Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8940
Peer-review started: June 17, 2016
First decision: July 12, 2016
Revised: August 11, 2016
Accepted: September 14, 2016
Article in press: September 14, 2016
Published online: October 28, 2016
Processing time: 133 Days and 20.7 Hours
To determine the non-biased prevalence and clinical significance of ansa pancreatica in patients with acute pancreatitis using magnetic resonance imaging (MRI).
Our institutional review board approved this cross-sectional study, which consisted of a community-based cohort of 587 consecutive participants in a whole-body health-check program, and 73 subjects with episode of acute pancreatitis (55 patients with a single episode of acute pancreatitis, and 18 patients with recurrent acute pancreatitis). All of the subjects underwent abdominal MRI including magnetic resonance cholangiopancreatography, medical examinations, and blood tests. Two board-certified, diagnostic, abdominal radiologists evaluated the images, and ansa pancreatica was diagnosed based on its characteristic anatomy on MRI.
Compared with the community group [5/587 (0.85%)], patients with recurrent acute pancreatitis had a significantly higher frequency of ansa pancreatica [2/18 (11.1%)] (P = 0.016; OR = 14.3; 95%CI: 1.27-96.1), but not compared with patients with single-episode acute pancreatitis [1/55 (1.8%)] (P = 0.42; OR = 2.1; 95%CI: 0.44-19.7). Multiple logistic regression analysis using age, alcohol intake, presence of ansa pancreatica, and presence of autoimmune disease as independent covariates, revealed a significant relationship between the presence of ansa pancreatica and recurrent acute pancreatitis. The presence of autoimmune disease was also significantly associated with the onset of recurrent acute pancreatitis. On the other hand, neither age nor alcohol intake were significantly related to the onset of recurrent acute pancreatitis.
The present study is the first to provide robust evidence that the presence of ansa pancreatica is significantly associated with recurrent acute pancreatitis.
Core tip: Ansa pancreatica is a rare anatomical variation of the accessory pancreatic duct and was hypothesized to be a predisposing factor for pancreatitis. However its in vivo prevalence was unknown and no case-control study has confirmed its clinical significance. This study is the first case-control study to determine the non-biased prevalence and provide robust evidence that the presence of ansa pancreatica is significantly associated with recurrent acute pancreatitis, using non-invasive magnetic resonance imaging.
- Citation: Hayashi TY, Gonoi W, Yoshikawa T, Hayashi N, Ohtomo K. Ansa pancreatica as a predisposing factor for recurrent acute pancreatitis. World J Gastroenterol 2016; 22(40): 8940-8948
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8940.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8940
Pancreatitis is a severe inflammatory disease that is critical in some patients. Furthermore, serious damage of pancreatic tissue leads to dysfunction of the endocrine and exocrine systems, particularly in the chronic phases of the disease. There are several known causes of pancreatitis, including excessive alcohol consumption, biliary stones, trauma, autoimmunity, metabolic disorders, drugs, iatrogenic, infection, genetic mutations, malignancy, and heredity factors[1-3]; and morphological aberrations such as atypical arrangement of the pancreaticobiliary ductal system[4,5], pancreas divisum[5-8], or a meandering main pancreatic duct[9]. However, recurrent episodes of acute pancreatitis is idiopathic in as many as 20% of patients[8,10,11].
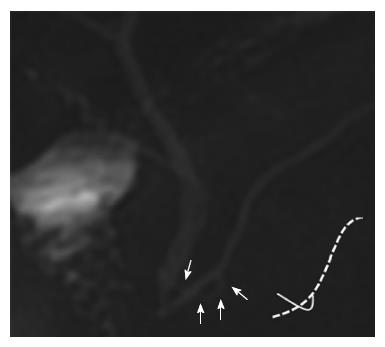
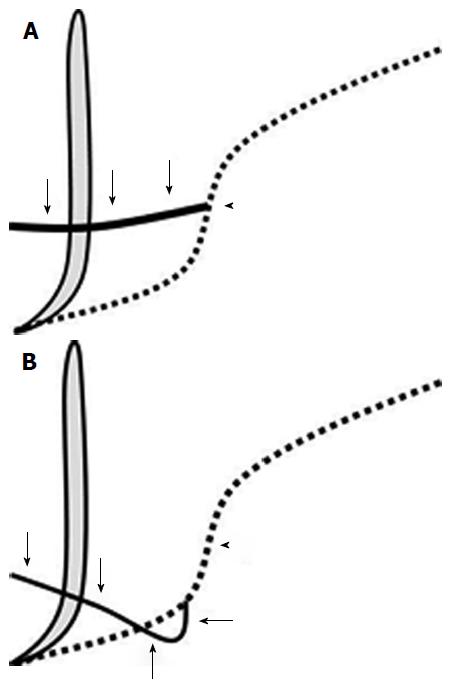
Dawson and Langman[12] first reported ansa pancreatica in 1961. In this pancreatic duct variant, the accessory duct is obliterated at its junction with the ventral duct, and is replaced with an additional curved communicating duct between the ventral and dorsal ducts at the pancreatic head. This additional duct arises from the ventral duct, runs into the caudal side of the ventral duct, turns to the ventral side with a reversed S-shaped curve, and finally terminates in and around the minor papilla (Figures 1 and 2)[4,12-14].
In several case reports, ansa pancreatica was hypothesized to be a predisposing factor for pancreatitis, particularly acute pancreatitis[14,15]. To date, however, no case-control study has confirmed its clinical significance. Therefore, the present study aimed to investigate the relationship between ansa pancreatica and acute pancreatitis.
This study conformed with the Declaration of Helsinki. The prospective and retrospective use of the clinical, biochemical, and radiographic data was approved by the Research Ethics Committee of the University of Tokyo Hospital for the present cross-sectional study.
The subjects were divided into a community group (group 1) and patients with acute pancreatitis (group 2); the latter group was divided into two subgroups. Group 1 included consecutive community residents who attended a paid health checkup program between October 12, 2006 and May 31, 2007 that was advertised via leaflets and the Internet. The program included blood testing; evaluation of drinking and smoking habits; a thorough medical and subjective symptom history; and a physical examination performed by a physician. Additionally, whole-body imaging studies were performed, and included magnetic resonance imaging (MRI) of the abdomen and magnetic resonance cholangiopancreatography (MRCP). Laboratory blood testing comprised WBC and platelet counts, hemoglobin, glycated hemoglobin, amylase, glucose, insulin, C-reactive protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ-glutamyltransferase, total bilirubin, and high- and low-density lipoprotein. All of the tests in individual subjects were performed on the same day. All of the subjects provided written informed consent for the comprehensive epidemiological study. Only subjects who underwent all of the examinations listed above were included in the study.
Group 2 included patients who were diagnosed with acute-type pancreatitis regardless of the cause. These patients were divided into two subgroups depending on whether they had single-episode acute pancreatitis or recurrent acute pancreatitis. We performed a retrospective medical chart review of all consecutive patients who were suspected of having single-episode or recurrent acute pancreatitis and who underwent abdominal MRI and MRCP between January 1, 2003 and October 17, 2013. To determine the type of pancreatitis, the entire medical records for all patients were reviewed to evaluate the type of onset and the cause of pancreatitis. The respective diagnoses were assessed using the latest criteria available as of March 2014. Single-episode acute pancreatitis was defined used the Japanese (JPN) Guidelines for the management of acute pancreatitis[16]. Recurrent acute pancreatitis was defined as the following: ≥ 2 well-documented episodes of abdominal pain typical of acute pancreatitis that were separated by > 2 mo; and ≥ 1 of (1) lipase or serum amylase elevation > 3 × the upper limit of normal, and/or (2) features consistent with acute pancreatitis on diagnostic imaging[11,17].
The cause of pancreatitis was also examined in the acute pancreatitis group. Idiopathic pancreatitis was diagnosed after excluding all established causes of pancreatitis, and according to the results of physical examination, imaging, and biochemical tests. Genetic testing and manometric assessments were conducted only if deemed necessary.
The exclusion criteria were as follows: incomplete clinical evaluations, pancreatitis with chronic onset (diagnosed using the latest criteria of the revised Japanese clinical diagnostic criteria for chronic pancreatitis)[18], the quality of MRI scans was inadequate to evaluate the accessory duct (we used stricter criteria compared with the usual clinical standard), post-pancreaticoduodenectomy status, and the presence of a pancreatic or biliary tumor occupying the head of pancreas. The region (undetectable, head, body, tail, or ≥ 2 of these pancreatic regions) and severity of inflammation were also assessed using the Japanese Ministry of Health, Labour, and Welfare severity scoring system for acute pancreatitis (JPN score 2008)[19].
Our institutional review board approved waiver of informed consent for the present cross-sectional study about the pancreatitis group.
For the community group, MRI was performed using 3 T scanners (GE Medical Systems, Waukesha, WI, United States). During breath hold, heavily T2-weighted MRCP images were acquired in the coronal plane using a two-dimensional (2D) half-Fourier fast spin echo (FSE) technique [repetition time/echo time (TR/TE), ∞/600 ms; slice thickness (ST), 40 mm]. Four coronal and oblique-coronal projection images were reconstructed. Transaxial FSE T2-weighted images [TR/TE, ∞/80 ms; ST, 3 mm (without gap)] and fat-suppressed T1-weighted images were also acquired for complementary interpretation, using a three-dimensional (3D) gradient echo technique [TR/TE, 3.5/1.5 ms; flip angle, 15°; ST, 3 mm (with 1.5 mm overlap)]. Subjects were not premedicated.
For the acute pancreatitis group, MRI was performed using either 3 T scanners (GE Medical Systems) or 1.5 T scanners (GE Medical Systems; Siemens AG, Erlangen, Germany; and Toshiba Medical Systems, Tochigi, Japan). With the patients in breath hold, heavily T2-weighted MRCP images were acquired by 2D half-Fourier FSE (TR/TE, 2400-∞/600-1100 ms; ST, 30-50 mm) and respiratory-gated 3D half-Fourier FSE [TR/TE, 1300-∞/500-900 ms; ST, 1.2-2.0 mm (without gap)]. Coronal and oblique-coronal projection images were reconstructed. We also acquired transaxial and coronal T2-weighted images [FSE; TR/TE, 1300-∞/80-150 ms; ST, 5 mm (without gap)] and fat-suppressed T1-weighted images [3D gradient echo; TR/TE, 3-840/1.5-140 ms; flip angle, 15°; ST, 1.5 mm (without gap)] for complementary interpretation. Before MRI, the patients were administered manganese chloride solution (Bothdel Oral Solution 10; Kyowa Hakko Kirin, Tokyo, Japan) as negative oral contrast agent.
All MRI scans were reviewed by two board-certified, qualified (6-8 years’ experience of pancreaticobiliary imaging), diagnostic, abdominal radiologists on picture archiving and communication system workstations (Centricity; GE Medical Systems). Both radiologists were blinded to the clinical information. One radiologist acted as the main interpreter and other supervised the image interpretation.
Pancreatic ductal anatomy was evaluated on MRI scans as follows. Ansa pancreatica was considered present if the oblique-coronal MRCP plane showed (1) the upstream accessory duct was obliterated; and (2) the additional duct arose from the ventral duct, ran caudally, then dextrad and ventrally, and finally terminated near the minor papilla. The radiologist was asked to state whether ansa pancreatica was present or not. All radiographic findings related to the pancreaticobiliary system were recorded (e.g., other variants of pancreatic ductal fusion, pancreatic ductal/ductile dilation or irregularity, gallstones, pancreatic cystic lesions, cystic polyps, pancreatic parenchymal atrophy, biliary morphological defects, adenomyomatosis, and juxtapapillary duodenal diverticulum). In suspected cases of ansa pancreatica, the two radiologists evaluated the images to reach a consensus on its presence or absence. When the opinions disagreed, the supervisor’s interpretation took precedence.
For univariate comparisons between groups, Welch’s t test was used for continuous variables and, for categorical values, Fisher’s exact test was used; 0.05 was set as the level of statistical significance. Bonferroni’s method was used to correct family-wise error. Multiple logistic regression analysis was used to identify factors that were associated with pancreatitis. To prevent overestimation of the number of predictive values, variables with P < 0.05 in the univariate analyses were selected before applying family-wise error correction. All statistical computations were performed using R Ver. 2.9 (free software; The R Foundation for Statistical Computing, Vienna, Austria; http://cran.r-project.org/).
In group 1674 subjects completed the study. Subjects were excluded because of post-pancreaticoduodenectomy (n = 1); incomplete MRI scans (n = 1); intraductal papillary mucinous neoplasm in the head of pancreas (n = 9); and inadequate image quality to evaluate the accessory duct, most commonly due to hindered visualization of the pancreatic ducts owing to artifact from gastrointestinal signal (n = 76). The final evaluable cohort of 587 community subjects included 250 women (mean age, 57.0 years; range, 31-84 years) and 337 men (mean age, 56.6 years; range, 40-86 years) (Table 1). The majority of subjects were Japanese (one subject was Korean). None of these subjects complained of pancreatic pain. Six subjects had a history of pancreatitis, of which four had acute pancreatitis and two had chronic pancreatitis. The medical records for the four subjects with acute pancreatitis were unavailable so we could not determine whether they had recurrent acute pancreatitis or single-episode acute pancreatitis.
| All subjects (n = 587) | Subjects without ansa pancreatica (n = 582) | Subjects with ansa pancreatica (n = 5) | P value | OR (95%CI) | |
| Age (yr) | 56.8 ± 10.4 | 56.8 ± 10.4 | 53.6 ± 10.7 | 0.601 | |
| Females | 250 (43) | 248 (43) | 2 (40) | 0.702 | |
| Brinkman index (cigarettes/d × years) | 244 ± 408 | 246 ± 409 | 120 ± 240 | 0.361 | |
| Alcohol intake (kg/yr) | 5.8 ± 7.8 | 5.7 ± 7.7 | 11.4 ± 12.9 | 0.431 | |
| Clinical history | |||||
| All cases of pancreatitis3 | 6 (1) | 5 (0.9) | 1 (20) | 0.0502 | 28 (0.49-364) |
| Acute pancreatitis | 4 (0.7) | 3 (0.5) | 1 (20) | 0.0342 | 46 (0.74-746) |
| Diabetes mellitus | 30 (5) | 30 (5) | 0 | 12 | |
| Hypertension | 109 (19) | 109 (19) | 0 | 0.592 | |
| Hyperlipidemia | 67 (11) | 67 (12) | 0 | 12 | |
| Any malignant neoplasm | 45 (8) | 45 (8) | 0 | 12 | |
| Autoimmune disease | 14 (2) | 14 (2) | 0 | 12 |
In group 2, a total of 6103 MRCP scans were performed between January 1, 2003 and October 17, 2013. After excluding overlapping subjects and patients without single-episode acute pancreatitis or recurrent acute pancreatitis, 102 patients remained, of which 78 had single-episode acute pancreatitis and 24 had recurrent acute pancreatitis. Patients were excluded because of incomplete clinical evaluation (n = 13), tumor in the head of pancreas (n = 3), and post-pancreaticoduodenectomy (n = 1). In addition, patients with insufficient image quality (n = 12) were also excluded. Of 73 evaluable patients, 55 had single-episode acute pancreatitis (mean age, 57.5 years; range, 26-85 years) and 18 had recurrent acute pancreatitis (mean age, 46.1 years; range, 26-82 years). The single-episode acute pancreatitis subgroup included 16 female patients (mean age, 57.8 years; range, 16-85 years) and 39 men (mean age, 57.4 years; range, 24-82 years). The recurrent acute pancreatitis subgroup included 9 women (age, 26-82 years; mean, 50.7 years) and 9 men (age, 29-67 years; mean, 41.6 years). All of the patients with acute pancreatitis were Japanese (Tables 2 and 3).
| All patients (n = 55) | Patients without ansa pancreatica (n = 54) | Patients with ansa pancreatica (n = 1) | |
| Age (yr) | 57.5 ± 18.5 | 57.6 ± 18.5 | 50 |
| Females | 16 (29) | 16 (30) | 0 (0) |
| Brinkman index (cigarettes/d × years) | 355 ± 887 | 364 ± 888 | 0 |
| Alcohol intake (kg/yr) | 8.1 ± 11.1 | 7.9 ± 11.1 | 16.1 |
| Clinical history | |||
| Diabetes mellitus | 11 (20) | 11 (22) | 0 (0) |
| Hypertension | 14 (25) | 14 (29) | 0 (0) |
| Hyperlipidemia | 12 (22) | 12 (25) | 0 (0) |
| Any malignant neoplasm | 8 (15) | 8 (16) | 0 (0) |
| Autoimmune disease | 5 (9) | 5 (10) | 0 (0) |
| All patients (n = 18) | Patients without ansa pancreatica (n = 16) | Patients with ansa pancreatica (n = 2) | P value | |
| Age (yr) | 46.1 ± 14.4 | 44.6 ± 14.1 | 58.5 ± 9.5 | 0.361 |
| Female | 9 (50) | 8 (50) | 1 (50) | 12 |
| Brinkman index (cigarettes/d × years) | 103.1 ± 174 | 122 ± 183 | 0 ± 0 | 0.061 |
| Alcohol intake (kg/yr) | 1.06 ± 2.01 | 1.14 ± 2.1 | 0 | 0.621 |
| Clinical history | ||||
| Diabetes mellitus | 1 (6) | 0 (0) | 1 (50) | 0.192 |
| Hypertension | 3 (17) | 3 (19) | 0 (0) | 112 |
| Hyperlipidemia | 5 (28) | 5 (31) | 0 (0) | 11 |
| Any malignant neoplasm | 0 (0) | 0 (0) | 0 (0) | 11 |
| Autoimmune disease | 4 (22) | 4 (25) | 0 (0) | 11 |
The accessory pancreatic duct was clearly visualized in 88.7% (587/663) of subjects in the community group and in 85.9% (73/85) of patients with acute pancreatitis, and was not significantly different between these two groups (P = 0.47; OR = 0.78; 95%CI: 0.40-1.67). In the community group, 0.85% (5/587) of subjects had ansa pancreatica; 17 (2.9%) had pancreas divisum, 37 (6.3%) had a meandering main pancreatic duct, 1 (0.017%) had an anomalous arrangement of the pancreaticobiliary ductal system, and 1 (0.017%) had a retroportal main pancreatic duct.
When we compared subjects with and without ansa pancreatica in the community group, we observed no significant differences between these two subgroups in terms of sex, clinical history, or hematologic and biochemical variables (Table 1). The incidence of acute pancreatitis, including single-episode acute pancreatitis and recurrent acute pancreatitis, was greater in subjects with ansa pancreatica (20.0%, 1/5) than in subjects without ansa pancreatica (0.52%, 3/582), although this difference did not reach statistical significance (Table 1). Two of the subjects (40%, 2/5) with ansa pancreatica in the community group presented with other radiological abnormalities in the pancreatic duct: one had slight dilation of main pancreatic duct (4 mm) and the other had Wirsungocele.
Among the patients with acute pancreatitis, 3 (4.1%, 3/73) had ansa pancreatica, of which 2 (11.1%, 2/18) had recurrent acute pancreatitis and 1 (1.8%, 1/55) had single-episode acute pancreatitis. Pancreatitis in patients with ansa pancreatica was caused by alcohol in two patients and was idiopathic in one (Table 4). None of the patients with ansa pancreatica in the acute pancreatitis group had other accompanying morphological pancreaticobiliary abnormalities.
| Cause | All patients with acute pancreatitis (n = 73) | Patients with single-episode acute pancreatitis (n = 55) | Patients with recurrent acute pancreatitis (n = 18) |
| Gallstones | 19 (26.0) | 18 (33.0) | 1 (5.6) |
| Alcohol | 14 (19.0)[2] | 12 (22.0)[1] | 2 (11.0)[1] |
| Idiopathic | 10 (14.0)[1] | 6 (11.0) | 4 (22.0)[1] |
| Iatrogenic | 6 (8.2) | 6 (11.0) | 0 (0) |
| Pancreas divisum1 | 6 (8.2) | 1 (1.8) | 5 (28.0) |
| Autoimmunity2 | 4 (5.6) | 2 (3.6) | 2 (5.6) |
| Meandering main pancreatic duct3 | 3 (4.2) | 1 (1.8) | 2 (11.0) |
| Pancreaticobiliary maljunction | 1 (1.4) | 1 (1.8) | 0 (0) |
| Alcohol and hyperlipidemia combined | 1 (1.4) | 1 (1.8) | 0 (0) |
| Choledocal cyst, pancreaticobiliary maljunction, and pancreas divisum combined | 1 (1.4) | 1 (1.8) | 0 (0) |
| Cholesterol embolism | 1 (1.4) | 1 (1.8) | 0 (0) |
| Crohn’s disease | 1 (1.4) | 1 (1.8) | 0 (0) |
| Drug induced | 1 (1.4) | 1 (1.8) | 0 (0) |
| Hyperlipidemia | 3 (4.2) | 2 (3.6) | 1 (5.6) |
| Hypothermia | 1 (1.4) | 1 (1.8) | 0 (0) |
| Sphincter of Oddi dysfunction | 1 (1.4) | 0 (0) | 1 (5.6) |
Compared with the community group, the recurrent acute pancreatitis subgroup showed significantly higher rates of ansa pancreatica after family-wise correction, with a very high OR (P = 0.016; OR = 14.3; 95%CI: 1.27-96.1). However, no difference was observed in the single-episode acute pancreatitis subgroup or the total group of patients with acute pancreatitis (Table 5). The age and alcohol intake were significantly lower and the frequency of autoimmune disease (including non-organ-specific autoimmune disorders and organ-specific autoimmune disorders like autoimmune pancreatitis) was significantly greater in the recurrent acute pancreatitis subgroup than in the community group, but no differences were observed in the other clinical features (Table 6). Based on the results of the univariate analyses, multiple logistic regression analyses were performed using age, alcohol intake, presence of ansa pancreatica, and presence of autoimmune disease as independent covariates. Considering that the exact type of pancreatitis (recurrent or single-episode) was unknown in four subjects with acute pancreatitis in the community group, we performed statistical analyses using all combinations of recurrent or single-episode acute pancreatitis. These analyses revealed a significant positive association between ansa pancreatica and the onset of recurrent acute pancreatitis in all combinations, with ORs ranging from 14.0 (P = 0.03; 95%CI: 3.0-25.0) to 79.3 (P = 0.0002; 95%CI: 69.5-89.1) depending on the combination tested. The presence of autoimmune disease was also significantly associated with the onset of recurrent acute pancreatitis in all combinations, with ORs ranging from 13.2 (P = 0.0030; 995%CI: 7.7-18.7) to 18.4 (P = 0.0028; 95%CI: 11.6-25.2). According to the results of multiple logistic regression analyses, neither age nor alcohol intake were significantly associated with the onset of recurrent acute pancreatitis.
| Community group (n = 587) | Patients with recurrent acute pancreatitis (n = 18) | P value | OR (95%CI) | |
| Age (yr) | 56.8 ± 10.4 | 46.1 ± 14.4 | 0.000112 | |
| Female | 250 (43) | 9 (50) | 0.633 | |
| Brinkman index (cigarettes/d × years) | 244 ± 408 | 103.1 ± 174 | 0.0191 | |
| Alcohol intake (kg/yr) | 5.8 ± 7.8 | 1.06 ± 2.0 | < 0.000112 | |
| Clinical history | ||||
| Diabetes mellitus | 30 (5) | 1 (6) | 13 | |
| Hypertension | 109 (19) | 3 (17) | 13 | |
| Hyperlipidemia | 67 (11) | 5 (28) | 0.0513 | |
| Any malignant neoplasm | 45 (8) | 0 (0) | 0.393 | |
| Autoimmune disease | 14 (2) | 4 (22) | 0.001023 | 11.6 (2.46-43.7) |
When we evaluated the prevalence of ansa pancreatica according to the cause of pancreatitis, we found that ansa pancreatica was most frequent in patients with alcoholic pancreatitis (14.3%, 2/14) (Table 4).
Among all patients with acute pancreatitis, MRCP scans were obtained in the acute phase of pancreatitis in 1 (33.3%) patient with ansa pancreatica. This patient had an idiopathic recurrent acute attack, which presented with pancreatitis limited to the head of pancreas and was classified as non-severe[19].
In all eight cases with ansa pancreatica, the duct arose from the papillary side of the flexion point of the ventral duct where the normal accessory duct arose (Figures 1 and 2).
To our knowledge, this is the first case-control study to focus on ansa pancreatica. We determined the prevalence of ansa pancreatica visible on MRCP in a community group and in patients with single-episode or recurrent acute pancreatitis, and revealed a significant association between the presence of ansa pancreatica and the onset of recurrent acute pancreatitis.
MRCP is a diagnostic technique that can image the pancreaticobiliary duct in a non-invasive manner; it does not require radiation exposure or injection of contrast media, and carries a low risk of complications. In recent years, technological advances mean that MRCP has started to compete with ERCP in terms of imaging quality[20,21]. In the present study, we used MRCP, which enabled us to acquire pancreaticobiliary images of the study groups in nearly identical conditions, which is necessary in a case-control study.
Ansa pancreatica was characterized by the absent accessory duct at the junction with the ventral duct and by the presence of an extra curved duct linking the ventral and dorsal pancreatic duct. In this study, we observed that all ansa pancreatica ducts arose from the papillary side of the flexion point of the ventral duct. This location was similar to the bifurcation of the lower branch of ventral duct, which arose from a point closer to the papillary side than the source of the normal accessory duct. Dawson proposed that ansa pancreatica was formed by the fusion of the proximal part of the dorsal duct with the lower branches of the dorsal and ventral ducts[12], and this hypothesis was consistent with our findings.
In several case reports, it was speculated that the presence of ansa pancreatica is a predisposing factor for pancreatitis. Kamisawa and Dawson reported a more frequent occurrence of impervious minor papilla in patients with ansa pancreatica (66.7%-79.3%[12,22]) than in healthy subjects (59%)[13], and this was assumed to be the cause of pancreatitis. In the present study, patients with recurrent acute pancreatitis had a significantly higher frequency of ansa pancreatica (11.1%) than seen in the community group (0.85%). This result indicates that the presence of ansa pancreatica is a predisposing factor for pancreatitis, as previously hypothesized.
Dawson and Kamisawa reported that in subjects without pancreatitis, the ansa pancreatica type of accessory duct was detected in 17% and 13.6% by ERCP and eosine injection, respectively[12,22]; these values are considerably higher than the values in our study. We found that most of the ansa pancreatica ducts presented as faint outlines on the MRCP images. Therefore, the detectability of ansa pancreatica may be influenced by differences in imaging methods, because intraductal pressure is higher during ERCP than in normal physiological conditions[23]. We also speculate that racial difference may have influenced the results. In addition, we think that the ansa pancreatica detected on MRCP are more dilated cases reflecting more severe congestion of pancreatic juice and high intraductal pressure, which are relevant to the etiology of pancreatitis.
Ansa pancreatica is widely assumed to be a predisposing factor for pancreatitis, particularly in heavy alcohol consumers[24-26]. Indeed, we found that the frequency of ansa pancreatica was highest in patients with alcoholic pancreatitis, although this was not statistically significant. This result may indicate that heavy alcohol consumers with ansa pancreatica should be advised to abstain from drinking. Nevertheless, the exact mechanism linking alcohol consumption and ansa pancreatica in the onset of pancreatitis is unknown.
We also observed that patients with recurrent acute pancreatitis were significantly more likely to have an autoimmune disease than subjects in the community group. This seems reasonable because autoimmune pancreatitis shows a strong tendency to recur.
The major limitations of the present study are as follows. First, the ability of MRCP to assess ductal anatomy might be imperfect. However, MRCP is the only pancreaticobiliary imaging method that can be used in healthy subjects and was therefore essential to ensure the procedures were comparable in this case-control study. Second, the subjects enrolled in this study were Asian: almost all subjects were Japanese. Therefore, careful consideration is needed in generalizing this result to the whole population.
In conclusion, this is the first study to investigate the clinical significance of ansa pancreatica using a case-control study design. Our results indicate that the presence of ansa pancreatica is a predisposing factor for the onset of recurrent acute pancreatitis.
Ansa pancreatica is a rare anatomical variation of the accessory pancreatic duct. Its in vivo prevalence and clinical significance were unknown.
Ansa pancreatica was hypothesized to be a predisposing factor for pancreatitis, however no case-control study has confirmed it. This study contributes to reveal the association of the onset of recurrent acute pancreatitis and the presence of ansa pancreatica.
This study is the first case-control study to determine the non-biased prevalence and provide evidence that the presence of ansa pancreatica is significantly associated with recurrent acute pancreatitis, using non-invasive magnetic resonance imaging.
This study investigated ansa pancreatica was a predisposing factor for recurrent acute pancreatitis. If a patient with ansa pancreatica has acute pancreatitis, the risk of recurrence should be noticed.
Ansa pancreatica is in fact said to be a rare anatomical variation of pancreatic ducts. This paper is the first in the world study of this subject, where it was indicated that ansa pancreatica is to be predisposing factor for recurrent acute pancreatitis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jagielski M, Liu B S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Garg PK, Khajuria R, Kabra M, Shastri SS. Association of SPINK1 gene mutation and CFTR gene polymorphisms in patients with pancreas divisum presenting with idiopathic pancreatitis. J Clin Gastroenterol. 2009;43:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Kume K, Masamune A, Mizutamari H, Kaneko K, Kikuta K, Satoh M, Satoh K, Kimura K, Suzuki N, Nagasaki Y. Mutations in the serine protease inhibitor Kazal Type 1 (SPINK1) gene in Japanese patients with pancreatitis. Pancreatology. 2005;5:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Weiss FU, Simon P, Bogdanova N, Mayerle J, Dworniczak B, Horst J, Lerch MM. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut. 2005;54:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Ishii H, Arai K, Fukushima M, Maruoka Y, Hoshino M, Nakamura A, Koike Y, Sakamoto N, Hanada H, Kusano M. Fusion variations of pancreatic ducts in patients with anomalous arrangement of pancreaticobiliary ductal system. J Hepatobiliary Pancreat Surg. 1998;5:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009;29:1003-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 259] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Reshef R, Shtamler B, Novis BH. Recurrent acute pancreatitis associated with pancreas divisum. Am J Gastroenterol. 1988;83:86-88. [PubMed] |
| 8. | Gonoi W, Akai H, Hagiwara K, Akahane M, Hayashi N, Maeda E, Yoshikawa T, Tada M, Uno K, Ohtsu H. Pancreas divisum as a predisposing factor for chronic and recurrent idiopathic pancreatitis: initial in vivo survey. Gut. 2011;60:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Gonoi W, Akai H, Hagiwara K, Akahane M, Hayashi N, Maeda E, Yoshikawa T, Kiryu S, Tada M, Uno K. Meandering main pancreatic duct as a relevant factor to the onset of idiopathic recurrent acute pancreatitis. PLoS One. 2012;7:e37652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Coyle WJ, Pineau BC, Tarnasky PR, Knapple WL, Aabakken L, Hoffman BJ, Cunningham JT, Hawes RH, Cotton PB. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy. 2002;34:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Sajith KG, Chacko A, Dutta AK. Recurrent acute pancreatitis: clinical profile and an approach to diagnosis. Dig Dis Sci. 2010;55:3610-3616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Dawson W, Langman J. An anatomical-radiological study on the pancreatic duct pattern in man. Anat Rec. 1961;139:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Kamisawa T, Tabata I, Tajima T, Tsushima K, Yoshida Y. Patency of the human accessory pancreatic duct as determined by dye-injection endoscopic retrograde pancreatography. Digestion. 1997;58:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Bhasin DK, Rana SS, Nanda M, Gupta R, Nagi B, Wig JD. Ansa pancreatica type of ductal anatomy in a patient with idiopathic acute pancreatitis. JOP. 2006;7:315-320. [PubMed] |
| 15. | Jarrar MS, Khenissi A, Ghrissi R, Hamila F, Letaief R. Ansa pancreatica: an anatomic variation and a rare cause of acute pancreatitis. Surg Radiol Anat. 2013;35:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Koizumi M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Takeda K. JPN Guidelines for the management of acute pancreatitis: diagnostic criteria for acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Li ZS. Progress in endoscopic management of pancreas diseases. World J Gastroenterol. 1998;4:178-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Shimosegawa T, Kataoka K, Kamisawa T, Miyakawa H, Ohara H, Ito T, Naruse S, Sata N, Suda K, Hirota M. The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol. 2010;45:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Takeda K, Yokoe M, Takada T, Kataoka K, Yoshida M, Gabata T, Hirota M, Mayumi T, Kadoya M, Yamanouchi E. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010;17:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Vitellas KM, Keogan MT, Spritzer CE, Nelson RC. MR cholangiopancreatography of bile and pancreatic duct abnormalities with emphasis on the single-shot fast spin-echo technique. Radiographics. 2000;20:939-957; quiz 1107-1108, 1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Soto JA. “MR cholangiopancreatography using HASTE (half-Fourier acquisition single-shot turbo spin-echo) sequences”--a commentary. AJR Am J Roentgenol. 2007;189:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Kamisawa T, Yuyang T, Egawa N, Ishiwata J, Okamoto A. Patency of the accessory pancreatic duct in relation to its course and shape: a dye-injection endoscopic retrograde pancreatography study. Am J Gastroenterol. 1998;93:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Tamura R, Ishibashi T, Takahashi S. Chronic pancreatitis: MRCP versus ERCP for quantitative caliber measurement and qualitative evaluation. Radiology. 2006;238:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Tanaka T, Ichiba Y, Miura Y, Itoh H, Dohi K. Pathogenesis of chronic alcoholic pancreatitis. Am J Gastroenterol. 1990;85:1536-1537. [PubMed] |
| 25. | Tanaka T, Ichiba Y, Miura Y, Itoh H, Dohi K. Variations of the pancreatic ducts as a cause of chronic alcoholic pancreatitis; ansa pancreatica. Am J Gastroenterol. 1992;87:806. [PubMed] |
| 26. | Tanaka T, Ichiba Y, Miura Y, Itoh H, Dohi K. Variations of the pancreatic ducts as a cause of chronic alcoholic pancreatitis. Am J Gastroenterol. 1991;86:792-793. [PubMed] |
| 27. | Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |