Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10166
Peer-review started: July 13, 2016
First decision: August 29, 2016
Revised: September 3, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: December 14, 2016
Processing time: 156 Days and 6.9 Hours
To investigate the diagnostic performance of liver stiffness measurement (LSM) by elastography point quantification (ElastPQ) in animal models and determine the longitudinal changes in liver stiffness by ElastPQ after splenectomy at different stages of fibrosis.
Liver stiffness was measured in sixty-eight rabbits with CCl4-induced liver fibrosis at different stages and eight healthy control rabbits by ElastPQ. Liver biopsies and blood samples were obtained at scheduled time points to assess liver function and degree of fibrosis. Thirty-one rabbits with complete data that underwent splenectomy at different stages of liver fibrosis were then included for dynamic monitoring of changes in liver stiffness by ElastPQ and liver function according to blood tests.
LSM by ElastPQ was significantly correlated with histologic fibrosis stage (r = 0.85, P < 0.001). The optimal cutoff values by ElastPQ were 11.27, 14.89, and 18.21 kPa for predicting minimal fibrosis, moderate fibrosis, and cirrhosis, respectively. Longitudinal monitoring of the changes in liver stiffness by ElastPQ showed that early splenectomy (especially F1) may delay liver fibrosis progression.
ElastPQ is an available, convenient, objective and non-invasive technique for assessing liver stiffness in rabbits with CCl4-induced liver fibrosis. In addition, liver stiffness measurements using ElastPQ can dynamically monitor the changes in liver stiffness in rabbit models, and in patients, after splenectomy.
Core tip: Elastography point quantification (ElastPQ) is a non-invasive technique for assessing tissue stiffness, and was used in this study. Splenectomy is a surgical intervention for liver cirrhosis patients with hypersplenism. The aim of the current study was to evaluate the diagnostic accuracy of liver stiffness measurement by ElastPQ in animal models and determine the longitudinal changes in liver stiffness by ElastPQ after splenectomy at different stages of fibrosis. We conclude that liver stiffness measurements using ElastPQ can be used to dynamically monitor the changes in liver stiffness in rabbit models, and in patients, after splenectomy.
- Citation: Wang MJ, Ling WW, Wang H, Meng LW, Cai H, Peng B. Non-invasive evaluation of liver stiffness after splenectomy in rabbits with CCl4-induced liver fibrosis. World J Gastroenterol 2016; 22(46): 10166-10179
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10166
Liver fibrosis, which is characterized by encapsulation or replacement of injured tissue by a collagenous scar[1], represents a common pathological process in chronic liver injury of varying etiologies. Cirrhosis, which is morphologically described as abnormal liver architecture encompassing fibrous bands surrounding regenerative nodules, is the end stage of liver fibrosis and has clinical complications, including liver failure, portal hypertension, and ultimately, hepatocellular carcinoma. A growing body of clinical evidence has indicated that liver fibrosis can reverse and possibly return to normal following the development of effective treatments for chronic hepatitis infection (B and C)[2-6], autoimmune hepatitis[7], and primary biliary cirrhosis[8].
In addition, improved results on the molecular mechanisms associated with the pathogenesis of hepatic fibrosis has led to growing acceptance of liver fibrosis as a potentially reversible process[9,10]. Hepatic stellate cells (HSCs) are a worldwide research focus based on their activation and transdifferentiation to myofibroblasts, which ultimately results in liver fibrosis in response to a variety of injuries; more interestingly, previous studies have indicated that macrophages can influence the process of liver fibrosis via different mechanisms[11,12]. Circulating macrophages arise from monocytes in the bone marrow (BM)[13], and Swirski et al[14] and other researchers[15,16] have indicated that numerous monocytes in the spleen could be mobilized in the pathological state such that the spleen can be considered a monocyte reservoir. BM cell infusion can improve liver function[17] and decrease liver fibrosis[18], while splenectomy can result in liver function improvements for patients with liver cirrhosis[19-21]. Furthermore, a previous study indicated that splenectomy attenuated murine liver fibrosis when accompanied by hypersplenism[22].
On the other hand, liver biopsy is traditionally regarded as the gold standard for staging fibrosis. Nevertheless, as an invasive procedure, liver biopsy is unwelcome in patients who need repeated examination to monitor fibrosis progression. Furthermore, liver biopsy is limited by serious complications[23,24], sampling errors[25], and both inter-pathologist and intra-pathologist variability[26]. Shear wave elastography, a reliable, rapid and non-invasive technique, has been used to evaluate tissue stiffness for many years and is increasingly important in the diagnosis of liver fibrosis[27-29]. Furthermore, an acoustic radiation force impulse (ARFI) technique, elastography point quantification (ElastPQ)[30], has been developed to measure the tissue[31-34]. However, no data are available on the changes in fibrotic liver stiffness after splenectomy at different pathological stages using ElastPQ.
We took advantage of a CCl4-induced liver fibrosis model in rabbits, from which liver biopsies were obtained at scheduled time points and ElastPQ was easily performed, to evaluate the correlation between liver fibrosis histological staging and liver stiffness measured by ElastPQ before splenectomy (Experiment 1). In addition, we determined the longitudinal changes in liver stiffness using ElastPQ after splenectomy at different pathological stages (Experiment 2).
One hundred and eight male New Zealand White rabbits weighing 2000-2500 g on arrival at the laboratory were purchased from the Experimental Animal Center of West China Medical Center, Sichuan University (Chengdu, China). All rabbits were acclimatized for one week to adapt to the new environment. Daily evaluation of rabbit health status was performed for one week to ensure the animals were clinically healthy prior to the experiments. The animals were individually housed in cages under a set temperature (22 ± 1 °C) and relative humidity (45% ± 10%) with a 12-h light/12-h dark cycle. Each animal was allowed free access to a standard diet for rabbits and fresh water. The experimental procedures were approved by the Institutional Animal Ethical Committee of Sichuan University (Chengdu, China), and all animals received humane care according to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Liver fibrosis was induced by intraperitoneal injection of CCl4, as described previously[35]. Unfortunately, in a pilot experiment (10 rabbits), using the regimen reported by Zhang et al[35], a mortality rate of 60% (6 of 10 rabbits) was observed. The pilot study was stopped, and a modified method for induction of liver fibrosis was explored and eventually adopted. The injection started with 50% CCl4, which was diluted in olive oil, in doses of 0.10 mL/kg body weight twice per week for the first two weeks, which allowed the rabbits to gradually adapt to the toxic agent. Then, 50% CCl4 was given intraperitoneally in doses of 0.20 mL/kg body weight twice a week for another 18 wk in Experiment 1, and the liver injury induced by 50% CCl4 lasted for ten weeks from the first operation in Experiment 2. This method was sufficient to produce all stages of liver fibrosis. Humane endpoints were established in the modeling process according to the guidelines for assessing discomfort in experiment animals[36]. No animals died in Experiment 1.
On the same day, just before surgery and blood collection, eight rabbits were chosen at random for preoperative examinations after at least four hours of fasting. The rabbits were anesthetized with a 40 mg/kg dose of pentobarbital via ear border vein injection and were then placed in the supine position with whole abdominal skin preparation. Liver stiffness measurements were then performed in or close to the subxiphoid region by two experienced examiners via ElastPQ with a 4-cm depth and a 0.5 cm × 1.5 cm region of interest on vessel-free areas at the end-inspiration phase with an iU22 ultrasound system (Royal Philips Electronics, the Netherlands) equipped with an ElastPQ feature and two transducers, C5-1 (1-5 MHz) (used in this study) and L9-3 (3-9 MHz) (not used in this study). Both examiners were blinded to the clinical, serological, and histological data. The results are expressed in kilopascals. ElastPQ results were obtained with 10 valid measurements from each operator; a success rate of at least 60% and an interquartile range of all successful measurements less than 30% of the median values were considered reliable. The successful measurements obtained by each operator were used for inter-examiner agreement analysis, while the median measurement obtained by both operators for each rabbit were used for other analyses in the current study.
After ultrasound-based examinations, peripheral blood was collected via the ear border vein. Levels of the following parameters were determined: (1) class I biomarkers of liver fibrogenesis, including type IV collagen, and hyaluronic acid[37], were quantified using a standardized and optimized commercial radioimmunoassay kit (Haiyan Biotechnology Center, Shanghai, China) and (2) conventional liver function tests, including total bilirubin (TB), albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels (Leadman Biochemistry Co., Ltd, Beijing, China).
In Experiment 1, after 4-wk of modeling, the eight rabbits were randomly divided into two equal groups following preoperative examinations (ultrasound-based examinations and the blood test mentioned above). After disinfection, the operation began with a midline abdominal incision. In one group (Group S, splenectomy group), total splenectomy was performed by ligature of the splenic vascular pedicle with 4-0 chromic catgut; then, a 1-cm × 1-cm piece of hepatic tissue from the subxiphoid region of the liver was cut for biopsy. In another group (Group L, liver biopsy group or sham group), the same process was performed with the exception of total splenectomy. The abdominal cavity was closed after confirming that there was no active hemorrhage in all rabbits. To obtain different stages of liver fibrosis at different time intervals, the same surgical process was repeated for the remaining rabbits every two weeks until the 20th wk. Due to humane endpoints and failed liver stiffness measurements, only seven rabbits underwent surgery at the 8th, 14th, 18th, and 20th wk (Table 1). In this case, four rabbits randomly underwent splenectomy plus liver biopsy, while the remaining three underwent liver biopsy alone.
| Modeling time | Distribution of operation | Humane | Rabbits | POW 2 | POW 4 | POW 6 | POW 8 | POW 10 | Humane | Exclusion | Death | Rabbits |
| Termination1 | left2 | Termination3 | left4 | |||||||||
| 0W | / | 0 | 90 | / | / | / | / | / | / | / | / | / |
| 2W | / | 0 | 90 | / | / | / | / | / | / | / | / | |
| 4W | AAAA | 0 | 82 | AAAA | AAAA | AAA5 | AA | AA | 1 | 1 | 1 | 5 |
| BBBB | BBB | BBB | BBB | BBB | BBB | |||||||
| 6W | AAAA | 0 | 74 | AAA | AAA | AAA | AA | AA | 2 | 0 | 2 | 4 |
| BBBB | BBBB | BBB | BBB | BB | BB | |||||||
| 8W | AAAA | 2 | 64 | AAA | AA | AA | AA | AA | 2 | 0 | 1 | 4 |
| BBBB5 | BBB | BBB | BB | BB | BB | |||||||
| 10W | AAAA | 3 | 53 | AAA | AAA | AA | AA | AA | 2 | 1 | 1 | 4 |
| BBBB | BBBB | BBB | BBB5 | BB | BB | |||||||
| 12W | AAAA | 3 | 42 | AAA | AAA | AA | AA | AA | 3 | 0 | 1 | 4 |
| BBBB | BBB | BB | BB | BB | BB | |||||||
| 14W | AAAA | 2 | 32 | AAA | AA | AA | AA | A | 5 | 0 | 0 | 2 |
| BBBB5 | BBB | BBB | BB | B | B | |||||||
| 16W | AAAA | 4 | 20 | AAAA | AAA | AAA | AA | AA | 4 | 0 | 1 | 3 |
| BBBB | BBB | BB | BB | BB | B | |||||||
| 18W | AAAA | 3 | 9 | AAA | AAA | AAA | AA | AA | 4 | 0 | 0 | 3 |
| BBBB5 | BBB | BB | BB | B | B | |||||||
| 20W | AAAA | 2 | 0 | AA | AA | AA | AA | A | 4 | 1 | 0 | 2 |
| BBB | BBB | BBB | BB | BB5 | B | |||||||
| Total | 68 | 19 | / | / | / | / | / | / | 27 | 3 | 7 | 31 |
In Experiment 2, after the first operation in each rabbit, a 1 cm × 1 cm piece of hepatic tissue was cut to dynamically monitor the changes in histological features according to the aforementioned ultrasound-based examinations and blood tests every two weeks for 10 wk. To avoid adhesions, chitosan (0.5 mL/surgery) was used. However, due to the increase in operation times, it was difficult to acquire liver tissue along the original midline incision. In this case, a left or right subcostal incision was needed. In Experiments 1 and 2, all animals were given penicillin intramuscularly at a dose of 40 U/rabbit to prevent infection during surgery, which was repeated once daily for a further two days. To reduce bias, only the hepatic tissue obtained in or very close to the subxiphoid region was included for analysis. In addition, due to humane endpoints and failed liver stiffness measurement (LSM) and death during the surgical procedure, only thirty-one rabbits with complete experimental data were available for analysis after the 10-wk surveillance period (Table 1).
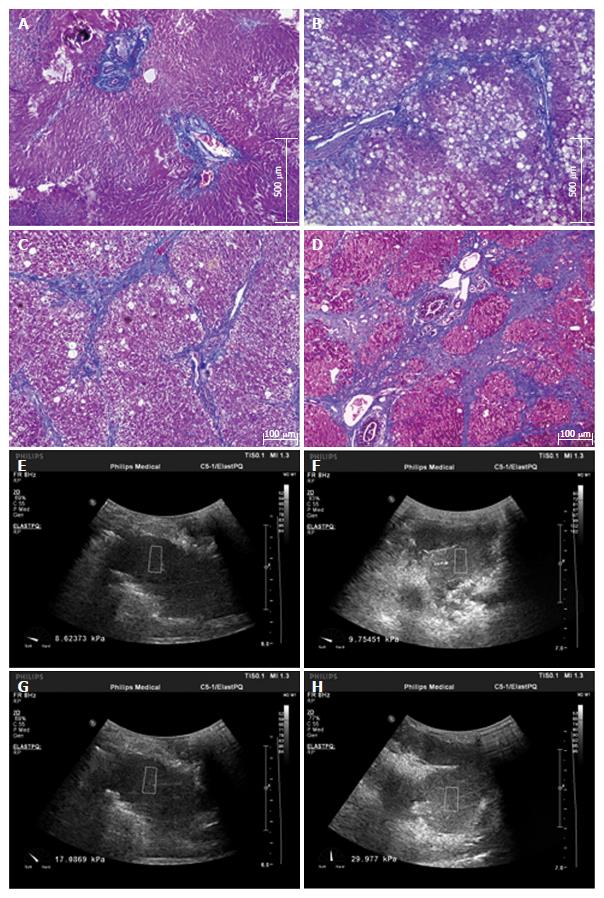
Liver biopsy samples taken at the time of the operation were fixed in formalin and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin and Masson trichrome. A biopsy sample with a minimum of 5 portal tracts was required for diagnosis. Two doctors with significant experience, who were blinded to all animal characteristics, were responsible for evaluating liver fibrosis, which was staged on a scale of 0-4 according to METAVIR[38] (F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and a few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis). The fibrosis stage was independently assessed on each histological section by both doctors. In the case of discrepancies, histological sections were simultaneously reviewed again by the two doctors to reach a final consensus. Typical liver fibrosis stages (F1-F4) are illustrated in Figure 1.
The quadratic-weighted k coefficient of Cohen was used to assess the consistency of the two doctors who were in charge of the pathological examinations, while the ICC (interclass correlation coefficient) was used to evaluate the agreement between the two examiners who performed the liver stiffness measurement via ElastPQ.
The median LSM obtained by both operators for each ElastPQ was calculated and used for further analyses. Because the LSM values were not normally distributed, the Kruskal-Wallis nonparametric analysis of variance test was used to compare these values with the categories of the consensus fibrosis stage. Correlations between the LSM and histologic fibrosis stage were further analyzed using Spearman correlation coefficients. The diagnostic performance of ElastPQ and serum fibrosis markers, including type IV collagen and hyaluronic acid, was assessed using receiver operating characteristic curves (ROC). The optimal cutoff values for predicting different fibrosis stages were chosen to maximize the sum of the sensitivity and specificity, and the corresponding positive predictive values (PPVs) and negative predictive values (NPVs) were computed. The AUC (area under ROC) values for the different diagnostic criteria for the same data were compared using the nonparametric DeLong test.
Quantitative data were presented as the mean ± SD or median (quartile), while categorical data were expressed as the number of cases with/without percentage. Statistical analyses also included the nonparametric Mann-Whitney U and Student’s t tests.
All statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, United States) for Windows and significance was set at a P value < 0.05.
The experimental details are presented in Table 1. In addition to eight controls, ninety rabbits were planned for inclusion. As mentioned in the Materials and methods section due to humane termination (n = 19) and failed LSM (n = 3) during Experiment 1, information on sixty-eight rabbits was available for analysis. Similarly, 10 wk after splenectomy or sham operation, complete data for only thirty-one rabbits were available for comparable analyses.
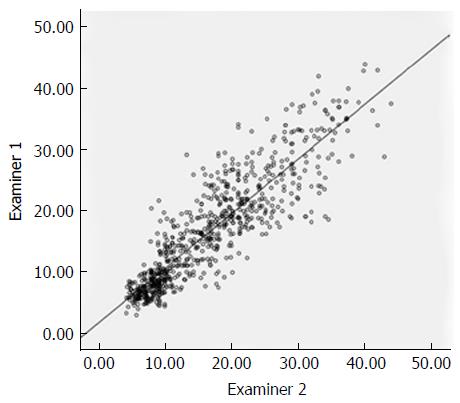
The two doctors responsible for pathological diagnosis were initially in agreement for 197 (85.3%) of the 231 liver samples (231 = 76 + 5 × 31) (k coefficient = 0.792, P < 0.01), and 100% agreement was reached after final reviews. The ElastPQ results identified by the two examiners were strongly correlated with an ICC value of 0.888, and are illustrated in Figure 2.
After 20 wk of medication, all fibrosis stages confirmed by pathological examinations were observed. As shown in Table 2, F1 was diagnosed in 11 cases (14.5%), F2 in 16 (21.1%), F3 in 16 (21.1%), and F4 in 25 (32.9%), and eight healthy rabbits (F0, n = 8, 10.4%) were included as controls. Table 3 includes the basic information on rabbits with different stages of fibrosis. Except for body weight and TB, AST, ALT, and albumin levels, a trend for a stepwise increase in liver fibrosis progression was found in the parameters, including type IV collagen (F0: 200.8 ± 131.5 μg/L, F1: 427.1 ± 226.2 μg/L, F2: 683.4 ± 332.5 μg/L, F3: 1161.4 ± 482.5 μg/L, and F4: 1292.0 ± 689.7 μg/L), hyaluronic acid (F0: 225.6 ± 117.1 μg/L, F1: 475.7 ± 296.4 μg/L, F2: 676.2 ± 274.8 μg/L, F3: 724.0 ± 264.5 μg/L, and F4: 1182.3 ± 1091.3 μg/L), and LSM [F0: 7.88 kPa (6.60-8.46 kPa), F1: 8.46 kPa (6.22-10.35 kPa), F2: 10.89 kPa (8.09-14.46 kPa), F3: 18.62 kPa (16.03-21.16 kPa), and F4: 25.10 kPa (20.28-30.95 kPa)].
| F0 | F1 | F2 | F3 | F4 | |
| 0 wk | 98 | / | / | / | / |
| 2 wk | / | / | / | / | |
| 4 wk | 5 | 3 | 0 | 0 | |
| 6 wk | 3 | 4 | 1 | 0 | |
| 8 wk | 2 | 4 | 1 | 0 | |
| 10 wk | 1 | 2 | 3 | 2 | |
| 12 wk | 0 | 2 | 1 | 5 | |
| 14 wk | 0 | 1 | 2 | 4 | |
| 16 wk | 0 | 0 | 4 | 4 | |
| 18 wk | 0 | 0 | 3 | 4 | |
| 20 wk | 0 | 0 | 1 | 6 | |
| Total | 8 | 11 | 16 | 16 | 25 |
| Parameters | F0 (n = 8) | F1 (n = 11) | F2 (n = 16) | F3 (n = 16) | F4 (n = 25) |
| Body weight (kg) | 2.34 ± 0.24 | 2.28 ± 0.27 | 2.39 ± 0.14 | 2.30 ± 0.38 | 2.24 ± 0.33 |
| Type IV collagen (μg/L) | 200.8 ± 131.5 | 427.1 ± 226.2 | 683.4 ± 332.5 | 1161.4 ± 482.5 | 1292.0 ± 689.7 |
| Hyaluronic acid (μg/L) | 225.6 ± 117.1 | 475.7 ± 296.4 | 676.2 ± 274.8 | 724.0 ± 264.5 | 1182.3 ± 1091.3 |
| TB (μmol/L) | 0.98 ± 0.53 | 1.91 ± 0.63 | 2.20 ± 0.85 | 1.81 ± 0.82 | 1.64 ± 0.91 |
| AST (IU/L) | 26.8 ± 14.7 | 345.0 ± 295.9 | 449.2 ± 304.7 | 666.4 ± 428.3 | 616.1 ± 609.2 |
| ALT (IU/L) | 14.0 ± 3.7 | 254.7 ± 194.0 | 301.5 ± 210.7 | 456.3 ± 316.0 | 486.5 ± 295.8 |
| Albumin (g/L) | 42.7 ± 4.9 | 40.4 ± 4.6 | 36.5 ± 4.3 | 32.3 ± 6.4 | 35.3 ± 5.9 |
| LSM (kPa) | 7.88 (6.60-8.46) | 8.46 (6.22-10.35) | 10.89 (8.09-14.46) | 18.62 (16.03-21.16) | 25.10 (20.28-30.95) |
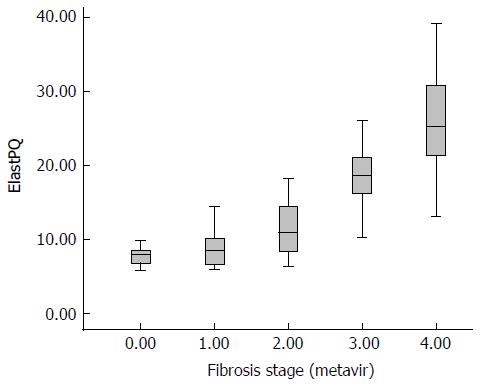
The median liver stiffness measured with ElastPQ in the eight controls was 7.88 kPa (6.60-8.46 kPa). The liver stiffness measured in the rabbits with fibrosis ranged from 5.86 kPa to 39.12 kPa. Based on the different fibrosis stages, the median liver stiffness values in the animals with F1 to F4 were 8.46 kPa (6.22-10.35 kPa), 10.89 kPa (8.09-14.46 kPa), 18.62 kPa (16.03-21.16 kPa), and 25.10 kPa (20.28-30.95 kPa), respectively, indicating a gradual increase in fibrosis progression, which is shown in Figure 3, with a Spearman correlation coefficient of 0.85 (P < 0.001). Given that the distributions of ElastPQ results for F0 and F1 were comparable and only eight F0 rabbits were included, F0 and F1 rabbits were combined as a single group for further analyses. Significant differences in the LSM by ElastPQ between each fibrosis stage were observed (F0-1 vs F2, P < 0.01; F2 vs F3, P < 0.01; and F3 vs F4, P < 0.01).
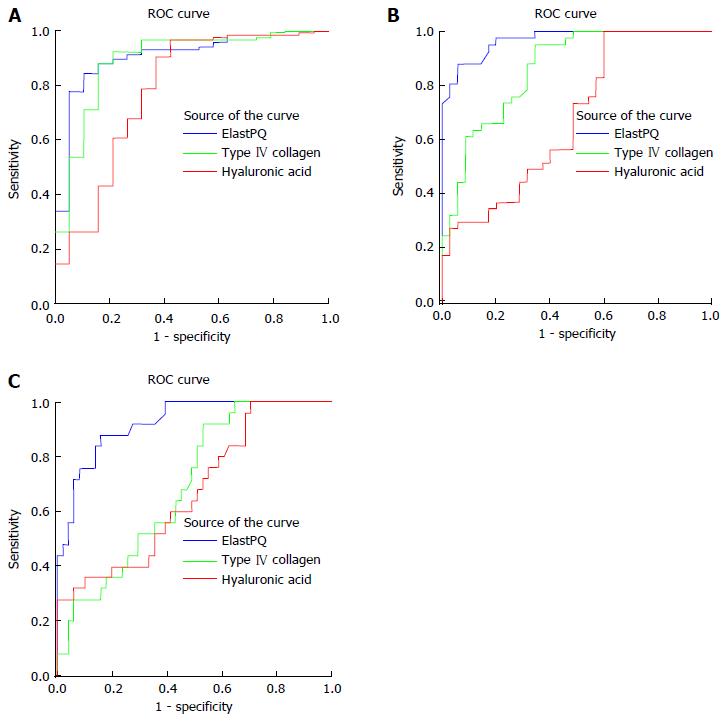
ROC curves of ElastPQ, hyaluronic acid, and type IV collagen for predicting minimal fibrosis (F0-F1 vs F2-F4), moderate fibrosis (F0-F2 vs F3-F4), and cirrhosis (F0-F3 vs F4) are shown in Figure 4A-C. The AUROC (area under ROC) of ElastPQ for predicting minimal fibrosis (0.931, 95%CI: 0.849-0.977) was comparable to those of hyaluronic acid (0.807, 95%CI: 0.700-0.889) and type IV collagen (0.919, 95%CI: 0.833-0.969), while the ElastPQ for predicting moderate fibrosis and cirrhosis (0.969, 95%CI: 0.901-0.995; 0.925, 95%CI: 0.841-0.973) was significantly superior to hyaluronic acid (0.677, 95%CI: 0.560-0.780; 0.670, 95%CI: 0.553-0.774) and type IV collagen (0.861, 95%CI: 0.762-0.930; 0.695, 95%CI: 0.578-0.795), which is summarized in Table 4. The ElastPQ critical values for differentiating fibrosis stages were subsequently confirmed by the ROC, and the corresponding specificities, sensitivities, PPVs, and NPVs are listed in Table 5.
| Parameter | F0-F1 vs F2-F4 | F0-F2 vs F3-F4 | F0-F3 vs F4 |
| AUROC | 0.931 (0.849-0.977) | 0.969 (0.901-0.995) | 0.925 (0.841-0.973) |
| Optimal cutoff value | 11.27 | 14.89 | 18.21 |
| Sensitivity (%) | 82.5 (70.1-91.3) | 87.8 (73.8-95.9) | 88.0 (68.8-97.5) |
| Specificity (%) | 94.7 (74.0-99.9) | 94.3 (80.8-99.3) | 84.3 (71.4-93.0) |
| PPV (%) | 97.9 (88.9-99.9) | 94.7 (82.3-99.4) | 73.3 (54.1-87.7) |
| NPV (%) | 64.3 (44.1-81.4) | 86.8 (71.9-95.6) | 93.5 (82.1-98.6) |
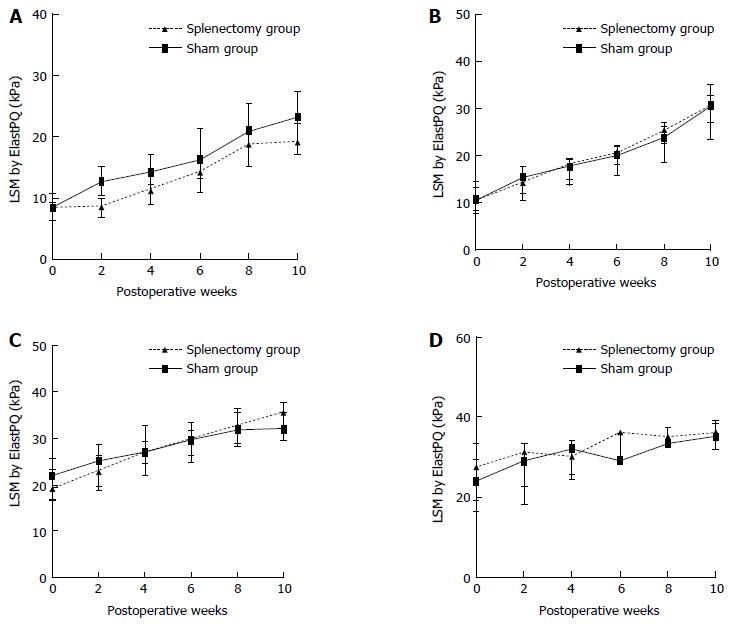
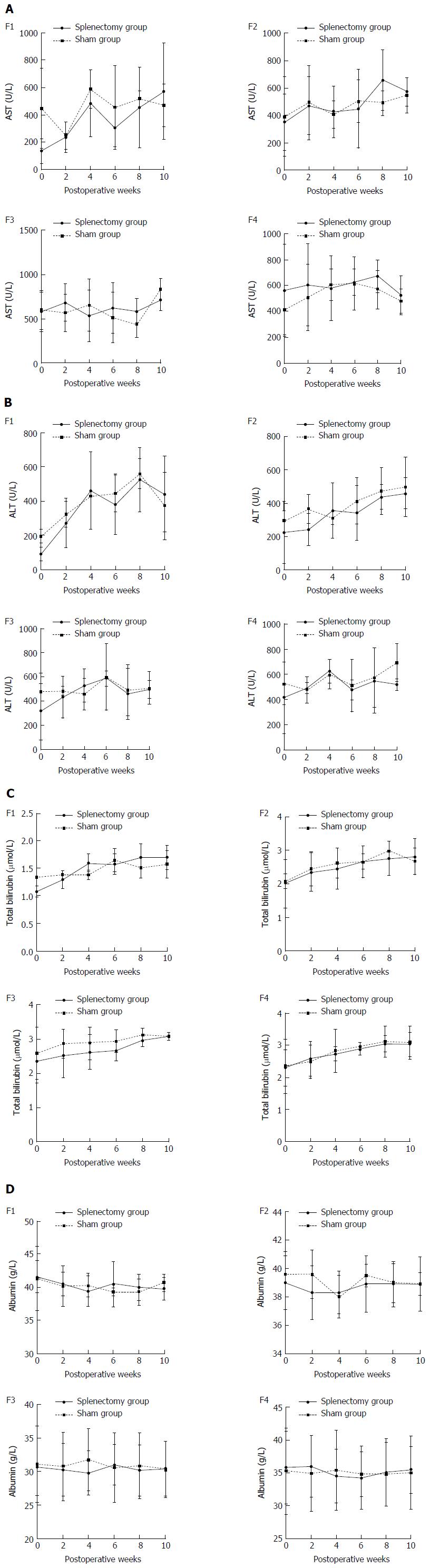
The longitudinal ElastPQ and laboratory data for rabbits with different stages of fibrosis after splenectomy and sham operation in Experiment 2 are shown in Tables 6 and 7. For the nine rabbits with F1 liver fibrosis (five in the splenectomy group vs four in the sham group), the increase in ElastPQ values was delayed in the splenectomy group compared with that in the sham group during the following the operations (Figure 5A), while the changes in other laboratory parameters, including AST, ALT, albumin, and TB levels, indicated otherwise (Figure 6, Table 7). For the rabbits with F2, F3, and F4 liver fibrosis, no favorable change in parameters, including the ElastPQ, AST, ALT, albumin, and TB levels, was detected in the splenectomy group compared with the sham group over the 10-wk period after the operations (Figure 5B-D, Figure 6 and Table 7).
| POW 0 | POW 2 | POW 4 | POW 6 | POW 8 | POW 10 | ||
| F1 | Group S (n = 5) | 8.46 (6.63-8.50) | 8.74 (6.97-8.91) | 11.34 (9.13-12.89) | 14.32 (11.45-15.45) | 18.89 (16.43-19.23) | 19.24 (17.78-21.09) |
| Group L (n = 4) | 8.59 (7.12-10.13) | 12.79 (11.01-14.62) | 14.35 (12.24-16.70) | 16.30 (13.25-20.07) | 21.01 (16.11-25.45) | 23.34 (20.66-26.15) | |
| F2 | Group S (n = 4) | 10.81 (8.34-13.48) | 14.45 (12.53-15.70) | 18.28 (16.72-18.56) | 20.73 (18.09-22.26) | 25.50 (23.10-25.89) | 30.79 (27.29-33.31) |
| Group L (n = 4) | 10.50 (9.31-11.96) | 15.39 (13.55-16.92) | 17.90 (16.01-19.27) | 20.25 (18.92-21.51) | 23.89 (23.15-25.24) | 30.60 (28.54-32.29) | |
| F3 | Group S (n = 4) | 19.19 (17.77-21.25) | 22.90 (20.70-25.17) | 26.99 (25.13-28.88) | 29.98 (26.14-33.23) | 32.38 (29.06-35.89) | 35.79 (33.79-37.31) |
| Group L (n = 4) | 22.08 (18.24-25.35) | 25.17 (21.79-27.59) | 27.21 (24.38-30.23) | 29.72 (28.27-30.70) | 31.89 (29.91-33.98) | 32.22 (30.70-34.71) | |
| F4 | Group S (n = 3) | 27.56 (23.40-28.34) | 31.23 (26.84-32.22) | 30.12 (27.89-32.12) | 36.12 (32.39-36.34) | 35.10 (34.12-36.27) | 36.12 (34.06-37.28) |
| Group L (n = 3) | 23.98 (20.21-28.66) | 29.12 (23.68-31.19) | 32.00 (28.17-33.00) | 29.00 (28.56-32.56) | 33.43 (33.32-35.32) | 35.30 (35.25-37.21) |
| POW 0 | POW 2 | POW 4 | POW 6 | POW 8 | POW 10 | ||
| AST (IU/L) | |||||||
| F1 | Group S (n = 5) | 138.0 ± 87.3 | 236.7 ± 110.6 | 484.3 ± 244.2 | 307.3 ± 138.9 | 454.0 ± 291.3 | 574.3 ± 350.6 |
| Group L (n = 4) | 447.0 ± 292.7 | 245.5 ± 98.3 | 589.5 ± 140.7 | 455.0 ± 309.7 | 517.5 ± 55.9 | 470.0 ± 155.6 | |
| F2 | Group S (n = 4) | 353.6 ± 208.4 | 473.4 ± 212.1 | 426.0 ± 186.4 | 448.6 ± 286.4 | 658.2 ± 221.9 | 575.6 ± 104.4 |
| Group L (n = 4) | 392.0 ± 290.5 | 493.5 ± 269.0 | 407.5 ± 100.4 | 506.3 ± 156.8 | 494.5 ± 88.8 | 550.0 ± 125.6 | |
| F3 | Group S (n = 4) | 578.3 ± 219.5 | 681.3 ± 208.9 | 534.0 ± 292.2 | 622.7 ± 286.2 | 584.7 ± 144.5 | 713.3 ± 122.8 |
| Group L (n = 4) | 598.8 ± 219.5 | 569.8 ± 208.9 | 651.5 ± 292.2 | 513.0 ± 286.2 | 435.5 ± 144.5 | 831.8 ± 122.8 | |
| F4 | Group S (n = 3) | 562.2 ± 356.6 | 604.2 ± 314.3 | 578.4 ± 247.7 | 623.8 ± 101.1 | 672.6 ± 122.6 | 525.6 ± 153.2 |
| Group L (n = 3) | 412.6 ± 190.9 | 508.7 ± 253.8 | 604.0 ± 121.8 | 616.7 ± 203.1 | 571.0 ± 145.7 | 480.3 ± 91.4 | |
| ALT (IU/L) | |||||||
| F1 | Group S (n = 5) | 88.7 ± 39.2 | 270.3 ± 144.9 | 460.7 ± 227.5 | 379.0 ± 179.2 | 526.7 ± 185.8 | 440.7 ± 220.4 |
| Group L (n = 4) | 195.0 ± 39.6 | 322.0 ± 76.4 | 430.5 ± 2.1 | 445.0 ± 108.9 | 560.5 ± 87.0 | 372.0 ± 198.0 | |
| F2 | Group S (n = 4) | 224.6 ± 187.8 | 240.8 ± 96.7 | 355.8 ± 165.8 | 342.6 ± 165.1 | 436.8 ± 74.9 | 458.0 ± 92.4 |
| Group L (n = 4) | 291.8 ± 65.9 | 364.3 ± 86.5 | 310.3 ± 35.8 | 414.5 ± 136.8 | 473.3 ± 140.5 | 498.3 ± 174.0 | |
| F3 | Group S (n = 4) | 315.7 ± 232.7 | 434.0 ± 171.8 | 528.3 ± 137.7 | 588.0 ± 61.6 | 461.3 ± 208.2 | 497.3 ± 73.6 |
| Group L (n = 4) | 477.8 ± 151.7 | 484.0 ± 34.4 | 459.3 ± 132.5 | 601.0 ± 274.9 | 488.8 ± 210.2 | 507.0 ± 133.2 | |
| F4 | Group S (n = 3) | 416.8 ± 286.2 | 490.6 ± 43.3 | 626.2 ± 93.0 | 478.0 ± 83.3 | 550.6 ± 252.5 | 519.2 ± 44.9 |
| Group L (n = 3) | 529.0 ± 167.0 | 475.8 ± 106.7 | 600.4 ± 116.3 | 512.6 ± 207.8 | 576.8 ± 233.1 | 695.4 ± 150.6 | |
| Albumin (g/L) | |||||||
| F1 | Group S (n = 5) | 41.6 ± 2.4 | 40.5 ± 1.8 | 39.4 ± 2.3 | 40.5 ± 3.4 | 40.0 ± 2.0 | 39.8 ± 1.7 |
| Group L (n = 4) | 41.3 ± 4.9 | 40.2 ± 3.0 | 40.3 ± 1.8 | 39.3 ± 0.6 | 39.3 ± 2.0 | 40.7 ± 1.3 | |
| F2 | Group S (n = 4) | 39.0 ± 1.9 | 38.3 ± 1.9 | 38.3 ± 1.5 | 38.9 ± 2.0 | 38.9 ± 1.6 | 38.9 ± 0.8 |
| Group L (n = 4) | 39.6 ± 1.6 | 39.6 ± 1.7 | 38.0 ± 1.5 | 39.5 ± 0.8 | 39.0 ± 1.4 | 38.9 ± 1.9 | |
| F3 | Group S (n = 4) | 30.7 ± 4.3 | 30.3 ± 3.9 | 29.8 ± 3.3 | 31.0 ± 3.0 | 30.2 ± 3.8 | 30.5 ± 4.1 |
| Group L (n = 4) | 31.1 ± 5.7 | 30.8 ± 5.1 | 31.8 ± 4.6 | 30.6 ± 5.2 | 30.9 ± 4.9 | 30.3 ± 4.2 | |
| F4 | Group S (n = 3) | 35.8 ± 5.6 | 36.0 ± 4.7 | 34.5 ± 4.1 | 34.2 ± 4.8 | 35.1 ± 5.1 | 35.5 ± 3.6 |
| Group L (n = 3) | 35.3 ± 6.6 | 34.9 ± 5.8 | 35.4 ± 6.1 | 34.8 ± 3.4 | 34.8 ± 4.9 | 35.0 ± 5.6 | |
| TB (μmol/L) | |||||||
| F1 | Group S (n = 5) | 1.08 ± 0.10 | 1.30 ± 0.16 | 1.60 ± 0.16 | 1.58 ± 0.18 | 1.71 ± 0.23 | 1.70 ± 0.22 |
| Group L (n = 4) | 1.34 ± 0.16 | 1.38 ± 0.08 | 1.39 ± 0.08 | 1.65 ± 0.20 | 1.51 ± 0.19 | 1.58 ± 0.25 | |
| F2 | Group S (n = 4) | 2.01 ± 0.72 | 2.35 ± 0.58 | 2.45 ± 0.61 | 2.65 ± 0.47 | 2.76 ± 0.51 | 2.81 ± 0.54 |
| Group L (n = 4) | 2.08 ± 0.23 | 2.45 ± 0.51 | 2.62 ± 0.45 | 2.67 ± 0.23 | 2.99 ± 0.15 | 2.68 ± 0.40 | |
| F3 | Group S (n = 4) | 2.36 ± 0.64 | 2.52 ± 0.64 | 2.62 ± 0.51 | 2.66 ± 0.29 | 2.97 ± 0.19 | 3.09 ± 0.10 |
| Group L (n = 4) | 2.59 ± 0.76 | 2.87 ± 0.42 | 2.88 ± 0.48 | 2.94 ± 0.33 | 3.13 ± 0.20 | 3.09 ± 0.12 | |
| F4 | Group S (n = 3) | 2.30 ± 0.56 | 2.58 ± 0.54 | 2.73 ± 0.22 | 2.88 ± 0.18 | 3.05 ± 0.26 | 3.03 ± 0.38 |
| Group L (n = 3) | 2.35 ± 0.84 | 2.49 ± 0.52 | 2.82 ± 0.67 | 2.96 ± 0.13 | 3.11 ± 0.48 | 3.08 ± 0.51 | |
In the current study, after four to twenty weeks of fibrosis induction, the LSM increased from 7.88 kPa to 5.86-39.12 kPa in all rabbits with different proven fibrosis stages. Two specific serum markers of liver fibrogenesis (type IV collagen and hyaluronic acid) were selected to reflect the progression of CCl4-induced liver fibrosis, and both markers showed a step-wise correlation with liver fibrosis stages compared to the LSM via ElastPQ, reinforcing that ElastPQ can reflect the severity of liver fibrosis. However, a comparison of the baseline values of liver stiffness in different studies cannot be performed mainly due to variations in the modeling methods and species.
Although there was a significant increase in the LSM via ElastPQ with increased fibrosis stage, there was a degree of overlap between consecutive stages. In this study, for F0-F2 liver fibrosis categories, the LSM was 7.88 (6.60-8.46), 8.46 (6.22-10.35), and 10.89 (8.09-14.46), respectively, which may have been due to an insufficient number of animals with F0-F2. A similar concern was reported in a previous study on the ARFI for assessing liver fibrosis[39]. The ElastPQ cutoff values for minimal fibrosis (F0-F1 vs F2-F4), moderate fibrosis (F0-F2 vs F3-F4), and cirrhosis (F0-F3 vs F4) were defined. As shown in Table 5, the ElastPQ cutoff values for predicting different stages of fibrosis can be clearly distinguished, which may be due to the relatively uniform distribution of rabbits with different stages.
The areas under the ROC curves were compared for ElastPQ, hyaluronic acid and type IV collagen. The ElastPQ prediction of minimal fibrosis was comparable to that for hyaluronic acid and type IV collagen, while the ElastPQ prediction of moderate fibrosis and cirrhosis was significantly superior to hyaluronic acid. This outcome demonstrates that this non-invasive technique could have clinical utility.
With continuing basic research, the concept of liver fibrosis has changed from static and progressive to dynamic and bidirectional, especially when the causes of liver damage have been removed. In addition, because there is a significant correlation between the ElastPQ values and liver fibrosis stages, it is theoretically possible to use ElastPQ to non-invasively assess the effect of anti-fibrosis treatments. Indeed, previous studies have reported on the clinical application of TE for dynamically monitoring fibrosis regression during antiviral treatment in chronic hepatitis B and C patients, indicating that the TE values seem to decrease during antiviral therapy[40,41].
Although splenectomy is performed for patients with hypersplenism in some institutions[21,42], hypersplenism in most patients should be considered a laboratory abnormality that does not require treatment or further consideration[43]. However, a previous well-designed study indicated that splenectomy attenuated murine liver fibrosis[22]. Therefore, splenectomy remains controversial for patients with hypersplenism. In the present study, splenectomy was only used to group rabbits and then determine whether splenectomy at different liver fibrosis stages will delay or reverse the progression of liver fibrosis. A previous study indicated that the spleen plays an important regulatory role in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet[44]. In this study, a trend suggesting that splenectomy can delay the progression of early liver fibrosis (especially F1) was detected. A previous study using a rat liver fibrosis model indicated that spleen-derived TGF-β1 is involved in the development of liver fibrosis such that decreasing the TGF-β1 level by splenectomy could inhibit hepatic stellate cell activation and then improve liver fibrosis[45]. However, in the present study, splenectomy did not seem to improve late liver fibrosis (especially types F3 and F4); therefore, there may be another potential mechanism for improving early liver fibrosis following splenectomy.
Although activated HSCs have a great impact on liver fibrogenesis, recent studies have suggested that monocytes and their progeny macrophages are also responsible for liver fibrosis[46,47]. Based on a study of monocytes derived from BM[13] as well as studies by Swirski et al[14] and other researchers[15,16], there are numerous monocytes in the spleen that could be mobilized in pathological states. As a result, the spleen can be considered a monocyte reservoir. An interesting previous study demonstrated that the spleen is a site for storing and rapidly deploying monocytes involved in inflammation regulation[14]. Therefore, performing splenectomy in the early stages of liver fibrosis would block the rapid deployment of monocytes to the liver, which may alleviate the inflammatory reaction and delay liver fibrosis. In contrast, performing splenectomy in the late stage would not help to postpone liver fibrosis, which may be explained by the hypothesis that during the late stage, monocytes from BM play a predominant role in liver fibrosis. However, it should be further determined whether the diversity of monocyte origin influences the different stages of liver fibrosis.
In summary, ElastPQ is an available, convenient, objective and non-invasive technique for assessing liver stiffness in rabbits with CCl4-induced liver fibrosis, and paves the way for its clinical application. Additionally, liver stiffness measurements with ElastPQ can dynamically monitor the changes in liver stiffness in rabbit models, or patients, after splenectomy. However, the underlying mechanism by which early splenectomy can decelerate liver fibrosis should be further studied.
Elastography point quantification (ElastPQ) is a non-invasive technique for assessing tissue stiffness and was used in this study. Splenectomy is a surgical intervention for liver cirrhosis patients with hypersplenism. However, no evidence of changes in fibrotic liver stiffness after splenectomy at different pathological stages using ElastPQ has previously been available.
Liver biopsy is the reference standard for staging fibrosis. Nevertheless, because of its invasive nature, liver biopsy is difficult to perform in patients who require repeated examination to monitor liver fibrosis progression. An acoustic radiation force impulse (ARFI) technique, ElastPQ, has been developed for measuring tissue stiffness. In this study, the author used a liver fibrosis animal model to demonstrate that ElastPQ is an available, convenient, objective and non-invasive technique for assessing liver stiffness in rabbits with CCl4-induced liver fibrosis. In addition, the changes in liver stiffness in rabbit models, or patients, after splenectomy can be dynamically monitored by ElastPQ.
This is the first animal experiment to confirm that liver stiffness measurements with ElastPQ could be used to dynamically monitor the changes in liver stiffness. The results provide good news for liver fibrosis patients who are in need of long-term follow-up.
Based on this study, ElastPQ could dynamically monitor the changes in liver stiffness after interventions.
ElastPQ is an ARFI technique that can non-invasively measure tissue stiffness.
This is a well-supported paper presenting a study on utilizing CCl4-induced liver fibrosis to evaluate liver stiffness measurement approaches.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bubnov RV, Ferraioli G, Gorrell MD S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Zhang FF
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Papatheodoridis GV, Petraki K, Cholongitas E, Kanta E, Ketikoglou I, Manesis EK. Impact of interferon-alpha therapy on liver fibrosis progression in patients with HBeAg-negative chronic hepatitis B. J Viral Hepat. 2005;12:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [PubMed] |
| 5. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399-403. [PubMed] |
| 7. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Kaplan MM, DeLellis RA, Wolfe HJ. Sustained biochemical and histologic remission of primary biliary cirrhosis in response to medical treatment. Ann Intern Med. 1997;126:682-688. [PubMed] |
| 9. | Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39 Suppl 1:S60-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 10. | Atta HM. Reversibility and heritability of liver fibrosis: Implications for research and therapy. World J Gastroenterol. 2015;21:5138-5148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Rantakari P, Patten DA, Valtonen J, Karikoski M, Gerke H, Dawes H, Laurila J, Ohlmeier S, Elima K, Hübscher SG. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci USA. 2016;113:9298-9303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Chu PS, Nakamoto N, Ebinuma H, Usui S, Saeki K, Matsumoto A, Mikami Y, Sugiyama K, Tomita K, Kanai T. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology. 2013;58:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2249] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 14. | Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1732] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 15. | Venosa A, Malaviya R, Gow AJ, Hall L, Laskin JD, Laskin DL. Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1487-L1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Kisseleva T, Brenner DA. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J Hepatol. 2012;56:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. Performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc. 2000;14:127-130. [PubMed] |
| 20. | Ushitora Y, Tashiro H, Takahashi S, Amano H, Oshita A, Kobayashi T, Chayama K, Ohdan H. Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function. Dig Surg. 2011;28:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Yamamoto N, Okano K, Oshima M, Akamoto S, Fujiwara M, Tani J, Miyoshi H, Yoneyama H, Masaki T, Suzuki Y. Laparoscopic splenectomy for patients with liver cirrhosis: Improvement of liver function in patients with Child-Pugh class B. Surgery. 2015;158:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yada A, Iimuro Y, Uyama N, Uda Y, Okada T, Fujimoto J. Splenectomy attenuates murine liver fibrosis with hypersplenism stimulating hepatic accumulation of Ly-6C(lo) macrophages. J Hepatol. 2015;63:905-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Procopet B, Bureau C, Métivier S, Selves J, Robic MA, Christol C, Grigorescu M, Vinel JP, Péron JM. Tolerance of liver biopsy in a tertiary care center: comparison of the percutaneous and the transvenous route in 143 prospectively followed patients. Eur J Gastroenterol Hepatol. 2012;24:1209-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 25. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1399] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 26. | Rousselet MC, Michalak S, Dupré F, Croué A, Bedossa P, Saint-André JP, Calès P. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology. 2005;41:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 411] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 27. | Ozturker C, Karagoz E, Mutlu H. Noninvasive Evaluation of Liver Fibrosis by Using Two-dimensional Shear-Wave Elastography. Radiology. 2016;280:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Kazemirad S, Zhang E, Nguyen BN, Bodson-Clermont P, Destrempes F, Trudel D, Cloutier G, Tang A. Detection of Steatohepatitis in a Rat Model by Using Spectroscopic Shear-Wave US Elastography. Radiology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Jansen C, Bogs C, Verlinden W, Thiele M, Möller P, Görtzen J, Lehmann J, Vanwolleghem T, Vonghia L, Praktiknjo M. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: A prospective multicentre study. Liver Int. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 30. | Xie H, Shamdasani V, Fernandez AT, Peterson R, Lachman M, Shi Y, Robert JL, Urban M, Chen S, Greenleaf J. Shear wave Dispersion Ultrasound Vibrometry (SDUV) on an ultrasound system: In vivo measurement of liver viscoelasticity in healthy animals. in Ultrasonics Symposium (IUS), 2010 IEEE. 2010. . |
| 31. | Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, Garra B, Myers RP, Wilson SR, Rubens D. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 434] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 32. | Ma JJ, Ding H, Mao F, Sun HC, Xu C, Wang WP. Assessment of liver fibrosis with elastography point quantification technique in chronic hepatitis B virus patients: a comparison with liver pathological results. J Gastroenterol Hepatol. 2014;29:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, Filice C. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Duan YY, Yin JK, Cui JH, Zhang Y, Cao TS. Grey scale enhancement by a new self-made contrast agent in early cirrhotic stage of rabbit liver. BMC Gastroenterol. 2007;7:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431-436. [PubMed] |
| 37. | Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of hepatic fibrosis, fibrogenesis and genetic pre-disposition pending between fiction and reality. J Cell Mol Med. 2007;11:1031-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 39. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 40. | Enomoto M, Mori M, Ogawa T, Fujii H, Kobayashi S, Iwai S, Morikawa H, Tamori A, Sakaguchi H, Sawada A. Usefulness of transient elastography for assessment of liver fibrosis in chronic hepatitis B: Regression of liver stiffness during entecavir therapy. Hepatol Res. 2010;40:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Arima Y, Kawabe N, Hashimoto S, Harata M, Nitta Y, Murao M, Nakano T, Shimazaki H, Kobayashi K, Ichino N. Reduction of liver stiffness by interferon treatment in the patients with chronic hepatitis C. Hepatol Res. 2010;40:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Yu H, Guo S, Wang L, Dong Y, Tian G, Mu S, Zhang H, Li D, Zhao S. Laparoscopic Splenectomy and Esophagogastric Devascularization for Liver Cirrhosis and Portal Hypertension Is a Safe, Effective, and Minimally Invasive Operation. J Laparoendosc Adv Surg Tech A. 2016;26:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Boyer TD, Habib S. Big spleens and hypersplenism: fix it or forget it? Liver Int. 2015;35:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Oishi T, Terai S, Iwamoto T, Takami T, Yamamoto N, Sakaida I. Splenectomy reduces fibrosis and preneoplastic lesions with increased triglycerides and essential fatty acids in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Hepatol Res. 2011;41:463-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Akahoshi T, Hashizume M, Tanoue K, Shimabukuro R, Gotoh N, Tomikawa M, Sugimachi K. Role of the spleen in liver fibrosis in rats may be mediated by transforming growth factor beta-1. J Gastroenterol Hepatol. 2002;17:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 813] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 47. | Baeck C, Wei X, Bartneck M, Fech V, Heymann F, Gassler N, Hittatiya K, Eulberg D, Luedde T, Trautwein C. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |