Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7518
Peer-review started: September 28, 2015
First decision: November 27, 2015
Revised: February 5, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: September 7, 2016
Processing time: 343 Days and 13 Hours
To investigate the hepatic microcirculatory changes due to Haemoxygenase (HO), effect of HO inhibition on remote ischemic preconditioning (RIPC) and modulation of CINC.
Eight groups of animals were studied - Sham, ischemia reperfusion injury (IRI) the animals were subjected to 45 min of hepatic ischemia followed by three hours of reperfusion, RIPC (remote ischemic preconditioning) + IRI group, remote ischemic preconditioning in sham (RIPC + Sham), PDTC + IR (Pyridodithiocarbamate, HO donor), ZnPP + RIPC + IRI (Zinc protoporphyrin prior to preconditioning), IR-24 (45 min of ischemia followed by 24 h of reperfusion), RIPC + IR-24 (preconditioning prior to IR). After 3 and 24 h of reperfusion the animals were killed by exsanguination and samples were taken.
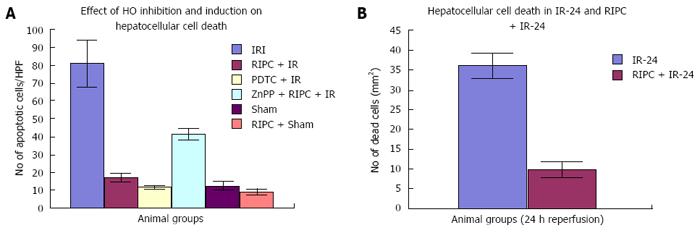
Velocity of flow (160.83 ± 12.24 μm/s), sinusoidal flow (8.42 ± 1.19) and sinusoidal perfusion index (42.12 ± 7.28) in hepatic IR were lower (P < 0.05) in comparison to RIPC and PDTC (HO inducer). RIPC increased velocity of flow (328.04 ± 19.13 μm/s), sinusoidal flow (17.75 ± 2.59) and the sinusoidal perfusion index (67.28 ± 1.82) (P < 0.05). PDTC (HO induction) reproduced the effects of RIPC in hepatic IR. PDTC restored RBC velocity (300.88 ± 22.109 μm/s), sinusoidal flow (17.66 ± 3.71) and sinusoidal perfusion (82.33 ± 3.5) to near sham levels. ZnPP (HO inhibition) reduced velocity of flow of RBC in the RIPC group (170.74 ± 13.43 μm/s and sinusoidal flow in the RIPC group (9.46 ± 1.34). ZnPP in RIPC (60.29 ± 1.82) showed a fall in perfusion only at 180 min of reperfusion. Neutrophil adhesion in IR injury is seen in both postsinusoidal venules (769.05 ± 87.48) and sinusoids (97.4 ± 7.49). Neutrophil adhesion in RIPC + IR injury is reduced in both postsinusoidal venules (219.66 ± 93.79) and sinusoids (25.69 ± 9.08) (P < 0.05). PDTC reduced neutrophil adhesion in both postsinusoidal venules (89.58 ± 58.32) and sinusoids (17.98 ± 11.01) (P < 0.05) reproducing the effects of RIPC. ZnPP (HO inhibition) increased venular (589.04 ± 144.36) and sinusoidal neutrophil adhesion in preconditioned animals (121.39 ± 30.65) (P < 0.05). IR after 24 h of reperfusion increased venular and sinusoidal neutrophil adhesion in comparison to the early phase and was significantly reduced by RIPC. Hepatocellular cell death in IRI (80.83 ± 13.03), RIPC + IR (17.35 ± 2.47), and PTDC + IR (11.66 ± 1.17) reduced hepatocellular death. ZnPP + RIPC + IR (41.33 ± 3.07) significantly increased hepatocellular death (P < 0.05 PTDC/RIPC vs ZnPP and IR). The CINC cytokine levels in sham (101.32 ± 6.42). RIPC + sham (412.18 ± 65.24) as compared to sham (P < 0.05). CINC levels in hepatic IR were (644.08 ± 181.24). PDTC and RIPC CINC levels were significantly lower than hepatic IR (P < 0.05). HO inhibition in preconditioned animals with Zinc protoporphyrin increased serum CINC levels (521.81 ± 74.9) (P < 0.05). The serum CINC levels were high in the late phase of hepatic IR (15306 ± 1222.04). RIPC reduced CINC levels in the late phase of IR (467.46 ± 26.06), P < 0.05.
RIPC protects hepatic microcirculation by induction of HO and modulation of CINC in hepatic IR.
Core tip: This study is novel in demonstrating the in vivo microcirculatory changes due to haemoxygenase (HO) induced by Remote ischemic preconditioning by brief hind limb ischemia (RIPC) in hepatic ischemia reperfusion (IR) injury. HO also decreased CINC levels (cytokine secreted from kupffer cells in hepatic IR) which is significant in reducing neutrophil recruitment and IR injury. HO inhibition abolished the protective effect of RIPC on hepatic microcirculation and was associated with significantly elevated CINC levels, serum transaminases and hepatocellular death. These findings are novel and have not been demonstrated in previous studies. RIPC may have a potential role in donor preconditioning.
- Citation: Tapuria N, Junnarkar S, Abu-amara M, Fuller B, Seifalian AM, Davidson BR. Haemoxygenase modulates cytokine induced neutrophil chemoattractant in hepatic ischemia reperfusion injury. World J Gastroenterol 2016; 22(33): 7518-7535
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7518.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7518
Ischemia reperfusion injury (IRI) in liver transplantation remains a concern since the incidence of primary nonfunction is 5%-10% in liver transplantation and the problem is aggravated in fatty livers. Recent animal experiments by our group have demonstrated that remote ischemic preconditioning (RIPC) by brief periods of limb ischemia and reperfusion significantly improved liver function, microvascular flow and modulation of hepatic microcirculation[1,2]. The role of haemoxygenase (HO)-1 in IR and RIPC has been investigated previously.
Observations in animal models of ischemia reperfusion injury of the liver and kidney suggest that there is an increase in microsomal haem content accompanied by a decrease in cytochrome-p450 content[3]. Haem is a source of reactive oxygen species i.e., free oxygen radicals which cause disruption of mitochondrial membranes associated with oxidative stress. The free radicals also inflict endothelial injury and swelling. This leads to reduced flow of red blood cells and sludging. Following reperfusion excessive oxygen results in free radical generation under the influence of the xanthine oxidase system in addition to haem. HO the rate limiting enzyme in the degradation of haem catalyzes the oxidative degradation of haem. There are three isoforms of haemoxygenase, HO-1 (inducible HO) also known as heat shock protein, HO-2 (constitutive HO found mainly in brain and testis) and HO-3 which is related to HO-2 but not well characterised. HO-1 is responsible for degradation of haem in senescent RBCs. Degradation of haem and formation of CO results in consumption of free radicals. Thus HO-1 enhances scavenging of free radicals and could potentially reduce hepatic IR injury by promoting haem degradation[4]. Based on this hypothesis experiments were conducted by Katori et al[4] and they found that the HO system was beneficial in reducing hepatic IR in animal liver transplant models. Kato showed that pre-treatment of donor rat livers with cobalt protoporphyrin (COPP) reduced hepatic IR after cold preservation and reperfusion in ex vivo models with significantly increased bile flow and portal flow as compared to non-treated livers[5]. In liver transplant models pre-treatment with COPP enhanced rat survival and decreased histological severity of IR injury in the liver as compared to nontreated rats. The beneficial effects of haemoxygenase have been demonstrated in genetically fat zucker rats[3] with significantly decreased hepatic IR injury in steatotic livers. Thus HO has a key role in the modulation of free radical generation and protection against IRI.
HO-1 has been shown to exert four major beneficial effects: (1) antioxidant function; (2) antiapoptosis; (3) anti-inflammatory function; and (4) maintenance of microcirculation. The functions of HO-1 and CO seem to be related to their ability to modulate immune function[6]. CO has been shown to exert anti-inflammatory actions, regulate cGMP activity through activation of guanylate cyclise which is known to regulate endothelial-dependent vasodilatation and inhibit platelet aggregation[7]. CO inhibits apoptosis by activating MAPK[8]. Endogenously generated CO rather than NO generated from iNOS has been shown to preserve sinusoidal perfusion and to limit hepatic dysfunction in a model of haemorrhagic shock in rats[9]. HO activity may be linked to iNOS (haem protein)[10]. INOS contains haem molecules. CO generated by HO-1 binds to haem ligands in iNOS and prevents binding of NO donor substrates thus producing an inhibitory effect on iNOS[11].
Further studies by McCarter et al[12] have shown that in ischemia reperfusion of the limbs there is remote organ injury and the expression of haemoxygenase in remote organs after 3-4 h of limb reperfusion was associated with remote organ protection[12]. Wunder et al[13] showed an increase in neutrophil adhesion in postsinusoidal venules and sinusoids following limb ischemia reperfusion injury in a rat model and the administration of chromium mesoporphyrin (HO-blocker) significantly enhanced the number of adherent neutrophils whereas administration of haemin(HO-inducer) significantly reduced the number of adherent neutrophils suggesting the role of HO-1 in remote organ protection[13,14].
Recently Lai et al[15] in a rat model of partial hepatic IR injury showed that remote ischemic limb preconditioning confers cytoprotection and protection of liver function against IRI due to HO-1 expression. In a rat model of partial hepatic IR, Lai et al[15] preconditioned the liver by four brief cycles of prior hind limb ischemia (10 min) followed by 10 min of reperfusion followed by hepatic ischemia and 240 min of reperfusion. Lai et al[15] demonstrated that RIPC decreased parenchymal injury, increased HO-1 expression in the liver and HO-1 inhibition by zinc protoporphyrrin abolished the protective effects of RIPC. They also showed increased HO-1 expression in Kupffer cells in preconditioned livers.
Functionally, CINC-1 is described as a major neutrophil chemoattractant and activator. CINC-1, induced by IL-1, TNF- and bacterial products, promotes both neutrophil rolling and adhesion, likely through the upregulation of surface integrins. It is also induced early in macrophages and declines more quickly in expression[16]. Kupffer cells, which comprise the largest fixed macrophage population in the liver are the prime source of CINC.
Hisama et al[16] demonstrated that serum CINC levels peaked 6 hours after reperfusion in animal models leading to activation and recruitment of neutrophils and CINC inhibition was associated with decrease in neutrophil recruitment in IR.
Lai et al[15] demonstrated increased HO-1 expression in kupffer cells in preconditioned livers. However they did not show the changes in microcirculation in hepatic IR or the effect of RIPC induced HO expression on Hepatic IR.
There has been no study which has demonstrated the effect of RIPC on CINC production. As Kupffer cells are known to produce CINC (Cytokine induced chemoattractant responsible for neutrophil recruitment) we investigated potential modulation of CINC production by RIPC induced HO.
All experiments were conducted under project license from home office United Kingdom in accordance with the animals’ scientific act 1986. Male Sprague - Dawley rats, weighing 250-300 gms were used. Animals were kept in a temperature controlled environment with a 12 h light-dark cycle and allowed tap water as well as standard rat chew pellets libitium. Animals were anaesthetized with 4 L/min of isoflurane (Baxter, Norfolk, United Kingdom) and maintained with 1-1.5 L/min of O2 and 0.5%-1.0% Isoflurane. They were allowed to breathe spontaneously through a concentric mask connected to an oxygen regulator and monitored with a pulse oximeter (Ohmeda biox 3740 pulse oximeter, Ohmeda, Louisville, United States).
Polyethylene catheters (0.76-mm inner diameter, Portex, Kent, United Kingdom) were inserted into the right carotid artery for monitoring of mean arterial blood pressure and the left jugular vein (0.40-mm inner diameter, Portex, Kent, United Kingdom) for administering normal saline (1 mL/100 gm/h body weight to compensate for intraoperative fluid loss. The animals were placed in supine position on a heating mat (Harvard apparatus Ltd., Kent, United Kingdom) to maintain their temperature.
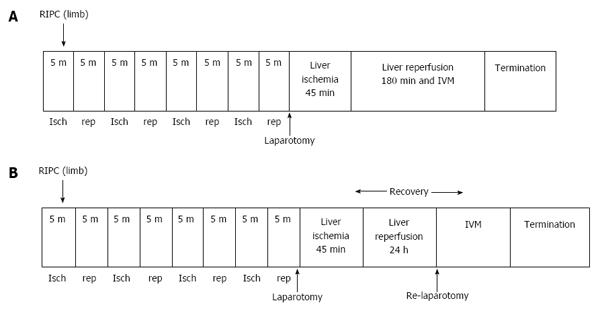
Hepatic IR models for early (Figure 1A) and late phase of IR (Figure 1B). Laparotomy was performed through a midline incision. The hepatic ligaments were cut and the liver was mobilised for exposure. A standard model of lobar hepatic ischemia of the left lateral and median lobes of (70% of liver)[17] was used. Ischemia was induced by clamping the corresponding vascular pedicle with an atraumatic microvascular clamp. Hepatic ischemia was induced for a period of 45 min followed by 3 h reperfusion to study the early phase of hepatic IR and 24 h of reperfusion to study the late phase of hepatic IR. Experiments were terminated by exsanguination of animals at the end of the reperfusion period. All animals had a bolus of heparin (20 U/kg, intravenously) prior to clamping to prevent potential thrombus formation in the hepatic artery. Global ischemia was not induced since the aim of this study was to investigate the effects of warm IR. Lobar ischemia in this model prevented splanchnic congestion and portal hypertension.
A tourniquet was applied around the thigh in one of the hind limbs. Limb perfusion was monitored by a laser Doppler (Moor instruments, Surrey, United Kingdom) and the tourniquet was tightened until no flow was detected. The procedure involved 5 min of ischemia followed by 5 min of reperfusion. This was repeated four times[18].
Animal behaviour was assessed every hour for 4 h and then at 22 and 24 h. Signs of poor clinical condition were lethargy, ruffled fur and guarding upon abdominal palpation, lack of grooming and decreased food intake. Animals which appeared to do poorly were killed before the 24 h reperfusion end point
The animals were re-anaesthetized after 24 h of reperfusion, homeostasis and monitoring was undertaken as described in the section on animal procedures. The abdominal sutures were carefully opened up and the liver was examined under the intravital microscope.
All animals were looked after according to home office United Kingdom guidelines ensuring that they did not suffer pain or distress during these experiments. They were anaesthetised with isoflurane and exsanguinated for collection of tissues and bloods.
Experimental groups (n = 6 in each group): Eight groups of animals were studied: (1) Group one (Sham) in which animals were subjected to laparotomy only and underwent an identical experimental protocol without clamping; (2) Group two (IRI) the animals were subjected to 45 min of ischemia followed by three hours of reperfusion; (3) Group three were preconditioned prior to IRI (RIPC + IRI) group. Protocols described above for preconditioning and inducing ischemia were used; (4) Group four sham animals were preconditioned (RIPC + Sham); (5) Group five animals were given Pyrrolidine dithiocarbamate (PDTC) 100 mg /kg 30 min prior to IR (PDTC + IR); (6) Group six animals were given Zinc protoporphyrin (ZNPP) 1.5 mg/kg (HO-1 blocker) by intraperitoneal route 1 h prior to RIPC and subsequent IRI (ZnPP + RIPC + IRI); (7) Group seven animals were subjected to ischemia for 45 min followed by reperfusion for 24 h and then subjected to intravital microscopy (IR-24); and (8) Group eight animals were preconditioned followed by ischemia for 45 min and reperfusion for 24 h (RIPC + IR-24).
The liver was placed upon a glass mount and covered with a cover slip along with continuous normal saline irrigation. A drop of saline was placed on the cover slip to enable immersion of the microscope lens tip. A Nikon (Tokyo, Japan) microscope (Nikon epi-illumination system with filter block set suitable for Texas Red, FITC and DAPI dyes) coupled to a CCD camera (JVC TK-C1360B (Osaka, Japan) colour video camera) was used. Magnification provided was 10 × and 40 ×. The microscopy images were transferred by camera to a video monitor and recorded for offline analysis. Frame by frame analysis of the recorded images for quantitative analysis was performed. Microcirculation was evaluated by measuring acinar perfusion in ten randomly chosen acini and leukocyte endothelial interaction in ten postsinusoidal venules. LUCIA (lab universal computer image analysis, Nikon, Tokyo, Japan) software was used to analyse the images.
RBC velocity (V): (1) 0.5 mL of FITC labelled red cells suspended in glucose saline buffer solution (20 mgFITC/mL of RBC) were given intravenously. Ten randomly chosen nonoverlapping rappaport acini were assessed[19]; and (2) The RBC velocity was assessed by measuring the length (L) of RBC movement (microns) in each sinusoid in subsequent frames. Twenty-five frames were captured per second. Hence the formula = L × 25/number of frames moved was used for calculating velocity.
The sinusoidal perfusion index was evaluated as ratio of perfused hepatic sinusoids Continuous perfusion (Scp) + intermittent perfusion (Sip) to the total visible sinusoids which includes non-perfused sinusoids (Snp). Perfusion index = (Scp + Sip/Scp + Sip + Snp).
Sinusoidal diameter (D): This was measured by assessing the length across the sinusoids and expressed in microns.
Sinusoidal blood flow: This was calculated using the formula- Velocity (V) ×22/7× (D/2)2.
Neutrophil adhesion: Rhodamine 6G (0.3 mg/kg)[20] was given intravenously for staining of neutrophils[13,14,21]. The numbers of stationary leuokocytes for a period of 30 s under green filter light were expressed as leukocytes/mm2. The area of the vessels was calculated using the product of diameter and length assuming cylindrical geometry (3.14 × D × L)[22,23].
Propidium iodide[12] (0.05 mg/kg i.v.) was injected intravenously to stain nuclei of dead hepatocytes which was expressed as number of dead cells/HPF[13,14,21].
Animals were killed by exsanguination and blood was collected in BD vacutainer tube SSTmII advance 5.0 mL tubes for Serum, BD vacutainer tube LH 102 I.U > (6 mL) for citrated plasma and BD vacutainer K 2E 7.2 mg (4 mL) for EDTA plasma and centrifuged at 3000 rpm for 10 min to sediment the RBC. Serum and plasma samples were frozen in liquid nitrogen and subsequently stored in -80 degees Celsius. Liver tissue was snap frozen and stored at -80degrees Celsius. Tissues were also fixed in 10% formalin and embedded in paraffin for histology.
Sections were cut at 5μ and stained with haematoxylin and eosin for histological analysis. Modified Suzuki criteria were used to describe the histological changes in the early phase of hepatic IR. The scoring used is as shown (Table 1).
| Numerical assessment | 0 | 1 | 2 | 3 | 4 |
| Sinusoidal congestion | None | Minimal | Mild | Moderate | Severe |
| Vacuolation | None | Minimal | Mild | Moderate | Severe |
| Necrosis | None | Single cell | 30% | 60% | > 60% |
The paraffin fixed slides were dewaxed in several changes of xylene and then rehydated through absolute alcohol, 90%alcohol, 70%alcohol to distilled water or deionised water (1-2 min in each solvent). Sections of 5μ were used. Mercury pigment was removed with iodine. In order to inactivate endogenous peroxidase and retrieve antigen the slides were immersed for 30 min in a solution of 0.3% H2O2 (4 mL of H2O2 per 100 mL) in methanol and were rinsed in phosphate buffer solution (PBS). This was followed by blocking of non-specific background with 100 mL of normal rabbit serum (diluted 1:30 with PBS) for 30 min. Primary rabbit polyclonal antibody was diluted in PBS containing 0.05% bovine serum albumin and 0.01% sodium azide at room temperature for 2 h. The slides were rinsed with PBS three times for 5 min each and then incubated for 30 min with 50-100 mL of 1:200 diluted biotinylated antibody solution(anti rabbit IgG). This was followed by three rinses with PBS for 5 min each. They were then incubated with secondary antibody for 30 min at room temperature.
To develop the reaction the sections were incubated with vectastain ABC reagent (Santa cruz technology) using peroxidise as substrate and 3, 3’ diaminobenzidine tetrahydrochloride as chromogen. Sections were then counterstained with hematoxylin.
The scoring system used for HO-1 expression is based on the system used for assessing oestrogen receptor status of invasive ductal carcinoma cells of the breast in paraffin embedded section[23-25]. The cells which are positive for HO-1 marker are graded into 4 grades depending on the intensity of staining: 0 = No staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining.
This was multiplied with the percentage of cells positive. The percentage was derived by counting the number of positive cells in a total of 100 cells in a high power field. Hence the minimum score possible was 0 and the maximum 100.
Haemoxygenase protein was identified using Western blotting. Frozen samples of Liver tissue (100-200 mg) were homogenised in TOXEX buffer (20 mmol/L HEPES [pH 7.9], 0.35 mol/L NaCl, 20% glycerole, 1% Nonidet P-40, 1 mmol/L MgCl2, 0.5 mmol/L ethylenediaminetetraacetic acid, 0.1 mmol/L ethylene glycolbis(b-aminoethyl ether)-N,N-tetraacetic acid, 100 mmol/L dithiothreitol, 0.1% phenylmethylsulfonyl fluoride, 10 mg/mL aprotinin) on ice, incubated for 30 min, and centrifuged at 13000 rpm for 5 min. For each lane, 100 mg of protein was dissolved in 10 mL of 13 sodium dodecyl sulfate loading dye and boiled for 5 min. A biotinylated protein marker (New England Biolabs, Schwalbach, Germany) was added. The samples were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore, Bedford, MA) by electroblotting for 2.5 h (Semidry Trans-Blot, BioRad, Hercules, CA). The membrane was blocked in a buffer containing 20 mmol/L Tris-base (pH 7.6), 137 mmol/L NaCl, 3.8 mL 1 mol/L HCl/L, 0.1% Tween (13 TBST), and 5% low-fat dry milk powder for 1 h and incubated with a rabbit polyclonal anti-HO-1 antibody (1:1000 dilution; SPA 895, StressGen, Biotechnologies, Victoria, British Columbia, Canada) in 13 TBST and 5% low-fat dry milk for 2 h at room temperature. After 3 washing steps with 1 X TBST, a secondary anti-rabbit (1:10000 dilution; ECL-detection kit, Amersham Pharmacia, Freiburg, Germany) and horseradish peroxidase- conjugated anti-biotin antibody (1:1000, New England Biolabs) was added and incubated for 1 h in 1 × TBST and 5% low-fat dry milk. Following 2 washing steps with 1 × TBST and 2 washing steps with 1 × TBS, detection was performed by the ECL detection kit (Amersham Pharmacia) according to the manufacturer’s instructions. After this the membrane was exposed to a digital camera as part of an electronic imaging system to visualise the proteins bound to the antibody.
This was done by using Adobe Photoshop. The JPEG picture of the western blot was analysed using this software. The maximum density of the film is 255. The density of the background on the film was measured and the density of the HO band obtained for each sample was measured. The density of the background was subtracted from the density of the HO band. The value obtained was subtracted from 255 to obtain the final density of the sample
Reagents: One 96 well polystyrene microplate coated with polyclonal antibody specific for rat CINC-1 and 12.5 mL polyclonal antibody against rat CINC-1 conjugated to horseshoeradish peroxidise. Rat CINC-1 Standard- 2.5 ng of recombinant rat CINC-1 in a buffered protein base. Rat CINC-1 control-1 vial of recombinant rat CINC-1 in a buffered protein base. Assay diluent RD1W- 12.5 mL of a buffered protein solution with preservatives. Calibrator diluent RD5-4-21 mL of a buffered protein solution with preservatives. Wash buffer concentrate-50 mL of a 25 fold concentrated solution of buffered surfactant with preservatives.
Colour reagent A-12.5 mL of stabilized hydrogen peroxide. Colour reagent B- 12.5 mL of stabilized chromogen (tetramethyl benzidine). Stop solution-23 mL of a diluted HCL. Plate covers- 4 adhesive strips.
Sample preparation: Rat serum samples were diluted 2-fold into calibrator diluent RD5-4 prior to assay.
50 μL of assay diluent RD1W added to each well. 50 μL of standard, control or sample per well added. The plate was gently tapped for 1 min to mix the contents and covered with adhesive strip. This was followed by incubation for 2 h at room temperature. Each well was aspirated and washed for a total of five times with wash buffer (400 μL). 100 μL of rat CINC-1 conjugate was added to each well. The plate was covered with adhesive strip and incubated for 2 h at room temperature. The wells were aspirated and washed five times. One hundred microliter of substrate solution was added to each well and incubated at room temp for 30 min under protection from light. One hundred microliter of stop solution was added to each well. Plate was gently tapped to ensure mixing. Optical density was measured within 30 min using a microplate reader set to 450 nm. (correction wavelength set at 540 nm or 570 nm).
All data are presented as mean plus minus standard error of the mean. Differences were considered significant for P < 0.05.Comparisons between groups were performed by one way analysis (ANOVA). Bonferroni correction was applied for ANOVA.
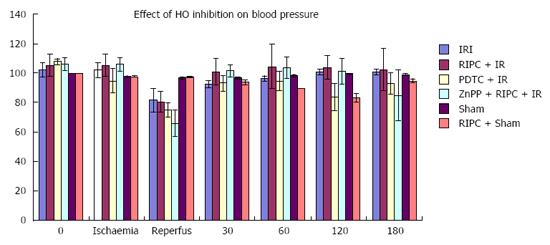
RIPC + IR group showed a drop in MAP similar to IR only group however MAP recovered to baseline in RIPC+IR rapidly (30-60 min) compared to IR (60-120 min). HO inhibition caused a further drop in B.P. and delayed recovery of blood pressure (Figure 2). There was no significant difference in pulse rate.
Of the six animals in each group, one animal in the IR-24 group died before the end of recovery period due to severe reperfusion injury. This animal was excluded from intravital analysis. All animals in the RIPC + IR-24 group survived after 24 h of reperfusion.
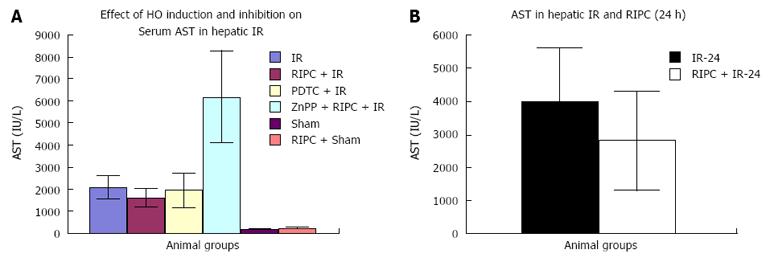
Effect of IR, RIPC, PDTC and ZnPP on hepatic transaminases (Early phase). Baseline transaminase levels in RIPC+sham were higher than sham. IRI produced a rise in transaminase levels (Figure 3A). Effect of IR and RIPC in the late phase of hepatic IR on hepatic transaminases (Figure 3B).
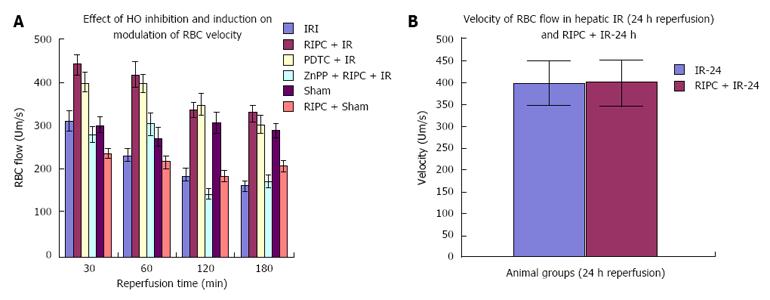
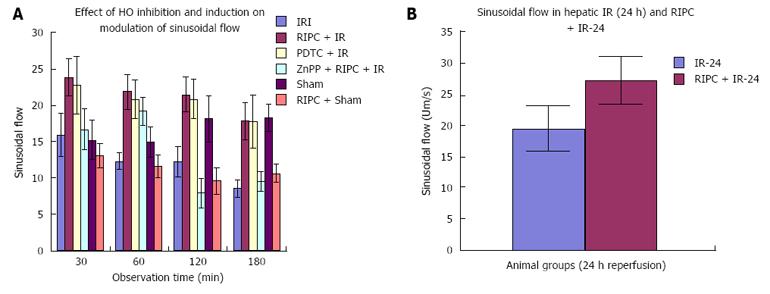
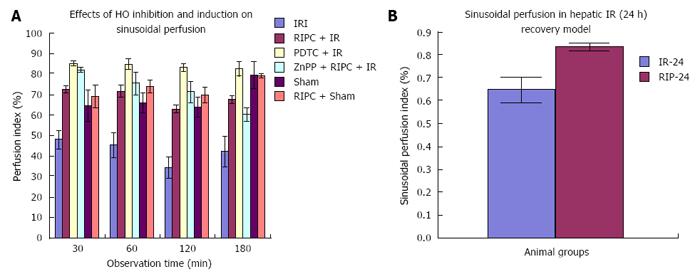
The sham group showed constant microcirculatory parameters. RIPC induced a low grade oxidative stress in the liver resulting in lower RBC flow and perfusion as compared to sham. The velocity of flow (160.83 ± 12.24 μm/s), sinusoidal flow (8.42 ± 1.19) and sinusoidal perfusion index (42.12 ± 7.28) in hepatic IR were significantly lower than RIPC + IR.
RIPC significantly increased velocity of flow (328.04 ± 19.13 μm/s), sinusoidal flow (17.75 ± 2.59) and the sinusoidal perfusion index was higher in RIPC group (67.28 ± 1.82). PDTC (HO induction) reproduced the effects of RIPC in hepatic IR. PDTC restored RBC velocity (300.88 ± 22.109 μm/s), sinusoidal flow (17.66 ± 3.71) and sinusoidal perfusion (82.33 ± 3.5) to near sham levels. ZnPP (HO inhibition) significantly reduced velocity of flow of RBC in the RIPC group (170.74 ± 13.43 μm/s and sinusoidal flow in the RIPC group (9.46 ± 1.34) However inhibition of HO by ZnPP in RIPC (60.29 ± 1.82) showed a fall in perfusion only at 180 min of reperfusion (Figure 4A, Figure 5A, Figure 6A and Figure 7A)
In the late phase of hepatic IR, velocity of flow was similar between preconditioned and IR -24 suggesting recovery of velocity of flow to near normal values after 24 h of reperfusion in the absence of preconditioning. However there was a significant increase in sinusoidal diameter, sinusoidal flow and perfusion in the RIPC + IR-24 group (Figure 4B, Figure 5B, Figure 6B and Figure 7B).
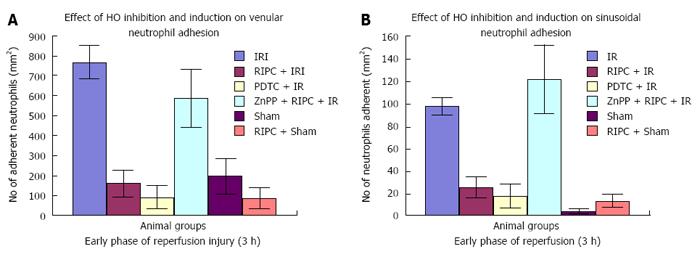
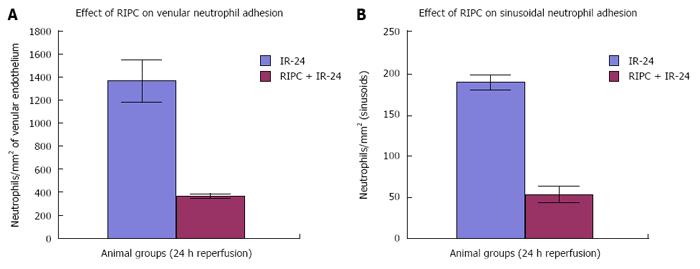
Significantly increased neutrophil adhesion in IR injury is seen in both postsinusoidal venules (769.05 ± 87.48) and sinusoids (97.4 ± 7.49). RIPC significantly reduced neutrophil adhesion in IR injury in both postsinusoidal venules (219.66 ± 93.79) and sinusoids (25.69 ± 9.08).
PDTC significantly reduced neutrophil adhesion in both postsinusoidal venules (89.58 ± 58.32) and sinusoids (17.98 ± 11.01) reproducing the effects of RIPC. HO inhibition with ZnPP significantly increased venular (589.04 ± 144.36) and sinusoidal neutrophil adhesion in preconditioned animals (121.39 ± 30.65). IR after 24 h of reperfusion significantly increased venular and sinusoidal neutrophil adhesion in comparison to the early phase and was significantly reduced by RIPC (Figure 8A and B, Figure 9A and B).
Hepatocellular cell death is significantly less on HO induction (PDTC + IR) in hepatic IR. Preconditioning (RIPC + IRI) reproduces effects of HO induction as Hepatocellular cell death in RIPC+IR is significantly less compared to IRI group. HO inhibition prior to preconditioning (ZnPP + RIPC + IR) significantly enhances hepatocellular death in preconditioned animals.
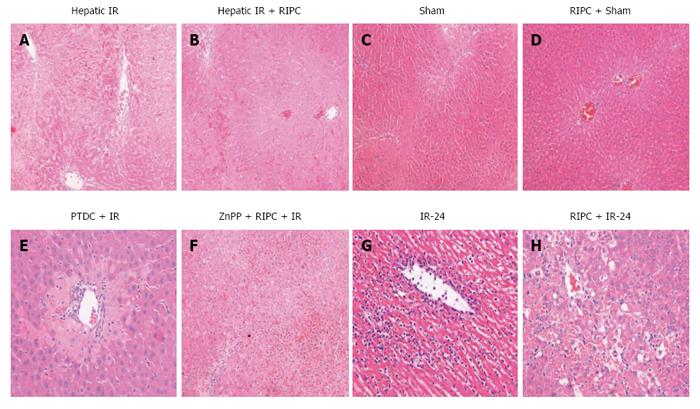
IR group showed diffuse and significant periportal congestion. There was severe necrosis in zones 2 and 3 and also sub capsular necrosis. RIPC+IR showed a significantly lower Suzuki score compared to the IR group. On inhibition of HO in RIPC+IR (ZNPP + RIPC + IR group) most of the sections showed diffuse and severe congestion and vacuolation. The necrosis is diffuse and focally associated with acute inflammatory cells. Changes in ZnPp + RIPC + IR were similar to IR group and significantly more compared to RIPC + IR. Only one animal showed extensive congestion and vacuolation but minimal necrosis. On HO induction (PTDC + IR) most animals showed diffuse congestion with patchy necrosis except for one which shows mild congestion. Sham animals did not show significant changes apart from congestion in both portal and central vein. Hepatocytes showed vacuolation. RIPC + Sham animals showed moreless similar changes with minimal vacuolation but no necrosis (Figures 10 and 11). Suzuki criteria see Table 2.
| Group | IR | RIPC + IR | ZnPP + RIPC + IR | PDTC + IR | Sham | RIPC + Sham |
| Suzuki score | 8.83 ± 0.7 | 6.2 ± 0.58 | 8.83 ± 0.6 | 4.5 ± 0.5 | 4 ± 0.31 | 1.5 ± 0.34 |
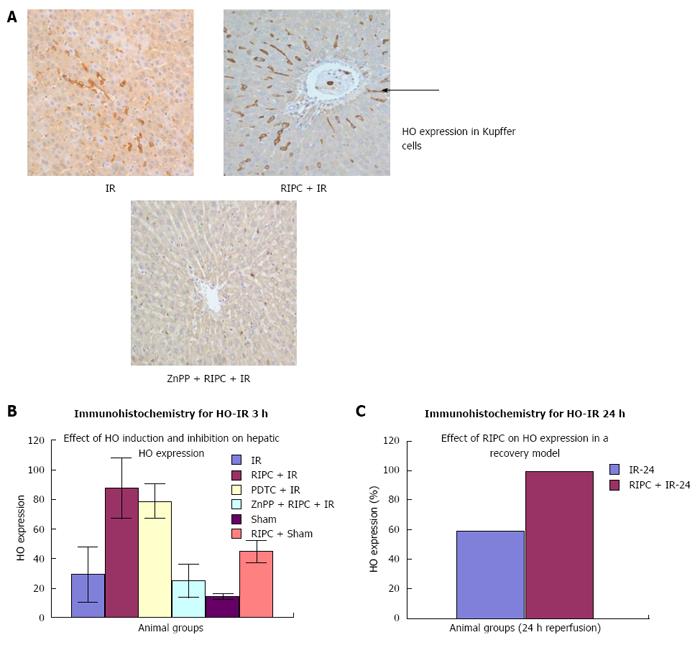
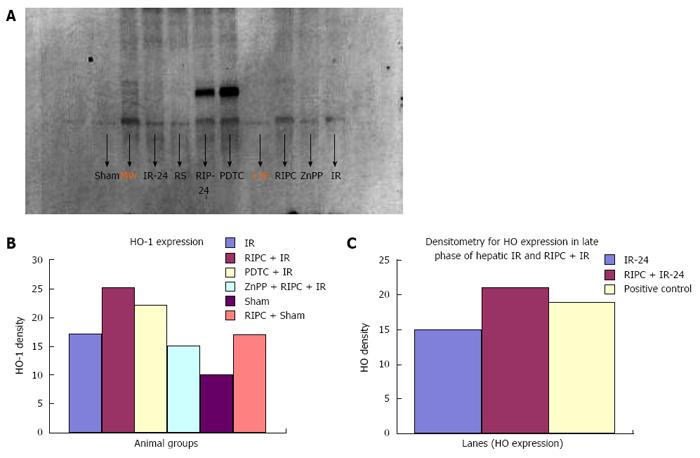
Immunohistochemistry revealed increased HO-1 expression in kupffer cells in RIPC + IR animals in comparison to IR group. Baseline HO expression is seen in kupffer cells in sinusoids of sham group. Haemoxygenase expression in IR (3 h of reperfusion) was more compared to sham. This is due to increased oxidative stress which acts a direct stimulus to HO-1 expression. The increased HO expression was observed in kupffer cells which are the primary source of HO production and degradation of haem to biliverdin and CO. At 24 h of reperfusion more HO was observed in Kupffer cells. PDTC induced HO-1 in macrophages in the early phase of hepatic IR. Inhibition of haemoxygenase by ZnPP (ZnPP inhibits both HO-1 and HO-2) showed decreased HO expression in macrophages in the early phase of hepatic IR. This explains the intravital findings of increased neutrophil adhesion and hepatocellular death in comparison to preconditioned animals.
In the late phase of hepatic IR (24 h) there was increased HO expression in both kupffer cells and hepatocytes (parenchymal cells) in preconditioned animals in comparison to IR only (Figure 12). Immunohistochemistry score for HO see Table 3.
Western blot analysis showed by densitometry significantly more HO expression in RIPC groups as compared to hepatic IR and PDTC reproduced the effects of RIPC. ZnPP inhibited HO expression in the RIPC group. Increased HO expression was seen in RIPC + IR-24 as compared to IR-24 (Figure 13).
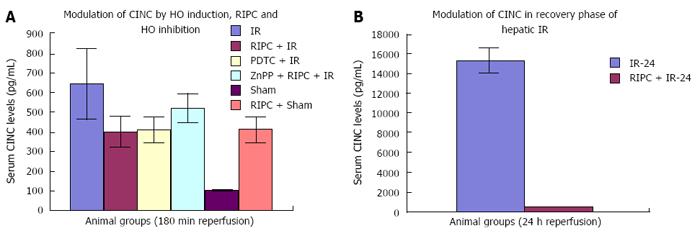
The CINC data shows significant difference between IR injury, RIPC + IR and ZnPP + RIPC + IR in the early phase of hepatic IR. In the late phase of IR there is a significant difference seen between IRI and RIPC+IRI suggesting that preconditioning modulates release of CINC-1 from kupffer cells.
The CINC cytokine levels in sham were low (101.32 ± 6.42). RIPC in sham led to relatively high CINC levels (412.18 ± 65.24) as compared to sham (P < 0.05). Hepatic IR injury produced high serum CINC level in comparison to sham animals in the early phase of hepatic IR (644.08 ± 181.24) (P < 0.05). RIPC reduced CINC -1 levels in the early phase (401.62 ± 78.56) in comparison to IR only. PDTC (HO inducer) reduced CINC-1 levels in serum in hepatic IR (413.36 ± 63.06). HO inhibition in preconditioned animals with Zinc protoporphyrin increased serum CINC levels (521.81 ± 74.9) (P < 0.05) (Figure 14A).
The serum CINC levels were high in the late phase of hepatic IR (15306 ± 1222.04). RIPC reduced CINC levels in the late phase of IR (467.46 ± 26.06, P < 0.05) (Figure 14B).
This is the first study to use intravital microscopy and demonstrate the effects of HO inhibition on the protective effect of RIPC in hepatic IR and that RIPC induced HO modulates cytokine release from Kupffer cells and subsequent neutrophil activation.
Blood pressure: The model used was haemodynamically stable. However a fall in blood pressure on reperfusion was seen in hepatic IR, RIPC + IR, PDTC + IR and ZnPP group with recovery to baseline in 30-60 min. The blood pressure thereafter was stable throughout the time course of observation. Hence the potential effect of hypotension leading to decreased parenchymal perfusion and confounding results were avoided.
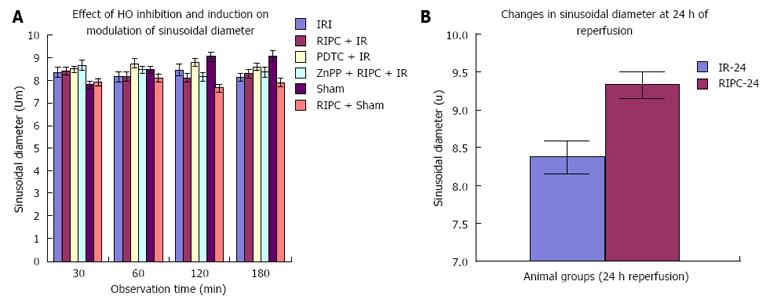
IR, RIPC + IR: RIPC + IR showed an increased velocity of RBC flow, sinusoidal flow and sinusoidal perfusion compared to IR group at all time points of observation and the administration of ZnPP (HO blockade) prior to RIPC abolished all protective effects. The loss of protective effects of RIPC after HO blockade suggests that HO pathways may have a role in modulation of hepatic IR. The lack of any change in sinusoidal diameter on HO inhibition suggests that RIPC modulates IR injury by anti-inflammatory properties of HO rather than sinusoidal dilatation in the early phase of hepatic IR. This observation was made in previous studies on ischemic preconditioning which have demonstrated no significant variation in sinusoidal diameter between HO induction and controls[26]. Moreover HO produced in the initial phase is in response to stress (inducible HO-1) and is responsible for anti-inflammatory functions[5]. Constitutive HO (HO-2) modulates sinusoidal tone and diameter and is produced in parenchmal cells after 6hrs of reperfusion as shown by Goda et al[27] and CO derived from an increase in HO-2 (Constitutive HO) in the parenchyma is responsible for modulation of sinusoidal tone and diameter.
This study shows increased RBC velocity and sinusoidal flow in PDTC+IR group as compared to IR only. The perfusion index in PDTC group is significantly higher than IRI at all time points. Inhibition of HO in preconditioned groups showed a significant fall in RBC velocity and sinusoidal flow in the RIPC + IR + ZnPP group. PDTC is known to induce HO-1, inhibit NF-κB and modulate IRI and increased HO-1 may be responsible for modulation of sinusoidal perfusion. This finding is supported by evidence from studies in animal models which have shown that HO-1 expression is associated with better sinusoidal perfusion[12,28,29]. However there was no significant difference in sinusoidal perfusion between HO induction and HO inhibition (ZnPP group) upto 120 min of reperfusion in our study suggesting that HO pathways maybe responsible for modulation of perfusion after 120 min of reperfusion. Previous studies have shown endogenous haemoxygenase expression to increase sinusoidal perfusion at 120 min of reperfusion[21] in animal models of hepatic IR secondary to prolonged limb ischemia. Hence the initial modulation of sinusoidal perfusion in hepatic IR by PDTC may be due to its anti-inflammatory action and inhibitory effect on NF-κB[29-31].
A previous study in a transplant model by Tsuchihashi demonstrated that PDTC given prior to harvest of liver reduced reperfusion injury after transplantation[30]. ZnPP given just before reperfusion blocked the protective effects of PDTC suggesting the role of PTDC induced HO-1. In our study low doses of PDTC (100 mg/kg) were used and no variation in sinusoidal diameter was observed however, recently Hata et al[26] have demonstrated an increase in sinusoidal diameter due to increase in parenchymal HO-1 on administration of higher doses of PDTC (150 mg/kg) intramuscularly in a rat model of hepatic IRI. Hata et al[26] has shown that CO derived from PDTC induced increase in parenchymal HO and modulated sinusoidal diameter in the early phase of hepatic IR.
RIPC significantly reduced neutrophil adhesion and hepatocellular death and increased sinusoidal perfusion as well as diameter. There was a significant increase in sinusoidal flow due to increase in diameter but not velocity. This increase in sinusoidal diameter has been demonstrated by our group in the late phase of RIPC[32] and other IPC studies.
Haemoxygenase expression in the liver was significantly more in the RIPC sham group as compared to shams. Increased HO-1 expression was predominantly observed in Kupffer cells in the early phase with additional parenchymal expression in the late phase of IR. HO-1 pathways are initiated after oxidative stress and previous studies[21] have shown expression of mRNA as early as 2 h after the initial oxidative stress. The increase in velocity and flow seen after 30 min of reperfusion corresponds to 2 h after the first cycle of preconditioning and the time point of earliest HO-mRNA expression suggesting that HO-1 pathways maybe responsible for increased flow seen in this study.
Interestingly the increased HO-1 expression was seen in kupffer cells in RIPC. This observation was also made by Lai et al[15]. Lai et al[15] demonstrated lack of increased HO-1 in the peripheral macrophages and hence it is unlikely that the increased haemoxygenase in the liver is from infiltrating macrophages. Hirano et al[33] have demonstrated that kupffer cells are the principal source of HO-1 in the liver. Previous studies have demonstrated increased free radicals in the blood following RIPC[34]. Increased ROS may induce a low grade oxidative stress in the liver and since kupffer cells are the key cells in the initial inflammatory response this may explain the increased HO-1 in the kupffer cells.
HO mediates breakdown of haem to biliverdin, iron and CO. Biliverdin and iron have antioxidant functions and CO has both vasodilatory and antiplatelet functions. HO-1 modulates flow, stabilizes membrane potential and most importantly degrades haem. It scavenges free radicals generated in IR injury thus mitigating the effects of IR injury[21]. The protective effects of RIPC, increased HO-1 expression in RIPC group and loss of protective effects of RIPC after inhibition of HO-1 suggest the role of RIPC induced HO-1 pathways in modulation of hepatic microcirculation. In addition the production of CO as a result of haem breakdown maybe responsible for reduced stasis and plugging due to its antiplatelet effects thereby leading to increased RBC flow. CO is known to modulate the function of hepatic sinusoidal pericytes called Ito cells which regulate sinusoidal tone and function. Future studies would need to investigate the role of CO releasing molecules (CORM) in modulation of hepatic IR.
Hepatic IR/RIPC + IR/PDTC + IR: The modified Suzuki score in IR injury was significantly higher (8.83 ± 0.7) than RIPC+IR group (6.2 ± 0.58) or PDTC+IR group (4.5 ± 0.5). This correlated with decreased flow and sinusoidal perfusion, increased hepatocellular death observed by propidium iodide staining under IVM in hepatic IR, increased sinusoidal and venular neutrophil adhesion, increased CINC levels and increased serum transminase levels. Increased HO expression was seen in kupffer cells in hepatic IR as compared to sham group. This is due to oxidative stress of IR and has been observed in hepatic IR in previous experiments by Katori et al[4] and Hata et al[26]. Both preconditioned animals (RIPC + IR) and PTDC+IR group showed improved blood flow, sinusoidal perfusion, decreased hepatocellular death , sinusoidal and venular neutrophil adhesion, decreased CINC levels and serum transaminase levels. Lai et al[15] showed that preconditioned animals (RIPC+IR) showed decreased histological evidence of injury and serum transminases supporting our findings in this study although they did not investigate microcirculatory flow. Increased HO expression was seen in RIPC as compared to IR only. Previous studies have shown by intravital microscopy improved flow and sinusoidal perfusion and decreased hepatocellular death following PDTC induced HO expression in the liver[12,20].
ZnPP + RIPC + IR: Inhibition of HO by ZnPP in the preconditioned group showed a significantly high Suzuki score (8.83 ± 0.6) suggestive of increased cell necrosis and congestion which correlated with decreased sinusoidal flow and perfusion, increased hepatocellular death on IVM, increased neutrophil adhesion, CINC levels and serum transaminase levels suggestive of significantly increased hepatocellular injury. Decreased HO expression was seen on immunohistochemistry and Western blot analysis in comparison to RIPC group. Previous studies by Katori et al[35] have shown that HO inhibition of the donor with chromium mesoporphyrin prior to harvest of the liver was associated with decreased recipient animal survival following subsequent implantation in recipient liver transplant models. Lai et al[15] showed that inhibition of HO by ZnPP in RIPC was associated with significantly increased histological evidence of cell necrosis and congestion and increased serum transaminases.
IR-24 and RIPC + IR-24: Increased HO protein correlated with decreased sinusoidal and venular neutrophil adhesion in RIPC in the late phase, decreased histological necrosis and congestion and hepatocellular death as observed by propidium iodide staining in the late phase of RIPC + IR. This suggests that HO protein is responsible for protection of hepatic parenchyma and hepatic microcirculation due to reduced injury in delayed phase of hepatic IR. Sinusoidal diameter increased in the late phase of hepatic IR suggesting that HO may be responsible for modulation of sinusoidal dilatation. Interestingly the difference in flow between IR and RIPC groups was due to modulation of flow due to changes in sinusoidal diameter and not RBC velocity.
ZnPP and its hepatotoxicity: Previous experiments in sham animals have shown that ZnPP inhibits haemoxygenase if used in the darkness. If ZnPP is exposed to light its effect of HO inhibition is lost however, ZnPP inhibits hepatic artery dilatation when exposed to light and this potentially leads to hepatotoxicity[36,37]. Hepatoxicity can confound the experimental data and hence in order to avoid this confounding effect ZnPP was prepared in the dark and administered through a syringe covered with silver foil with minimal laboratory light. The dose used was 1.5 mg/kg (2.5 μmol/kg) and this is much lower than the hepatotoxic toxic dose of ZnPP (5-10 μmol/kg) as shown by Greenbaum et al[36]. Amersi et al[3] (1999) showed that ZnPP treatment in shams led to undetectable baseline HO and reduced portal flow and bile flow.
Tsuchihashi et al[30] (2003) demonstrated in an animal model that doses in excess of 600 mg/kg killed animals but animals did not die by injection of any dose upto 600 mg/kg. Doses more than 200 mg/kg offset the beneficial effects of PDTC as demonstrated by Liu et al[29] (1999). Hence we chose a dose of 100 mg/kg as used in experiments by Tsuchihashi et al[30]. PDTC shows a dose dependent relationship with regard to its beneficial effects. At doses of 100 mg/kg PDTC induces HO in kupffer cells but does not influence sinusoidal dilatation. Hata et al[26] showed that at doses of 150 mg/kg PDTC induced sinusoidal dilatation in sham animals, increased HO-1 with peak mRNA levels detectable at 3 h after PDTC injection and then declined. HO Protein expression peaked at 24-48 h after injection and was dose dependent. Immunohistochemistry revealed that PDTC at higher doses induced HO-1 in both periportal kupffer cells and hepatocytes in pericentral areas.
In a recent animal rat model of orthotopic liver transplantation RIPC applied to the recipient in the anhepatic recipient phase significantly improved liver graft function[38].
This study has demonstrated the hepatic microcirculatory changes which are associated with the early and late phases of liver warm IR injury. RIPC induced increased sinusoidal perfusion and sinusoidal flow in both phases of IR but increased velocity of RBCs in the early phase in contrast to increased sinusoidal diameter in the late phase. The effect of RIPC was reproduced by HO-1 induction prior to the IR injury and inhibited by a HO-1 blocker in the early phase of hepatic IR. Increased HO production was observed in the late phase of hepatic IR both in kupffer cells and parenchymal cells.
These data would suggest that HO-1 is a key pathway responsible for modulation of microcirculatory changes and protection from the deleterious effects of IR. In the early period inducible HO-1 rather than constitutive HO-1 through its antinflammatory actions is likely to be the key candidate molecule responsible for protection of microcirculation. In the late phase it is likely that parenchymal HO is responsible for sinusoidal dilatation and protection of hepatic microcirculation. This study could not distinguish between the subtypes of HO.
Another Key finding of the study was demonstration of CINC modulation and neutrophil activation by HO.
Further investigation in HO knockout mice to clarify the mechanism of HO and the role of different subtypes is needed. The role of HO inducers in the clinical setting of hepatic IR and use of CORM (CO releasing molecules), curcumin and simvastatin (HO inducer) needs to be further investigated. Translation to clinical application would be the next step.
This original research article has focussed on investigating the role of haemoxygenase pathways in remote ischemic preconditioning (RIPC) induced modulation of hepatic ischemia reperfusion injury. Intravital microscopy in this study has demonstrated significantly improved hepatic microcirculation following haemoxygenase (HO) induction and loss of protective effects of RIPC following HO inhibition.
This study is Novel in demonstrating the protective effect of RIPC on hepatic IR through modulation of haemoxygenase pathways and Serum CINC (Cytokine induced neutrophil chemoattractant) levels. Significantly increased flow, perfusion and decreased neutrophil adhesion as well as hepatocellular death co-related with decreased CINC levels and increased HO production in RIPC.
This study highlights the modulation of CINC by RIPC induced HO. CINC is responsible for neutrophil activation and modulation of neutrophil activation is a key mechanism in protection of hepatic microcirculation.
RIPC can be translated to donor preconditioning and preconditioning prior to liver resection to minimise ischemia reperfusion injury and manage small for size syndrome.
This article is to study the hepatic microcirculatory changes due to HO induced by RIPC, effect of HO inhibition and modulation of CINC. The study finding is new and interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Xu Q, Zhang YP S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Tapuria N, Junnarkar SP, Dutt N, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford). 2009;11:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 406] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, Geller DA, Murase N. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236-G244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Brüne B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497-504. [PubMed] |
| 8. | Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109-5114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Rensing H, Bauer I, Datene V, Pätau C, Pannen BH, Bauer M. Differential expression pattern of heme oxygenase-1/heat shock protein 32 and nitric oxide synthase-II and their impact on liver injury in a rat model of hemorrhage and resuscitation. Crit Care Med. 1999;27:2766-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1997] [Cited by in RCA: 1975] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 11. | Wang J, Stuehr DJ, Ikeda-Saito M, Rousseau DL. Heme coordination and structure of the catalytic site in nitric oxide synthase. J Biol Chem. 1993;268:22255-22258. [PubMed] |
| 12. | McCarter SD, Akyea TG, Lu X, Bihari A, Scott JR, Badhwar A, Dungey AA, Harris KA, Feng Q, Potter RF. Endogenous heme oxygenase induction is a critical mechanism attenuating apoptosis and restoring microvascular perfusion following limb ischemia/reperfusion. Surgery. 2004;136:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wunder C, Scott JR, Lush CW, Brock RW, Bihari A, Harris K, Eichelbrönner O, Potter RF. Heme oxygenase modulates hepatic leukocyte sequestration via changes in sinusoidal tone in systemic inflammation in mice. Microvasc Res. 2004;68:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Wunder C, Brock RW, McCarter SD, Bihari A, Harris K, Eichelbrönner O, Potter RF. Inhibition of haem oxygenase activity increases leukocyte accumulation in the liver following limb ischaemia-reperfusion in mice. J Physiol. 2002;540:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Hisama N, Yamaguchi Y, Ishiko T, Miyanari N, Ichiguchi O, Goto M, Mori K, Watanabe K, Kawamura K, Tsurufuji S. Kupffer cell production of cytokine-induced neutrophil chemoattractant following ischemia/reperfusion injury in rats. Hepatology. 1996;24:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, Nielsen TT, Bøtker HE. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2005;288:H1252-H1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol. 2003;38:59-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Nie RG, McCarter SD, Harris KA, Lee PJ, Zhang X, Bihari A, Gray D, Wunder C, Brock RW, Potter RF. The role of endogenous heme oxygenase in the initiation of liver injury following limb ischemia/reperfusion. J Hepatol. 2002;36:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 22. | Vollmar B, Lang G, Post S, Menger MD, Messmer K. Microcirculation of the liver in hemorrhagic shock in the rat and its significance for energy metabolism and function. Zentralbl Chir. 1993;118:218-225. [PubMed] |
| 23. | Wood B, Junckerstorff R, Sterrett G, Frost F, Harvey J, Robbins P. A comparison of immunohistochemical staining for oestrogen receptor, progesterone receptor and HER-2 in breast core biopsies and subsequent excisions. Pathology. 2007;39:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Leake R. Contents of HRT and mechanisms of action. J Epidemiol Biostat. 1999;4:129-133; discussion 133-139. [PubMed] |
| 25. | Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474-1481. [PubMed] |
| 26. | Hata K, Yamamoto Y, Nakajima A, Taura K, Yonezawa K, Uchinami H, Ikeda F, Yamaoka Y. Induction of heme oxygenase-1 and dilatation of hepatic sinusoids by an administration of pyrrolidine dithiocarbamate in rat livers. J Surg Res. 2003;115:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, Tamatani T, Suematsu M. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998;101:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Liu SF, Ye X, Malik AB. Pyrrolidine dithiocarbamate prevents I-kappaB degradation and reduces microvascular injury induced by lipopolysaccharide in multiple organs. Mol Pharmacol. 1999;55:658-667. [PubMed] |
| 30. | Tsuchihashi S, Tamaki T, Tanaka M, Kawamura A, Kaizu T, Ikeda A, Kakita A. Pyrrolidine dithiocarbamate provides protection against hypothermic preservation and transplantation injury in the rat liver: the role of heme oxygenase-1. Surgery. 2003;133:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Hartsfield CL, Alam J, Choi AM. Transcriptional regulation of the heme oxygenase 1 gene by pyrrolidine dithiocarbamate. FASEB J. 1998;12:1675-1682. [PubMed] |
| 32. | Tapuria N, Junnarkar S, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning. HPB (Oxford). 2012;14:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Hirano K, Kobayashi T, Watanabe T, Yamamoto T, Hasegawa G, Hatakeyama K, Suematsu M, Naito M. Role of heme oxygenase-1 and Kupffer cells in the production of bilirubin in the rat liver. Arch Histol Cytol. 2001;64:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Chen YS, Chien CT, Ma MC, Tseng YZ, Lin FY, Wang SS, Chen CF. Protection “outside the box” (skeletal remote preconditioning) in rat model is triggered by free radical pathway. J Surg Res. 2005;126:92-101. [PubMed] |
| 35. | Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Greenbaum NL, Kappas A. Comparative photoactivity of tin and zinc porphyrin inhibitors of heme oxygenase: pronounced photolability of the zinc compounds. Photochem Photobiol. 1991;54:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Zygmunt PM, Högestätt ED, Grundemar L. Light-dependent effects of zinc protoporphyrin IX on endothelium-dependent relaxation resistant to N omega-nitro-L-arginine. Acta Physiol Scand. 1994;152:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Jia J, Li J, Jiang L, Zhang J, Chen S, Wang L, Zhou Y, Xie H, Zhou L, Zheng S. Protective effect of remote limb ischemic perconditioning on the liver grafts of rats with a novel model. PLoS One. 2015;10:e0121972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |