44, 95%CI: 0.27-0.71). Overall, DBCM analyses indicated an average cost savings of $1027 among cases when accounting for the proportion within each “hospital deviation” class, with significantly more patients in the SCBS cohort with an “on course” hospitalization (defined as no complications and a LOS less than the median). We believe this study, despite the lack of clinical detail on number of lobes resected, provides information on “real-world” practice outside of a controlled prospective study or randomized controlled trial.
This study adds to a growing body of research evaluating the safety and efficacy of SCBS in liver resections. Authors at the University of Pittsburgh Starzl Transplant Institute performed a single-arm study evaluating the safety of the SCBS (formerly of “TissueLink Medical”) in 170 open liver resection procedures performed between 2001 and 2004[20]. Overall, 3.5% of patients were transfused and 2.4% developed a postoperative bile leak. There were no cases of postoperative hemorrhage, hepatic failure, liver abscess, or reoperation. The authors concluded the SCBS was effective in achieving intraoperative hemostasis in hepatic resection. The observed transfusion rate was much lower than in our present study, however this is likely due to comparing outcomes from a single high-volume hospital vs our present study, which includes data from 284 hospitals.
In a prospective single-arm study in Italy, the incidence of early surgical complications (including bleeding, biliary leakage, and abscess development) following 12 partial hepatectomies with the SCBS was evaluated[21]. Mean blood loss was 20 mL (range 5 to 80 mL), with no transfusions and a mean LOS of six days[21]. This LOS is similar to the 7.4 d observed in the case cohort of our study.
Lastly, two studies have examined the combined use of the SCBS and CUSA. In the largest study of SCBS use in liver resection to date, authors at four hepatopancreaticobiliary units in Europe evaluated the safety and efficacy of combined use of SCBS plus CUSA during 114 minor and 199 major hepatectomies. Authors reported a transfusion rate of 10.5% and two postoperative deaths (0.6%), concluding the combined method is associated with decreased blood loss[9]. A similar Japanese study also evaluated the combined use of CUSA and SCBS (n = 55) vs CUSA with traditional bipolar electrosurgery (n = 54)[22]. The SCBS and CUSA cohort demonstrated significantly lower total blood loss (677 mL vs 1076 mL, P = 0.0486), shorter transection time (81 min vs 115 min, P = 0.0025) and fewer ties required (13.1 vs 22.8, P < 0.001) vs the traditional electrosurgery and CUSA cohort[22]. While the combined use of SCBS and CUSA is evaluated in these studies, other device combinations or techniques may provide equivalent outcomes at lower cost. This is an area for future research.
Although it was observed in the present study that a greater proportion of the case cohort had concurrent use of topical hemostats during the procedure (25.0% vs 17.2%, P = 0.038), it appears hemostat use was reserved for the most severe cases. We infer this due to the incidence of transfusion being greater in both univariate and multivariate analyses among those with topical hemostat use vs no use, regardless of SCBS. However, there is likely an unmeasured confounder that is not readily observed in insurance claims data that may have influenced surgeon selection of both the SCBS and a hemostat. Nonetheless, incidence of transfusion remained numerically lower in the case cohort vs controls both when topical hemostats were used during the procedure and when they were not.
Limitations of this study center on the lack of detailed clinical detail in the insurance claims dataset used for analysis, which included only diagnosis and procedure codes, and items listed in the hospital Chargemaster. Therefore, we could not evaluate the number of liver segments resected, the relative complexity of the procedure, pre- and post-operative hemoglobin levels, the Hg level triggering a transfusion, or number of units of blood transfused. Also, as noted, specific line-item costs for blood were not available for approximately two-thirds of patients. However, blood costs were captured in the next level roll-up of cost reporting under OR costs. During patient selection we did not attempt to query the Chargemaster file to evaluate concurrent devices used with the SCBS, as the only comparison in this study was at the highest level of use vs no use. Given the array of device choices during hepatic resection, and the variance of names listed in the Chargemaster file, we did not attempt to compare concurrent device use. Future studies may address the question of device synergy in influencing clinical outcomes (e.g., SCBS plus CUSA). Finally, while we observed a reduction in the incidence of transfusion associated with use of SCBS in the present study, the SCBS is not designed to provide hemostasis in the event of bleeding from large vessels - thus additional technology or techniques to control bleeding that cannot be accounted for may have been present.
This retrospective database analysis demonstrated that use of the SCBS in open partial liver resection for hepatic malignancy is associated with reduction in the need for transfusion, and is cost-effective in a deviation-based cost modeling analysis. This technology provides an alternative solution for bleeding control in partial liver resection compared to traditional methods.
The authors thank Jeanne McAdara-Berkowitz PhD for professional assistance with manuscript preparation.
Despite advances in surgical technique over the past two decades, blood transfusions are still required in a proportion of patients undergoing liver resection, varying by the extent of the procedure and device combinations used. Predictors of transfusion include factors related to the operative procedure as well as patient-specific characteristics.
Prior research has demonstrated the effects of surgical technique, peri-operative blood management protocols, and use of surgical technologies on the risk of transfusion. The majority of these studies examined clinical outcomes alone, with few examining the total cost of the procedure or incremental costs associated with complications. Two prior cost studies applied a novel methodology, deviation-based cost modeling (DBCM), however the primary comparison was of open vs laparoscopic approach rather than specific surgical technologies utilized during the procedure.
This retrospective database analysis evaluated the use of a saline-coupled bipolar sealer (SCBS) in open partial liver resection for hepatic malignancy. After matching, patients treated with the SCBS had a lower incidence of transfusions (18.1% vs 29.4%, P = 0.007). Controlling for topical hemostat use, the reduction of transfusion incidence was confirmed in multivariate analysis, with SCBS use associated with a lower odds of transfusion (OR = 0.44, 95%CI: 0.27-0.71). Overall, DBCM cost analyses indicated an average cost savings of $1027 among cases when accounting for the proportion falling into each “hospital deviation” class, with significantly more patients in the SCBS cohort with an “on course” hospitalization (defined as no complications and a length of stay less than the median).
This analysis demonstrated that use of the SCBS in open partial liver resection for hepatic malignancy is associated with reduction in the need for transfusion, and is cost-effective in a deviation-based cost modeling analysis. This technology provides an alternative solution for bleeding control in partial liver resection compared to traditional electrosurgical methods.
A DBCM approach was employed in this study. This methodology measures the frequency and severity of deviations from an “expected” postoperative course and calculates the economic consequences of hospitalizations that do not follow expected outcomes. The benefits of this approach are the incorporation of complications, length of stay, and costs into one measure, providing a single outcomes-based metric that provides more information than simply clinical or cost data alone.
This is an interesting paper and well written. The current results will be great helpful to the surgical fields when evaluating the benefits and costs of alternative blood-management technologies during liver resection.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Morales-Gonzalez JA S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
| 1. | Guo JY, Li DW, Liao R, Huang P, Kong XB, Wang JM, Wang HL, Luo SQ, Yan X, Du CY. Outcomes of simple saline-coupled bipolar electrocautery for hepatic resection. World J Gastroenterol. 2014;20:8638-8645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, Kokudo N, Makuuchi M. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Nanashima A, Abo T, Arai J, Takagi K, Matsumoto H, Takeshita H, Tsuchiya T, Nagayasu T. Usefulness of vessel-sealing devices combined with crush clamping method for hepatectomy: a retrospective cohort study. Int J Surg. 2013;11:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, Yamaguchi T, Yamaguchi T, Muto T, Makuuchi M. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg. 2006;192:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Xia F, Wang S, Ma K, Feng X, Su Y, Dong J. The use of saline-linked radiofrequency dissecting sealer for liver transection in patients with cirrhosis. J Surg Res. 2008;149:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Cockbain AJ, Masudi T, Lodge JP, Toogood GJ, Prasad KR. Predictors of blood transfusion requirement in elective liver resection. HPB (Oxford). 2010;12:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Lucas DJ, Schexneider KI, Weiss M, Wolfgang CL, Frank SM, Hirose K, Ahuja N, Makary M, Cameron JL, Pawlik TM. Trends and risk factors for transfusion in hepatopancreatobiliary surgery. J Gastrointest Surg. 2014;18:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Pulitanò C, Arru M, Bellio L, Rossini S, Ferla G, Aldrighetti L. A risk score for predicting perioperative blood transfusion in liver surgery. Br J Surg. 2007;94:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Sima CS, Jarnagin WR, Fong Y, Elkin E, Fischer M, Wuest D, D’Angelica M, DeMatteo RP, Blumgart LH, Gönen M. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250:914-921. [PubMed] |
| 10. | Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, Zhou Y, Zhou Y, Zhang Y. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e64261. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;CD005033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Sugita S, Sasaki A, Iwaki K, Uchida H, Kai S, Shibata K, Ohta M, Kitano S. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: a retrospective study. Eur J Surg Oncol. 2008;34:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Felekouras E, Petrou A, Neofytou K, Giakoustidis A, Bagenal J, Cananzi F, Pikoulis E, Mudan S. Combined ultrasonic aspiration and saline-linked radiofrequency precoagulation: a step toward bloodless liver resection without the need of liver inflow occlusion: analysis of 313 consecutive patients. World J Surg Oncol. 2014;12:357. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Cannon RM, Scoggins CR, Callender GG, Quillo A, McMasters KM, Martin RC. Financial comparison of laparoscopic versus open hepatic resection using deviation-based cost modeling. Ann Surg Oncol. 2013;20:2887-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Vanounou T, Steel JL, Nguyen KT, Tsung A, Marsh JW, Geller DA, Gamblin TC. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol. 2010;17:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Crawshaw BP, Chien HL, Augestad KM, Delaney CP. Effect of laparoscopic surgery on health care utilization and costs in patients who undergo colectomy. JAMA Surg. 2015;150:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 18. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [PubMed] |
| 19. | Vanounou T, Pratt W, Fischer JE, Vollmer CM, Callery MP. Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg. 2007;204:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Geller DA, Tsung A, Maheshwari V, Rutstein LA, Fung JJ, Marsh JW. Hepatic resection in 170 patients using saline-cooled radiofrequency coagulation. HPB (Oxford). 2005;7:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Currò G, Lazzara S, Barbera A, Cogliandolo A, Dattola A, De Marco ML, De Leo E, Rampulla V, Lazzara C, Navarra G. The Aquamantys® system as alternative for parenchymal division and hemostasis in liver resection for hepatocellular carcinoma: a preliminary study. Eur Rev Med Pharmacol Sci. 2014;18:2-5. [PubMed] |
| 22. | Kaibori M, Matsui K, Ishizaki M, Sakaguchi T, Matsushima H, Matsui Y, Kwon AH. A prospective randomized controlled trial of hemostasis with a bipolar sealer during hepatic transection for liver resection. Surgery. 2013;154:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |