Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10140
Peer-review started: July 2, 2016
First decision: October 11, 2016
Revised: October 24, 2016
Accepted: November 14, 2016
Article in press: November 16, 2016
Published online: December 14, 2016
Processing time: 165 Days and 4.4 Hours
To explore the relationship between colonic secretory function and colonic motility.
Using a rat model chronically implanted with intracerebroventricular (ICV) and cecal catheters, we validated the correlation between colonic secretion and colonic motor functions, as well as the role of ICV injection volume.
Compared to saline controls (5 μL/rat), ICV acyl ghrelin at 1 nmol/5 μL enhanced the total fecal weight, accelerated the colonic transit time, and increased the fecal pellet output during the first hour post-injection, while ICV des-acyl ghrelin at 1 nmol/5 μL only accelerated the colonic transit time. These stimulatory effects on colonic motility and/or secretion from acyl ghrelin and des-acyl ghrelin disappeared when the ICV injection volume increased to 10 μL compared with saline controls (10 μL/rat). Additionally, the ICV injection of 10 μL of saline significantly shortened the colonic transit time compared with the ICV injection of 5 μL of saline. The total fecal weight during the first hour post-injection correlated with the colonic transit time and fecal pellet output after the ICV injection of acyl ghrelin (1 nmol/5 μL), whereas the total fecal weight during the first hour post-injection correlated with the fecal pellet output but not the colonic transit time after the ICV injection of des-acyl ghrelin (1 nmol/5 μL).
Colonic secretion does not always correlate with colonic motility in response to different colonic stimulations. Acyl ghrelin stimulates colonic secretion.
Core tip: The colokinetic effects of acyl ghrelin and des-acyl ghrelin depend on the intracerebroventricular (ICV) injection volume, and the acute increase of the ICV volume accelerates the colonic transit time. In addition, acyl ghrelin, rather than des-acyl ghrelin, stimulates colonic secretion. Colonic secretion does not always correlate with colonic motility in response to different colonic stimulations.
- Citation: Huang HH, Ting CH, Syu YF, Chang SC, Chen CY. Correlation between colonic secretion and colonic motility in rats: Role of ghrelin. World J Gastroenterol 2016; 22(46): 10140-10147
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10140
The colonic motor function is assessed by the colonic transit time (CTT), geometric center of colonic motility, and fecal pellet output. The fecal pellet output is a simple and easy method to measure colonic motor function[1,2]. Recently, the geometric center method has been validated as a good measure to evaluate colonic transit; however, it requires the use of radiochromium which limits its application. The CTT using trypan blue dye is another option to measure the entire colonic motor function. This method has the advantage of no radioactivity. Our recent study showed that human/rat corticotropin releasing factor (h/r CRF)[3,4], in addition to ghrelin as demonstrated by other studies, accelerated the CTT in conscious fed rats, using the trypan blue dye method. Peripheral CRF injection was also shown to increase the fecal output and secretion[5].
Ghrelin is a 28-amino acid peptide that is mainly synthesized in the gastric oxyntic glands[6,7]. Acyl ghrelin and des-acyl ghrelin are the two major molecular forms of ghrelin found in the stomach and plasma. Acyl ghrelin is a ghrelin peptide acylated by ghrelin O-acyl transferase[8,9], and des-acyl ghrelin is produced with lacking O-n-octanoylation at serine 3[10,11]. Acyl ghrelin is well known as an orexigenic gut-brain peptide[12] and has the ability to regulate the gastrointestinal motility[13] and energy balance[14,15]. In contrast, des-acyl ghrelin is known to decrease food intake and disrupt the gastric motility[16,17]. Intracerebroventricular (ICV) injection of acyl ghrelin has been reported to speed the CTT[18], but the impact of des-acyl ghrelin through ICV injection on the colonic motor function is still unexplored.
In the current study, first, we aimed to investigate the effects of des-acyl ghrelin on colonic secretory and motor functions, as well as to validate the relationship between colonic secretion and motility. Second, we aimed to investigate the role of ICV injection volume in our unique rat model which can simultaneously measure the colonic motility and secretion in conscious rats.
Male Sprague-Dawley rats (National Laboratory Animal Center, Taipei, Taiwan) weighing 250-320 g at the initial period of the experiment were used and housed in group cages under controlled illumination (light cycle: 08:00-20:00), humidity and temperature of 22.5 ± 1.5 °C, and free access to water and laboratory chow pellets (LabDiet®, Brentwood, MO, United States). All experiments were performed starting at 9 a.m. in freely moving conscious rats, in accordance to guidelines which have been approved by the Institutional Animal Care and Use Committee, Taipei Veterans General Hospital.
Implantation of the ICV catheter: For ICV implantation, the rats were anesthetized with sodium pentobarbital (50 mg/kg, Nembutal; Abbott Laboratories, Abbott Park, IL, United States) by intraperitoneal injection, placed in a stereotaxic apparatus (Benchmark™, myNeuroLab, St. Louis, MO, United States), and implanted with a guide cannula (25-gauge; Eicom, Kyoto, Japan), which reached the right lateral ventricle. The stereotaxic apparatus was placed 0.8 mm posterior to the bregma, 1.4 mm right lateral to the midline, and 4.5 mm below the outer surface of the skull using a stereotaxic frame with the incisor bar set at the horizontal plane passing through the bregma and lambda[19,20]. The guide cannula was secured, a dummy cannula (Eicom) was inserted into the guide cannula, and a screw cap (Eicom) was placed as described in our previous study[19,20]. The rats were allowed 7 d for full recovery before food intake measurement after the implantation of the ICV catheters. If the rats did not increase their body weight 7 d after the operation, they were considered to have been injured during the operation and were excluded. All ICV injections with a total volume of 10 μL were administered over 60 s via the AMI-5 (Eicom).
Implantation of the colonic catheter: The rats were anesthetized with sodium pentobarbital (50 mg/kg, Nembutal; Abbott) by intraperitoneal injection. After laparotomy of the lower abdomen, the proximal colon was exposed and a catheter (3 Fr, 1-mm diameter; ATOM) was implanted into the proximal colon, 2 cm distal from the cecocolonic junction[3,4,21]. The catheter was fixed with a purse-string suture at the colonic wall and routed subcutaneously to the interscapular region, exteriorized through the skin, and secured together with an intravenous catheter for intracolonic administration of the dye marker[3,4,21]. The animals were allowed to recover for 7 d before simultaneous measurement of colonic motor and secretory functions.
Rat O-n-octanoylated ghrelin (Peptides International, Inc., Luisville, KY, United States) and rat des-acyl ghrelin (Peptides International) were kept in powder form at -20 °C, and dissolved in sterile, pyrogen-free 0.9% saline (Otsuka, Tokyo, Japan) immediately before use.
Measurement of the CTT: The measurement of colonic motor and secretory function was modified from our previous studies[3,4,21]. The CTT was calculated using an enteral non-absorbable dye marker, trypan blue (Sigma Chemical Co., St. Louis, MO, United States). The dye (0.2 mL) was injected through the catheter positioned in the proximal colon, followed by a 0.2 mL saline flush 10 min after the ICV injection of acyl ghrelin and des-acyl ghrelin (1.0 nmol/rat). The CTT was defined as the time interval between the dye injection and the discharge of the first blue pellet.
Measurements of fecal pellet output and total fecal weight: The rats were accustomed to single housing for 7 d before the experiment. The total number of pellets was recorded every hour for 2 h following an intracolonic injection of trypan blue. The total fecal material was collected every hour for 2 h following the intracolonic injection of trypan blue, and its content was weighed as the total fecal weight[3,4,21].
All the results are expressed as mean ± standard error of the mean. One-way analysis of variance followed by the Student-Newman-Keuls post-hoc test was used to detect the differences among groups. The relationship between total fecal weight, CTT, and the fecal pellet output in response to the ICV injection of either saline, acyl ghrelin, or des-acyl ghrelin was analyzed by Spearman’s nonparametric correlation. Differences were considered statistically significant when P < 0.05.
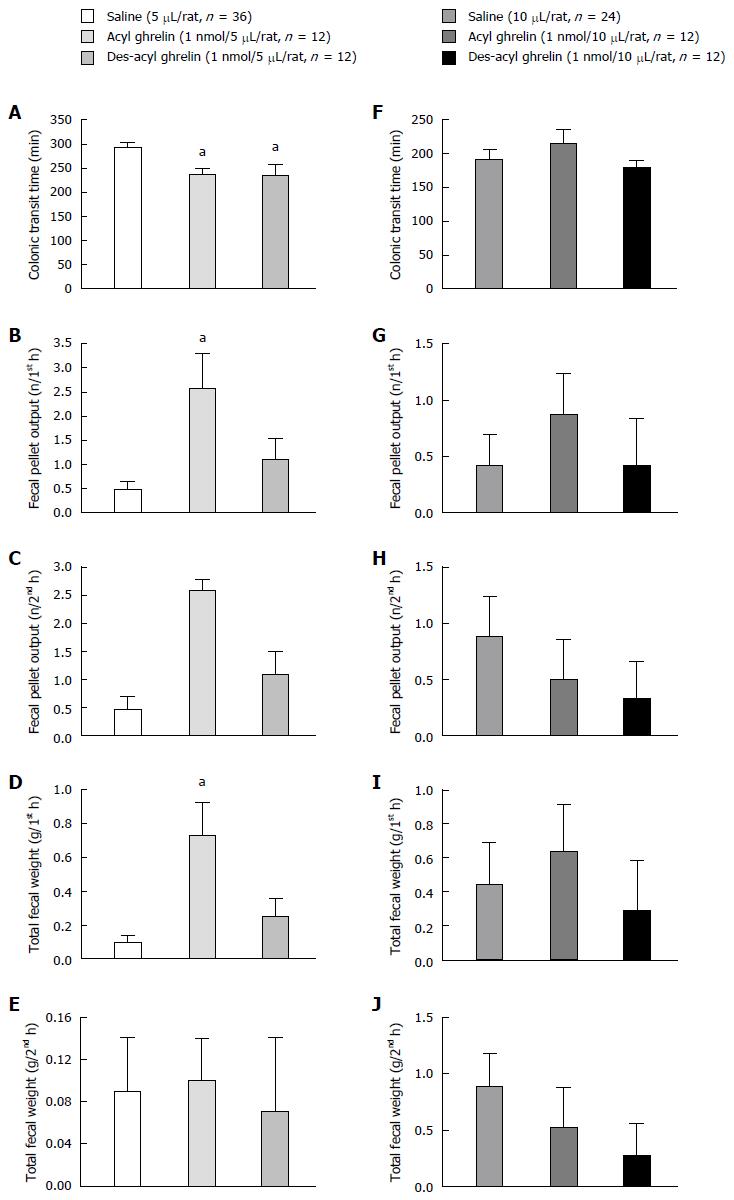
As compared with saline controls (5 μL/rat), ICV acyl ghrelin (1 nmol/5 μL/rat) and des-acyl ghrelin (1 nmol/5 μL/rat) significantly accelerated the mean CTT from 292 to 236 and 234 min, respectively (P < 0.05, Figure 1A). During the first hour post-injection, ICV acyl ghrelin (1 nmol/5 μL/rat) also significantly increased the fecal pellet output and total fecal weight (P < 0.05, Figure 1B and D). ICV acyl ghrelin (1 nmol/5 μL/rat) did not affect the fecal pellet output and total fecal weight during the second hour post-injection (P > 0.05, Figure 1C and E). ICV des-acyl ghrelin (1 nmol/5 μL/rat) did not affect either the fecal pellet output or total fecal weight during first and second hour post-injection (P > 0.05, Figure 1C and E).
An ICV injection of 10 μL of saline significantly shortened the mean CTT from 292 to 191 min compared to the ICV injection of 5 μL of saline (P < 0.05, Figure 1A and F). Moreover, the ICV injection of acyl ghrelin (1 nmol/10 μL/rat) and des-acyl ghrelin (1 nmol/10 μL/rat) did not have any effects on the CTT, fecal pellet output, and total fecal weight, compared to saline controls (10 μL/rat, Figure 1F-J).
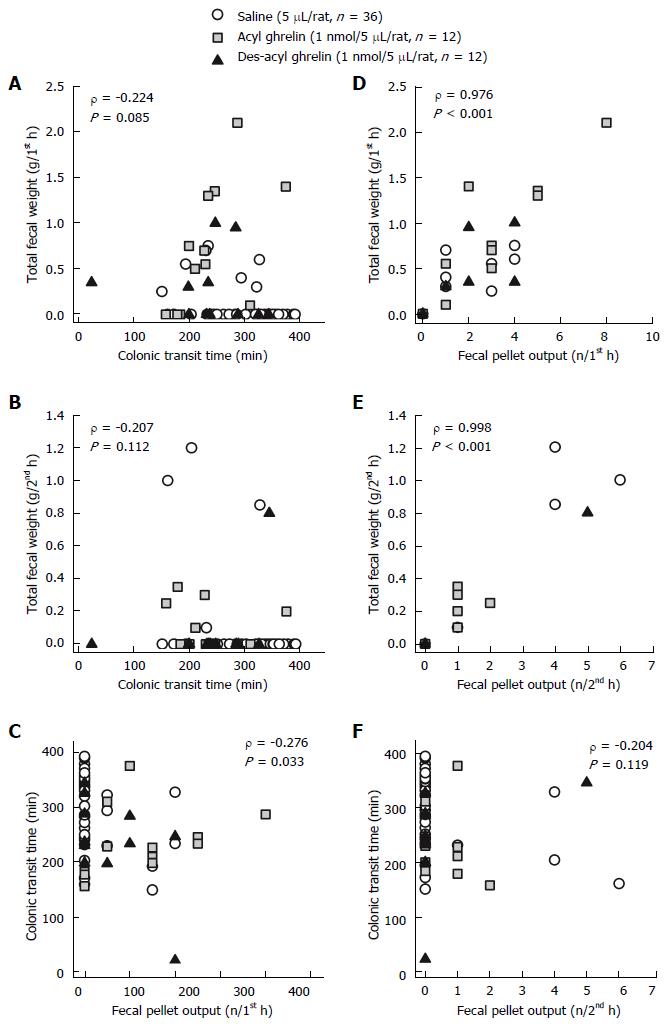
We pooled the data from the ICV injection of saline, acyl ghrelin, and des-acyl ghrelin at 5 μL, and analyzed the correlations among total fecal weight, CTT, and fecal pellet output. The total fecal weight did not correlate with the CTT during the first hour and second hour post-injection (P > 0.05, Figure 2A and B), whereas the total fecal weight exhibited a significantly positive correlation with the fecal pellet output (P < 0.001, Figure 2D and E). The CTT had a negative correlation with the fecal pellet output during the first hour (P < 0.05, Figure 2C) but not the second hour post-injection (P > 0.05, Figure 2F).
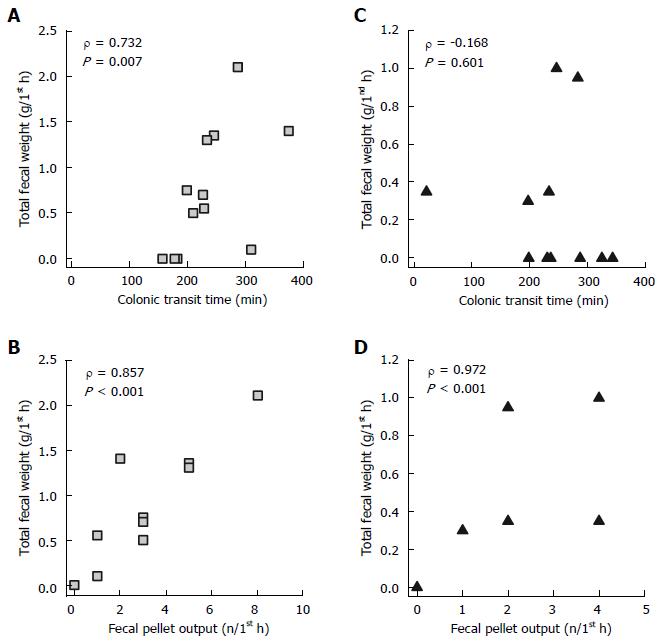
We analyzed the correlations between total fecal weight, CTT, and fecal pellet output during the first hour post-injection, in response to ICV injection of either acyl ghrelin or des-acyl ghrelin with the volume at 5 μL. The total fecal weight significantly correlated with the CTT and fecal pellet output during the first hour after ICV acyl ghrelin (1 nmol/5 μL/rat) injection (P < 0.01, Figure 3A and B). The total fecal weight significantly correlated with the fecal pellet output, but not the CTT, during the first hour after ICV des-acyl ghrelin (1 nmol/5 μL/rat) injection (P < 0.001, Figure 3C and D).
In the present study, we first demonstrated the in vivo effects of ICV des-acyl ghrelin on colon motor and secretory functions. ICV injection of des-acyl ghrelin at 1 nmol/5 μL accelerated the CTT without altering the fecal pellet output and total fecal weight during the first hour and second hour post-injection. Acyl ghrelin and ghrelin mimetics have been previously shown to exhibit colokinetic effects such as shortening the CTT[22] and decreasing the time to the first bowel movement[23], and may have the clinical implication in relieving diet-induced constipation in a rat model[24]. In addition to accelerating the CTT and increasing the fecal pellet output during the first hour post-injection, we also showed that the ICV injection of acyl ghrelin at 1 nmol/5 μL enhanced the total fecal weight during the first hour post-injection, which is consistent with the results that intrathecal but not intravenous application of acyl ghrelin to the L6-S1 region of the spinal cord increased the fluid output through the anal cannula[25]. Therefore, in our current study, the stimulatory properties of acyl ghrelin on the colonic secretion and motility are confirmed.
Although an acute increase of the ICV pressure has been reported to immediately suppress the amplitude of gastric and duodenal contractions in rabbits[26], the effects of the ICV injection volume on colonic secretion and motility still remain obscure. Our study was the first to demonstrate that the increased ICV injection volume (from 5 μL to 10 μL) shortened the CTT in saline controls. Because the CTT has been shortened in the saline controls, the stimulating effects on colonic motility and/or secretion by either acyl ghrelin or des-acyl ghrelin disappeared when the ICV injection volume increased to 10 μL at the same dose (1 nmol/rat). Acute moderate to severe head injury patients have been shown to have prolonged gastric emptying[27]. An increased intracranial pressure is proposed to be the major cause of gastric motility dysfunction[28]. The finding that an acutely increased ICV injection volume shortened the CTT may be explained by the increased intracranial pressure in a limited intracerebroventricular space, though we did not measure the increased intracranial pressure in our current study.
The CTT is considered a reflection of the motor function in the entire colon, while the fecal pellet output is the reflection of the distal colonic motor function[29]. This means that the acceleration of the colonic transit is not always equal to the increase of fecal pellet output. A recent rodent study indicated that the central administration of CRF and restraint stress accelerate the colonic transit but do not always correlate with the increase of fecal pellet output[28]. Our results are in accordance with this point (Figure 2B and C). We also showed that the ICV injection of acyl ghrelin enhanced the CTT, fecal pellet output, and total fecal weight, while the ICV injection of des-acyl ghrelin only accelerated the CTT. Therefore, we propose that acyl ghrelin accelerates the entire colon and distal colonic motor functions, whereas des-acyl ghrelin, lacking O-n-octanoylation at serine 3, may have greater effects in stimulating the proximal colon motor function without altering the distal colonic motility. In addition, our findings provide new information regarding the relationship between colonic secretion and motility. The finding that the total fecal weight during the first hour post-injection correlated with the CTT stimulated by the ICV injection of acyl ghrelin (Figure 3A) but not des-acyl ghrelin (Figure 3B) suggests that colonic secretion does not always correlate with colonic motility in response to different colonic stimulations.
In conclusion, the colokinetic effects of acyl ghrelin and des-acyl ghrelin depend on the ICV injection volume, and the acute increase of the ICV volume accelerates the CTT. In addition, acyl ghrelin, rather than des-acyl ghrelin, stimulates colonic secretion. Colonic secretion does not always correlate with colonic motility in response to different colonic stimulations.
We would like to thank Miss Yu-Chi Lee for her secretarial assistance in manuscript editing, as well as the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing experimental space and facilities.
Acyl ghrelin is well known as an orexigenic gut-brain peptide and has the ability to regulate the gastrointestinal motility and energy balance. In contrast, des-acyl ghrelin is known to decrease food intake and disrupt the gastric motility.
The intracerebroventricular (ICV) injection of acyl ghrelin has been reported to speed the colonic transit time (CTT), but the impact of des-acyl ghrelin through ICV injection on the colonic motor function is still unexplored.
This is the first study to investigate the effects of des-acyl ghrelin on colonic secretory and motor functions, as well as to validate the relationship between colonic secretion and motility. This is also the first study to evaluate the role of ICV injection volume on the colonic motility and secretion in conscious rats.
Using a rat model chronically implanted with ICV and cecal catheters, the authors validated the correlation between colonic secretion and colonic motor functions, as well as the role of ICV injection volume.
The colokinetic effects of acyl ghrelin and des-acyl ghrelin depend on the ICV injection volume, and the acute increase in ICV volume accelerates the CTT. In addition, acyl ghrelin, rather than des-acyl ghrelin, stimulates colonic secretion. Colonic secretion does not always correlate with colonic motility in response to different colonic stimulations.
Colonic secretion does not always correlate with colonic motility in response to different colonic stimulations in rats: role of intracerebroventricular injectional volume. The aims of this study were to investigate the influences of des-acyl ghrelin on colonic secretory and motor functions, as well as to validate the relationship between colonic secretion and motility. Also the authors aimed to appraise the role of ICV injectional volume in our unique rat model which can simultaneously measure the colonic motility and secretion in conscious rats.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huerta-Franco MR S- Editor: Yu J L- Editor: A E- Editor: Liu WX
| 1. | Forbes SC, Cox HM. Peptide YY, neuropeptide Y and corticotrophin-releasing factor modulate gastrointestinal motility and food intake during acute stress. Neurogastroenterol Motil. 2014;26:1605-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Nozu T, Kumei S, Takakusaki K, Ataka K, Fujimiya M, Okumura T. Central orexin-A increases colonic motility in conscious rats. Neurosci Lett. 2011;498:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Chen CY, Doong ML, Li CP, Liaw WJ, Lee HF, Chang FY, Lin HC, Lee SD. A novel simultaneous measurement method to assess the influence of intracerebroventricular obestatin on colonic motility and secretion in conscious rats. Peptides. 2010;31:1113-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Chen CY, Chien EJ, Chang FY, Lu CL, Luo JC, Lee SD. Impacts of peripheral obestatin on colonic motility and secretion in conscious fed rats. Peptides. 2008;29:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Saunders PR, Maillot C, Million M, Taché Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur J Pharmacol. 2002;435:231-235. [PubMed] |
| 6. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5887] [Article Influence: 226.4] [Reference Citation Analysis (0)] |
| 7. | Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-4261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 960] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 8. | Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 873] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 9. | Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105:6320-6325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 622] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 11. | Chen CY, Fujimiya M, Laviano A, Chang FY, Lin HC, Lee SD. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J Chin Med Assoc. 2010;73:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kojima M, Kangawa K. Ghrelin, an orexigenic signaling molecule from the gastrointestinal tract. Curr Opin Pharmacol. 2002;2:665-668. [PubMed] |
| 13. | Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 270] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Chen CY, Tsai CY. From endocrine to rheumatism: do gut hormones play roles in rheumatoid arthritis? Rheumatology (Oxford). 2014;53:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8-25. [PubMed] |
| 17. | Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 352] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 18. | Tebbe JJ, Tebbe CG, Mronga S, Ritter M, Schäfer MK. Central neuropeptide Y receptors are involved in 3rd ventricular ghrelin induced alteration of colonic transit time in conscious fed rats. BMC Gastroenterol. 2005;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Ting CH, Chi CW, Li CP, Chen CY. Differential modulation of endogenous cannabinoid CB1 and CB2 receptors in spontaneous and splice variants of ghrelin-induced food intake in conscious rats. Nutrition. 2015;31:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yeh C, Ting CH, Doong ML, Chi CW, Lee SD, Chen CY. Intracerebroventricular urocortin 3 counteracts central acyl ghrelin-induced hyperphagic and gastroprokinetic effects via CRF receptor 2 in rats. Drug Des Devel Ther. 2016;10:3281-3290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Ting CH, Chen YC, Liaw WJ, Lin HC, Chen CY. Peripheral injection of pancreatic polypeptide enhances colonic transit without eliciting anxiety or altering colonic secretion in rats. Neuropeptides. 2016;55:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schäfer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Venkova K, Mann W, Nelson R, Greenwood-Van Meerveld B. Efficacy of ipamorelin, a novel ghrelin mimetic, in a rodent model of postoperative ileus. J Pharmacol Exp Ther. 2009;329:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Pustovit RV, Furness JB, Rivera LR. A ghrelin receptor agonist is an effective colokinetic in rats with diet-induced constipation. Neurogastroenterol Motil. 2015;27:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Hirayama H, Shiina T, Shima T, Kuramoto H, Takewaki T, B Furness J, Shimizu Y. Contrasting effects of ghrelin and des-acyl ghrelin on the lumbo-sacral defecation center and regulation of colorectal motility in rats. Neurogastroenterol Motil. 2010;22:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Garrick T, Mulvihill S, Buack S, Maeda-Hagiwara M, Tache Y. Intracerebroventricular pressure inhibits gastric antral and duodenal contractility but not acid secretion in conscious rabbits. Gastroenterology. 1988;95:26-31. [PubMed] |
| 27. | Kao CH, ChangLai SP, Chieng PU, Yen TC. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | McArthur CJ, Gin T, McLaren IM, Critchley JA, Oh TE. Gastric emptying following brain injury: effects of choice of sedation and intracranial pressure. Intensive Care Med. 1995;21:573-576. [PubMed] |
| 29. | Nakade Y, Mantyh C, Pappas TN, Takahashi T. Fecal pellet output does not always correlate with colonic transit in response to restraint stress and corticotropin-releasing factor in rats. J Gastroenterol. 2007;42:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |