Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5790
Peer-review started: March 4, 2016
First decision: April 14, 2016
Revised: April 26, 2016
Accepted: May 23, 2016
Article in press: May 23, 2016
Published online: July 7, 2016
Processing time: 124 Days and 1.9 Hours
AIM: To evaluated patterns and outcomes of hepatocellular carcinoma (HCC) recurrence after living donor liver transplantation (LDLT).
METHODS: From 2001 to 2014, 293 patients underwent LDLT for HCC at our transplant center. We retrospectively reviewed 54 (18.4%) patients with HCC recurrence after LDLT. We evaluated patterns and outcomes of HCC recurrence after LDLT, with particular attention to the Milan criteria at transplantation, treatments for HCC-recurrent patients, and factors related to survival after HCC recurrence. Furthermore, we evaluated the efficacy of combination treatment of sorafenib and an mTOR inhibitor.
RESULTS: The 1-, 2-, and 3-year overall survival rates after HCC recurrence were 41.1%, 20.5%, and 15.4%, respectively. The median time interval between LDLT and HCC recurrence was 6.5 mo. Although recurrence rates according to the Milan criteria at LDLT were significantly different, HCC recurrence patterns and survival rates after HCC recurrence were not significantly different between the two groups. Time to recurrence < 12 mo (P = 0.048), multiple recurrences at HCC recurrence (P = 0.038), and palliative treatment for recurrent tumors (P = 0.003) were significant independent prognostic factors for poor survival after HCC recurrence in a multivariate analysis. The combination treatment of sorafenib and sirolimus showed survival benefits in the palliative treatment group (P = 0.005).
CONCLUSION: Curative treatment for recurrent HCC after LDLT is the most important factor in survival rates after HCC recurrence and combination treatments of sorafenib and an mTOR inhibitor could have survival benefits in patients with HCC recurrence after LT in the palliative treatment group.
Core tip: Although survival rates after liver transplantation (LT) have improved dramatically, hepatocellular carcinoma (HCC) recurrence remains a significant problem and studies regarding the clinical outcomes and treatments of patients with HCC recurrence after LT are rare. In this study, satisfying the Milan criteria at living donor liver transplantation (LDLT) was important for recurrence rates after LDLT, but was not important for survival rates after HCC recurrence. Curative treatment for recurrent HCC after LDLT is the most important factor in survival rates after HCC recurrence. Combination treatments of sorafenib and an mTOR inhibitor could have survival benefits in patients with HCC recurrence after LT in the palliative treatment group.
- Citation: Na GH, Hong TH, You YK, Kim DG. Clinical analysis of patients with hepatocellular carcinoma recurrence after living-donor liver transplantation. World J Gastroenterol 2016; 22(25): 5790-5799
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5790.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5790
Among the several treatment modalities for hepatocellular carcinoma (HCC), liver transplantation (LT) is a preferred treatment for selected patients with HCC because it targets not only the tumor but also the underlying liver disease. Since the introduction of the Milan criteria by Mazzaferro et al[1] in 1996, disease-free survival and overall survival after LT for patients HCC meeting the Milan criteria have been equivalent to those of non-HCC patients. HCC has become a major indication for LT, and due to the increasing number of HCC patients, the number of LTs will likely increase further[2]. However, although survival rates after LT have improved dramatically, HCC recurrence remains a significant problem. Furthermore, because the outcomes were disappointing when patients with HCC not meeting the Milan criteria were treated with current treatment modalities other than LT[3], many centers are making efforts to expand the selection criteria[4,5]. For living-donor liver transplantation (LDLT), there has been a tendency to accept extended criteria in comparison with deceased-donor liver transplantation (DDLT)[6]. Thus, an evaluation of results after LDLT with such expanded criteria is important. In particular, it would be meaningful to compare HCC recurrence patterns and clinical outcome after LT according to the Milan criteria.
Treatment strategies for HCC-recurrence patients after LT are not well established, and treatment options are limited[7]. Due to the use of immunosuppressive agents, the prognosis of recurrent HCC after LT is poor, and its progression is typically very rapid. Additionally, recurrent HCC after LT tends to recur in multifocal and extra-hepatic sites. Nevertheless, some patients with HCC recurrence after LT have favorable prognoses and long-term survival after recurrence treatment[8]. However, few reports have examined patients with recurrent HCC after LT. Furthermore, LDLT differs from DDLT in terms of treatments after HCC recurrence, and there are a few reports about recurrent HCC after LDLT. Because currently used immunosuppressive agents, such as calcineurin inhibitors, make tumor cells proliferate, mammalian target of rapamycin (mTOR) inhibitors are recommended in patients at high risk of recurrent HCC after LT[9]. Due to compromised liver function in cirrhotic patients and the low efficacy of chemotherapy in HCC, chemotherapy treatments in HCC are not used commonly. Sorafenib is a small-molecule inhibitor of several tyrosine protein kinases (VEGFR and PDGFR) and Raf kinases. The SHARP trial showed the efficacy of sorafenib in HCC, with both median survival and time to progression showing 3-mo improvements[10]. Although combination treatments with mTOR inhibitors and sorafenib had some favorable results in HCC-recurrent patients after LT, there is currently no consensus on the most reliable treatment approach.
Thus, we examined patterns and outcomes of recurrent HCC after LDLT, particularly with regard to the Milan criteria at transplantation, treatments for HCC-recurrent patients after LDLT, and factors related to survival after HCC recurrence. Furthermore, we evaluated the effectiveness of combination treatment with sorafenib and mTOR inhibitors for recurrent HCC patients.
From January 2001 to June 2014, 293 patients underwent LDLT for HCC at our transplant center. Among them, 54 (18.4%) patients experienced HCC recurrence after LDLT. We retrospectively reviewed HCC recurrence patients after LDLT. This study was approved by the institutional review board of our center.
Because of a shortage of deceased donor in my country, most patients with HCC could not undergo DDLT, and usually underwent LDLT. Therefore, For LDLT, relatively expanded selection criteria could be adopted, if liver transplantation was not contraindicated such as distant metastasis, regional lymph node metastasis, and macroscopic main portal vein invasion. The availability of liver donor is most important in LDLT. When a live donor is available, we performed LDLT. If not, we performed locoregional treatments regardless of the Milan criteria. In case of HCC patients within Milan criteria, we performed LT first, if the donor is available. In case of HCC patients beyond Milan criteria, when the tumor biology is expected to good and the donor is available, we performed LT first, but otherwise we performed locoregional treatments with the purpose of bridging or down-staging.
LDLT was performed according to a standard technique using a modified right lobe with middle hepatic vein reconstruction. For patients with ascites, aspiration and cytology were performed before the operation. When lymph node enlargement was present and in cases with suspected metastatic disease, an intraoperative biopsy was performed. The operation was performed only in cases with negative biopsy results. Immunosuppression regimens after LT consisted of a triple-drug regimen that included tacrolimus or cyclosporin, mycophenolate mofetil (MMF), and prednisolone. Steroids were withdrawn 3 mo after surgery, and MMF was withdrawn 6 mo after surgery. An interleukin-2 receptor blocker was administered on the day of surgery and on the fourth postoperative day. Patients were followed monthly for the first year, every 2 mo for 5 years, and then every 3 mo. Tumor markers such as AFP was measured monthly during the first year, and then every 2 mo. Abdomen CT, chest CT, and bone scintigraphy were performed routinely every 4 mo for the first year, every 6 mo for the second year, and then annually. When tumor recurrence was suspected, MRI and/or PET-CT were performed. The median follow up period was 18.5 mo (range: 3-170 mo).
Upon HCC recurrence after LDLT, the immunosuppressive agent was discontinued immediately or used at a reduced dose. Since the introduction of sorafenib in 2008 and sirolimus in 2012 in our hospital, the current standard treatment for recurrent HCC after LT has been a combination treatment of sorafenib and an mTOR inhibitor after locoregional treatment for suitable recurrent lesions. As an immunosuppressant, sirolimus was used instead of calcineurin inhibitors. The initial dose of sirolimus was 2 mg/d orally, and sirolimus whole blood concentrations were measured by immunoassay methodologies to adjust the dose to maintain whole blood trough concentrations at 5-10 ng/mL. The dose of sorafenib was 400 mg orally twice daily, and sorafenib was continued until the patient no longer benefitted or unacceptable toxicity emerged. Treatment modalities for recurrence were divided into curative intent and palliative intent treatments. Curative intent treatment was defined as surgical resection or ablation therapy, such as radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI), intended to achieve no evidence of known recurrence. All patients considered unsuitable for curative treatment were enrolled in the palliative treatment group. Palliative treatments included transarterial chemoembolization (TACE), chemotherapy, and radiation.
Continuous variables are reported as mean ± SD; they were compared using Student’s t-test. Categorical variables were analyzed using Pearson’s χ2 test. Overall survival was calculated using the Kaplan-Meier method. To evaluate risk factors for survival in HCC recurrence patients, univariate analysis was performed using the Kaplan-Meier method and evaluated using the log-rank test. Candidate predictors associated with a P value < 0.2 in univariate analyses were entered into a multivariate analysis using Cox regression analysis. Furthermore, comparative study was done between recurrent HCC patients regarding Milan criteria at transplantation, also, in curative and palliative treatment groups, comparative studies were done between sorafenib and sirolimus treatment group and other treatment group. Statistical analyses were performed using the SPSS software (ver. 18.0 for Windows; SPSS, Inc., Chicago, IL, United States). A P value < 0.05 was considered to indicate statistical significance.
The mean age of patients with HCC recurrence after LDLT was 52.0 ± 8.1 years, and 46 (85.2%) patients were males. The most common reason for LT was hepatitis B (n = 46, 85.2%), followed by alcohol (n = 5, 9.3%), hepatitis C (n = 2, 3.7%), and other causes (n = 1, 1.9%). The mean Child-Pugh score was 7.5 ± 2.4, and the mean model for end-stage liver disease (MELD) score was 11.7 ± 8.5. Of the patients, 48 (88.9%) received pretransplant locoregional treatments. The mean tumor number and maximal tumor size at LT were 2.4 ± 1.9 and 4.85 ± 4.07 cm, respectively. Of the patients, 38 (70.4%) did not meet the Milan criteria. The median follow-up periods after LDLT and after HCC recurrence were 18.5 (range, 3-170) mo and 8.5 (range, 0-122) mo, respectively (Table 1).
| Factors | Data, n (%) |
| Recipient age1 | 52.0 ± 8.1 |
| Recipient sex, male | 46 (85.2) |
| Etiology | |
| Hepatitis B | 46 (85.2) |
| Hepatitis C | 2 (3.7) |
| Alcohol | 5 (9.3) |
| Other | 1 (1.9) |
| MELD score1 | 11.7 ± 8.5 |
| GRWR1 | 1.28 ± 0.24 |
| Pre-transplant locoregional treatment | 48 (88.9) |
| Tumor marker at transplantation | |
| AFP1 | 717.3 ± 1748.1 (median: 43.3) |
| HCC characteristics at pathology | |
| Number1 | 2.4 ± 1.9 (median: 2.0) |
| Maximal tumor size (cm)1 | 4.85 ± 4.07 (median: 3.8) |
| Microvascular invasion | 30 (60.0) |
| E-S grade III-IV | 25 (52.1) |
| Beyond Milan criteria | 38 (70.4) |
| Patterns of HCC recurrence | |
| Time between LDLT and recurrence (mo)1 | 15.3 ± 23.9 (median: 6.5, range: 1-150) |
| Single vs multiple | 14 (25.9) vs 40 (74.1) |
| Intrahepatic vs extrahepatic vs both | 12 (22.2) vs 37 (68.5) vs 5 (9.3) |
| Recurrence organ | |
| Lung | 24 (44.4) |
| Liver | 17 (31.3) |
| Bone | 10 (18.5) |
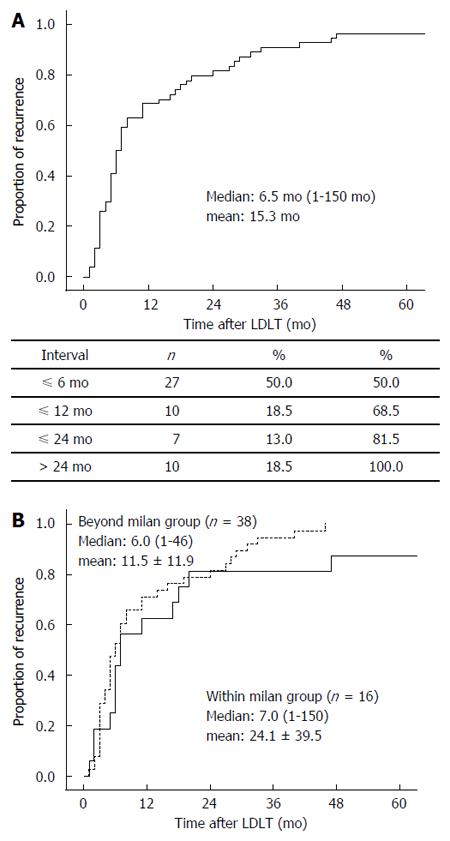
The median time interval between LDLT and HCC recurrence was 6.5 mo (range, 1-150 mo, mean: 15.3 mo). Most HCC recurrence (n = 44, 81.5%) occurred within 2 years, with 37 (68.5%) patients experiencing HCC recurrence within 1 year (Figure 1A). At the time of HCC recurrence after LDLT, 14 (25.9%) patients had a solitary recurrent tumor, but 40 (74.1%) patients had multiple recurrent tumors. The most frequently involved organs were the lung (n = 24, 44.4%), followed by the liver (n = 17, 31.5%), bone (n = 10, 18.5%), lymph node (n = 6, 11.1%), brain (n = 2, 3.7%), and chest wall (n = 2, 3.7%).
In this study, 15 (27.8%) patients were managed with curative intent treatment, and the remaining 39 (72.2%) were managed with palliative intent treatments. Among the curative treatment group, 13 patients received only the operation for the first treatment of recurrent HCC, one patient underwent the operation and TACE, and one patient underwent TACE and RFA. Among the palliative group, TACE was the most common treatment modality for the first treatment of recurrent HCC: 10 patients received TACE as monotherapy or combined therapy (Table 2).
| Treatments | Curative | Palliative |
| (n = 15, 27.8%) | (n = 39, 72.2%) | |
| Operation | 13 (86.7) | 1 (2.6) |
| Operation and radiation | 1 (6.7) | 4 (10.3) |
| Operation and TACE | 1 (2.6) | |
| TACE | 8 (20.5) | |
| TACE and RFA | 1 (6.7) | 1 (2.6) |
| Radiation | 7 (17.9) | |
| Intravenous chemotherapy | 5 (12.8) | |
| Sorafenib | 8 (20.5) | |
| Conservative treatment | 4 (10.3) |
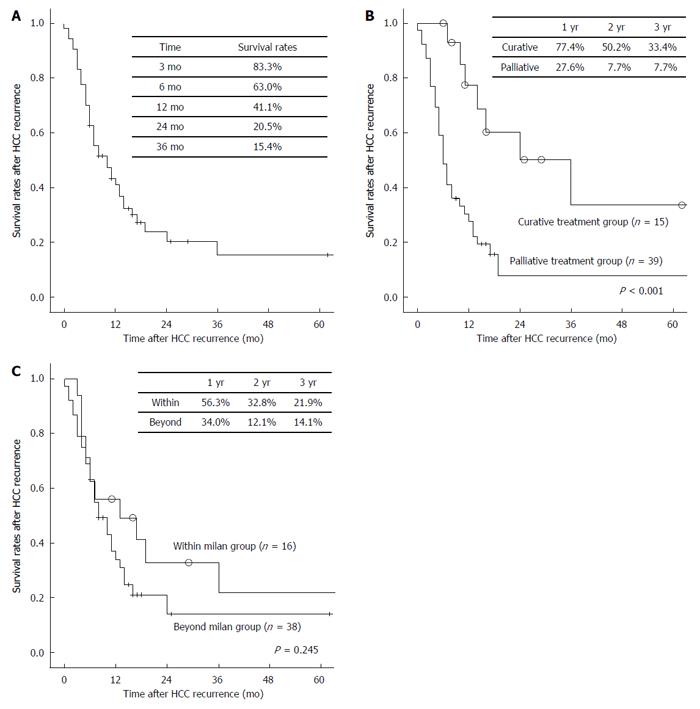
We performed subgroup analysis to compare patterns of recurrent HCC according to the Milan criteria at LDLT. The Milam criteria were based on pathology results. During the study period, 293 patients underwent LDLT for HCC at our transplant center. Among the 180 (61.4%) patients who met the Milan criteria at LDLT, 16 (8.9%) experienced HCC recurrence. Among the 113 (38.6%) patients who did not meet the Milan criteria, 38 (33.6%) experienced HCC recurrence. Recipient age in the group not meeting the Milan criteria was significantly higher than that in the group meeting them (P = 0.005). Tumor number (P < 0.001) and size (P < 0.001) and AFP (P = 0.043) in the outside-the-Milan group were significantly higher than those in the within Milan group. Other clinicopathological factors at transplantation, such as recipient gender, etiology for transplantation, MELD score, GRWR, and history of pre-transplant locoregional treatments, were not significantly different between the two groups. Recurrence rates according to the Milan criteria at LDLT were significantly different (P < 0.001). However, in terms of tumor recurrence patterns, the time interval between LDLT and HCC recurrence (P = 0.157, Figure 1B), tumor number at HCC recurrence (P = 0.735), and HCC recurrence site (P = 0.555), there was no significant difference between the groups (Table 3). Furthermore, survival rates after HCC recurrence were not significantly different between the groups (P = 0.245, Figure 2C).
| Factors | Within Milan (n = 16) | Beyond Milan (n = 38) | P value |
| Recipient age1 | 47.4 ± 5.9 | 53.9 ± 8.1 | 0.005 |
| Recipient sex, male | 13 (81.3) | 33 (86.8) | 0.682 |
| Etiology, hepatitis B | 12 (75.0) | 34 (89.5) | 0.127 |
| MELD score1 | 10.9 ± 9.1 | 12.0 ± 8.2 | 0.692 |
| GRWR1 | 1.27 ± 0.20 | 1.28 ± 0.26 | 0.919 |
| Pre-transplant locoregional treatment | 13 (81.3) | 35 (92.1) | 0.346 |
| Tumor marker at transplantation | |||
| AFP1 | 213.0 ± 371.8 | 829.6 ± 2040.7 | 0.043 |
| HCC characteristics at pathology | |||
| Number1 | 1.2 ± 0.6 | 2.9 ± 2.0 | < 0.001 |
| Maximal tumor size1 | 2.33 ± 1.10 | 5.93 ± 4.41 | < 0.001 |
| Microvascular invasion | 7 (50.0) | 23 (63.9) | 0.368 |
| E-S grade III-IV | 6 (46.2) | 19 (54.3) | 0.616 |
| Recurrent HCC patterns | |||
| Recurrence rates | 8.90% | 33.60% | < 0.001 |
| Time interval, median (mo) (LDLT-HCC recurrence) | 7.0 (1-150) | 6.0 (1-46) | 0.157 |
| mean: 24.1 ± 39.5 | mean: 11.5 ± 11.9 | ||
| Single or Multiple | 0.735 | ||
| Single | 5 (31.3) | 9 (23.7) | |
| Multiple | 11 (68.7) | 29 (76.3) | |
| Recurrence site | 0.555 | ||
| Intrahepatic | 5 (31.3) | 7 (18.4) | |
| Extrahepatic | 10 (62.5) | 27 (71.1) | |
| Both | 1 (6.3) | 4 (10.5) | |
| Survival after HCC recurrence, median (mo) | 12.0 (3-122) | 8.0 (1-62) | 0.224 |
| mean: 24.0 ± 33.5 | mean: 10.4 ± 10.5 |
The 6-, 12-, 24-, and 36-mo overall survivals after HCC recurrence were 63.0, 41.1, 20.5, and 15.4%, respectively (Figure 2A). In univariate analysis, time to recurrence < 12 mo (P = 0.010), multiple HCC recurrences (P < 0.001), brain metastasis (P < 0.001), and palliative treatment for recurrent tumors (P < 0.001; Figure 2B) were statistically associated with poor overall survival after HCC recurrence. Among them, time to recurrence < 12 mo [hazard ratio = 2.408 (1.007-5.756), P = 0.048], multiple HCC recurrences [hazard ratio = 3.438 (1.072-11.025), P = 0.038], and palliative treatment for recurrent tumors [hazard ratio = 3.886 (1.591-9.490), P = 0.003] were independent predictors of poor survival in a multivariate analysis. However, tumor state at transplantation, such as meeting the Milan criteria (P = 0.245), microvascular invasion (P = 0.384), and tumor grade (P = 0.227), were not significantly associated with survival rate after HCC recurrence (Table 4).
| Factors | Univariate analysis | Multivariate analysis | |
| P value | Harzard ratio (95%CI) | P value | |
| Age > 60 yr | 0.124 | ||
| Male sex | 0.739 | ||
| Etiology | 0.291 | ||
| MELD score > 15 | 0.153 | ||
| GRWR < 1.0 | 0.658 | ||
| Pre-transplant treatments | 0.170 | ||
| Tumor state at transplantation | |||
| AFP > 100 | 0.575 | ||
| Beyond Milan criteria | 0.245 | ||
| Microvascular invasion | 0.384 | ||
| E-S grade III-IV | 0.227 | ||
| Time to recurrence < 12 mo | 0.010 | 2.408 (1.007-5.756) | 0.048 |
| Multiple recurrence | < 0.001 | 3.438 (1.072-11.025) | 0.038 |
| Recurrence site | |||
| Liver | 0.824 | ||
| Lung | 0.937 | ||
| Bone | 0.695 | ||
| Brain | < 0.001 | 2.966 (0.565-15.583) | 0.199 |
| Palliative treatment for recurrent tumors | < 0.001 | 3.886 (1.591-9.490) | 0.003 |
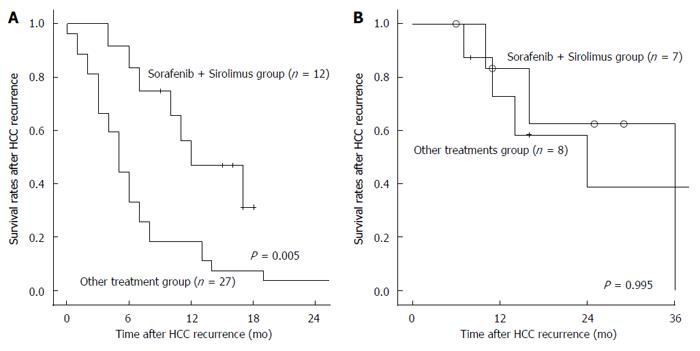
We evaluated the efficacy of the combination treatment of sorafenib and sirolimus. We divided patients into a curative treatment group and a palliative group. In the palliative group, 12 patients received a combination treatment of sorafenib and sirolimus; the remaining 27 patients did not. Although tumor characteristics at transplantation and at HCC recurrence were not significantly different between the combination treatment group and the other treatment group (Table 5), survival rates after HCC recurrence in the combination treatment group were significantly higher than in the other treatment group among the palliative group (P = 0.005; Figure 3A). However, combination treatment with sorafenib and sirolimus did not show survival benefits in the curative treatment group (P = 0.955; Figure 3B).
| Factors | Sorafenib + Sirolimus | Other treatments | P value |
| Recipient age1 | 55.3 ± 9.1 | 51.6 ± 6.9 | 0.179 |
| Recipient sex, male | 9 (75.0) | 24 (88.9) | 0.348 |
| Etiology, Hepatitis B | 10 (83.3) | 22 (81.5) | 0.369 |
| MELD score1 | 9.3 ± 3.3 | 14.0 ± 9.7 | 0.033 |
| GRWR1 | 1.40 ± 0.27 | 1.23 ± 0.25 | 0.065 |
| AFP at transplantation1 | 1038.3 ± 1849.2 | 256.5 ± 361.1 | 0.173 |
| HCC characteristics at pathology | |||
| Number1 | 2.42 ± 2.02 | 2.38 ± 2.09 | 0.965 |
| Maximal tumor size1 | 4.20 ± 2.06 | 5.44 ± 5.36 | 0.444 |
| Beyond Milan criteria | 9 (75.0) | 17 (65.4) | 0.714 |
| Time between LDLT and recurrence, median | 6 (range: 3-150) | 5 (range: 1-46) | 0.270 |
| Patterns of HCC recurrence | |||
| Single or Multiple | 0.219 | ||
| Single | 2 (16.7) | 1 (3.7) | |
| Multiple | 10 (83.3) | 26 (96.3) | |
| Intrahepatic or Extrahepatic | 0.074 | ||
| Intrahepatic | 0 | 9 (33.3) | |
| Extrahepatic | 10 (83.3) | 15 (55.6) | |
| Both | 2 (16.7) | 3 (11.1) | |
| AFP at HCC recurrence1 | 7913.2 ± 16023.5 | 6139.5 ± 17794.2 | 0.772 |
During the combination treatments of sorafenib and sirolimus, adverse effects included 8 cases of diarrhea and 3 cases of hand-foot syndrome. Although the combination treatment was generally well tolerated, there were adverse events exceeding grade 3 were 3 cases of diarrhea. Among them, one patient discontinued the combination treatment, the remaining two patient reduced the dose of sorafenib.
LT is a preferred treatment for selected patients with HCC because it targets not only the tumor but also the underlying liver disease. The 5-year survival rate for HCC patients who meet the Milan criteria is 70%-85%, with a recurrence-free survival rate of 75%[1]. Many centers are making efforts to expand the selection criteria because of the strictness of the Milan criteria[4,5]. However, HCC recurrence after LT remains an important problem in clinical practice. Indeed, the number of patients with HCC recurrence after LT continues to increase. In addition, for LDLT, there has been a tendency to accept extended criteria in comparison with DDLT[6]. In one meta-analysis, disease-free survival was significantly shorter in patients receiving LDLT[11]. The inferior oncological outcomes in the LDLT group may have been caused by more aggressive tumor biology and small-for-size graft injuries and regeneration[12]. In this study, 54 (18.4%) patients experienced HCC recurrence after LDLT. HCC recurrence rates after LDLT in this study were higher than that in previous DDLT studies, especially in the group that did not meet the Milan criteria (33.6% for the group outside the Milan criteria vs 8.9% for the group within them). Although the risk factors for HCC recurrence after LT have been studied, there are few studies about the clinical outcomes and treatments of patients with HCC recurrence after LT. Furthermore, studies about the effects of meeting the Milan criteria at transplantation on clinical outcomes after HCC recurrence are even rarer. Thus, the primary aim of this study was to assess the clinical outcome in patients with HCC recurrence after LDLT and to identify factors affecting survival after HCC recurrence following LDLT. A secondary aim was to compare recurrence patterns and outcomes according to the Milan criteria at transplantation. The last aim was to examine the effectiveness of new modalities for patients with HCC recurrence after LDLT.
Although HCC patients are selected for LT according to standard criteria, previous studies have reported that 10%-40% of them experience HCC recurrence[13]. Most HCC recurrence develops within 2 years after LT, and the lung is the most frequent site of recurrence after LT[14,15]. Similarly, in this study, most HCC recurrence (n = 44, 81.5%) occurred within 2 years after LDLT, with 37 (68.5%) patients experiencing HCC recurrence within 1 year; where the most frequently involved organ was the lung (n = 24, 44.4%). The 1-, 2-, and 3-year overall survival rates after HCC recurrence were 41.1%, 20.5%, and 15.4%, respectively. These findings were consistent with previous studies. This study also showed that time to recurrence < 12 mo, multiple recurrences at HCC recurrence, and palliative treatment for recurrent tumors were significant independent predictors of poor survival after HCC recurrence. These findings reinforce previous studies suggesting that the time between LT and HCC recurrence and curative treatment for recurrent HCC after LT are keys for predicting outcomes after HCC recurrence[16-18]. This study provides an important guide for the management for recurrent HCC in patients after LT and supports our treatment policy indicating that when a patient is diagnosed with HCC recurrence after LT, resection or ablative treatments should be considered first in the treatment algorithm if possible.
Because previous reports about patients with HCC recurrence after LT involved patients receiving DDLT, most patients met the Milan criteria at transplantation. There are some differences between LDLT and DDLT, and there has been a tendency to accept extended criteria for LDLT compared with DDLT. Our transplant center mainly performs LDLT. Thus, this study included many HCC patients who did not meet the Milan criteria at transplantation in contrast with most previous reports. Additionally, previous studies comparing recurrence patterns and survival rates after HCC recurrence according to the Milan criteria at LT are rare. Thus, we analyzed clinical outcomes and HCC-recurrent patterns according to the Milan criteria at transplantation. Our study showed that although recurrence rates after LDLT were significantly different, HCC recurrence patterns and survival rates after HCC recurrence were not significantly different between the two groups. Because of the small number of patients included in this study and the scarcity of comparable previous studies, further prospective studies are needed.
Treatment of recurrent HCC after LT is difficult, and the prognosis is poor, with a median survival of less than 1 year[18]. In principal, all treatment options currently available for advanced HCC are also potentially feasible in recurrent HCC after LT. However, HCC recurrence after LT is considered a “systemic disease”, and the efficacy of locoregional treatment for a systemic disease is doubtful. Thus, new treatment strategies in recurrent HCC after LT are needed. Sorafenib is the treatment of choice for advanced HCC because survival in sorafenib patients with underlying liver cirrhosis is longer than that in placebo controls[10,19]. Theoretically, such a systemic therapy could be the best approach for HCC recurrence after LT. Sposito et al[20] reported that sorafenib seemed to be associated with an acceptable safety profile and provided benefit in survival in HCC patients suffering recurrence after LT. Survival of patients in the sorafenib group was improved significantly (median survival from recurrence 21.3 mo vs 11.8 mo, HR = 5.2, P = 0.0009). The only factor associated with survival after HCC recurrence in a multivariate analysis was treatment with sorafenib (HR = 4.0; P = 0.0325).
The growth rate of recurrent HCC after LT is significantly faster than that in non-transplanted patients with HCC who underwent surgical resection, presumably due to ongoing immunosuppression and reduced host immunity against micrometastasis[21,22]. Over recent decades, the mTOR inhibitors everolimus and sirolimus has been introduced, and attention has turned to the question of whether their use could ameliorate the risk of post-transplant HCC recurrence. Combination treatments of sorafenib and an mTOR inhibitor as a new treatment modality have been studied, but data about combination treatments of sorafenib and mTOR inhibitors in patients with HCC recurrence after LT are limited. It has been hypothesized that combination treatments could have synergistic effects. Recently, some studies reported that combination treatments of sorafenib and mTOR inhibitors showed survival benefits in patients with HCC recurrence after LT. Gomez-Martin et al[23] reported that the combination treatment of sorafenib and an mTOR inhibitor could be effective in recurrent HCC after LT in the palliative treatment group. In a total of 26 patients, there was one partial response and 13 cases with disease stabilization as the best response. Our study also showed that combination treatments of sorafenib and mTOR inhibitors had significant survival benefits in the palliative treatment group (P = 0.005).
In conclusion, meeting the Milan criteria at LDLT is associated with significant less recurrence rates after LDLT, but is not associated with survival rates after HCC recurrence. However, curative treatment for recurrent HCC after LDLT is the most important factor in survival rates after HCC recurrence. Furthermore, combination treatments of sorafenib and an mTOR inhibitor could have survival benefits in patients with HCC recurrence after LT in the palliative treatment group. Lastly, prospective studies with larger cohorts are required to address these issues more fully.
Liver transplantation (LT) is a preferred treatment for selected patients with hepatocellular carcinoma (HCC). Many centers are making efforts to expand the selection criteria. For living donor liver transplantation (LDLT), there has been a tendency to accept extended criteria in comparison with deceased-donor liver transplantation. However, HCC recurrence remains an important problem, and studies regarding the clinical outcomes and treatments of patients with HCC recurrence after LT are rare.
The authors evaluated patterns and outcomes of HCC recurrence after LDLT, with particular attention to the Milan criteria at transplantation, treatments for HCC-recurrent patients, and factors related to survival after HCC recurrence. Furthermore, we evaluated the efficacy of combination treatment of sorafenib and an mTOR inhibitor.
In present study, curative treatment for recurrent HCC after LDLT is the most important factor in survival rates after HCC recurrence and combination treatments of sorafenib and an mTOR inhibitor could have survival benefits in patients with HCC recurrence after LT in the palliative treatment group.
Although the risk factors for HCC recurrence after LT have been studied, there are few studies about the clinical outcomes and treatments of patients with HCC recurrence after LT. Therefore, our study may assist the treatments for patients with HCC recurrence after LT.
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase, which belongs to phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs) family. Sorafenib is a small-molecule inhibitor of several tyrosine protein kinases (VEGFR and PDGFR) and Raf kinases.
This is a retrospective study of the recurrent (HCC after LDLT among 54 patients with recurrent HCC out of 293 LDLT recipients with HCC. The study followed the principle of retrospective clinical research with some useful information, and seems suitable for the future publication.
Manuscript Source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: South Korea
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gad EH, Sugawara Y S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5308] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 2. | Ioannou GN, Perkins JD, Carithers RL. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33 Suppl 1:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 5. | Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Xiang ZW, Sun L, Li GH, Maharjan R, Huang JH, Li CX. Progress in the treatment of pulmonary metastases after liver transplantation for hepatocellular carcinoma. World J Hepatol. 2015;7:2309-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10261] [Article Influence: 603.6] [Reference Citation Analysis (2)] |
| 11. | Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, Lee TK, Tsui SH, Ng IO, Zhang ZW. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 14. | Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, Soejima Y. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 18. | Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4648] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 20. | Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095-2100. [PubMed] |
| 22. | Cheng JW, Shi YH, Fan J, Huang XW, Qiu SJ, Xiao YS, Wang Z, Dai Z, Tang ZY, Zhou J. An immune function assay predicts post-transplant recurrence in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011;137:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |