Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5364
Peer-review started: February 15, 2016
First decision: March 7, 2016
Revised: April 4, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: June 21, 2016
Processing time: 119 Days and 20.5 Hours
AIM: To investigate whether Tg737 is regulated by microRNA-548a-5p (miR-548a-5p), and correlates with hepatocellular carcinoma (HCC) cell proliferation and apoptosis.
METHODS: Assays of loss of function of Tg737 were performed by the colony formation assay, CCK assay and cell cycle assay in HCC cell lines. The interaction between miR-548a-5p and its downstream target, Tg737, was evaluated by a dual-luciferase reporter assay and quantitative real-time polymerase chain reaction. Tg737 was then up-regulated in HCC cells to evaluate its effect on miR-548a-5p regulation. HepG2 cells stably overexpressing miR-548a-5p or miR-control were also subcutaneously inoculated into nude mice to evaluate the effect of miR-548a-5p up-regulation on in vivo tumor growth. As the final step, the effect of miR-548a-5p on the apoptosis induced by cisplatin was evaluated by flow cytometry.
RESULTS: Down-regulation of Tg737, which is a target gene of miR-548a-5p, accelerated HCC cell proliferation, and miR-548a-5p promoted HCC cell proliferation in vitro and in vivo. Like the down-regulation of Tg737, overexpression of miR-548a-5p in HCC cell lines promoted cell proliferation, increased colony forming ability and hampered cell apoptosis. In addition, miR-548a-5p overexpression increased HCC cell growth in vivo. MiR-548a-5p down-regulated Tg737 expression through direct contact with its 3’ untranslated region (UTR), and miR-548a-5p expression was negatively correlated with Tg737 levels in HCC specimens. Restoring Tg737 (without the 3’UTR) significantly hampered miR-548a-5p induced cell proliferation, and rescued the miR-548a-5p induced cell proliferation inhibition and apoptosis induced by cisplatin.
CONCLUSION: MiR-548a-5p negatively regulates the tumor inhibitor gene Tg737 and promotes tumorigenesis in vitro and in vivo, indicating its potential as a novel therapeutic target for HCC.
Core tip: Tg737 gene functions as a tumor suppresser in hepatocellular carcinoma (HCC). However, studies based on regulation of Tg737 were rare. MiR-548a-5p is a novel miRNA which negatively regulates Tg737 and promotes tumorigenesis in vitro and in vivo, indicating its potential as a novel therapeutic target for HCC.
- Citation: Zhao G, Wang T, Huang QK, Pu M, Sun W, Zhang ZC, Ling R, Tao KS. MicroRNA-548a-5p promotes proliferation and inhibits apoptosis in hepatocellular carcinoma cells by targeting Tg737. World J Gastroenterol 2016; 22(23): 5364-5373
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5364.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5364
Primary hepatocellular carcinoma (HCC) is currently one of the most common and lethal malignancies, and the leading cause of death among patients with cirrhosis[1]. Despite advances in surgical therapies, the reason for frequent recurrence remains mostly obscure. Several studies showed that some genes may play a major role in cell proliferation and migration in HCC cells[2]. However, little information is available about the regulation of these genes.
The Tg737 gene, first found in algae[3], is now identified as a tumor suppressor gene in multiple cancers, including cancers of the liver, kidney and pancreas[4-5]. In liver tissues from HCC patients, a 59% down-regulation of the Tg737 was observed[6]. This gene may participate in alterations in a series of human or rodent liver tumors and tumorigenic cell lines[7]. In the previous investigation, we found that Tg737 contributed to hypoxia-induced invasion and migration in HepG2 and MHCC97H cells[8], and that Tg737 inhibition resulted in malignant transformation in fetal liver stem/progenitor cells by promoting cell-cycle progression and differentiation arrest[9]. However, the regulatory factor for Tg737 is not yet clear.
In recent years, microRNAs (miRNAs) emerged as a group of important endogenous modulators of gene function at the posttranscriptional level. It has been revealed that many miRNAs are underscored by the promotion of tumorigenesis and cancer progression[10-11]. More importantly, in HCC, several miRNAs have been demonstrated to contribute to the tumor regulation, including, but not limited to, migration, invasion and proliferation[12-13]. Multiple miRNAs have been identified as down-regulated tumor-suppressing genes involved in cellular processes, including miR-34a[14], miR-122[15], miR-199a[16] and miR-200[17]. Contrarily, miR-21[18], miR-148a[19] and miR-221[20], as oncogenic miRNAs, showed up-regulated expression which potentially targeted many tumor-suppressive genes. These results suggest the involvement of miRNAs in HCC.
MiR-548 is a big, poorly conserved primate-specific miRNA family. There are 68 members of the hsa-miR-548 family recorded in the miRBase database[21]. The products of miR-548c-5p and miR-548c-3p show discrepant evolutionary patterns, which brings about great genetic distances between pre-miRNAs to some extent and might contribute to dynamic expression profiles and regulatory network[22]. MiR-548c-3p was identified as a source of functional biomarkers for the primary prostate cancer progression[23]. It also significantly increased in Helicobacter pylori-negative cancer tissues[24]. In addition, our previous studies found the high expression of miR-548c-3p and miR-548c-5p in side population cells from HCC[25]. However, like the homologous gene, the exact effect of miR-548a-5p on HCC is still unknown. The direct aim of this study was to investigate whether Tg737 is regulated by miR-548a-5p, and correlates with HCC cell proliferation and apoptosis.
HCC cell lines HepG2 and MHCC97-H were maintained in DMEM medium (Invitrogen, United States) supplemented with 10% fetal bovine serum (FBS, Invitrogen, United States), 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cells were cultured in a humidified cellular incubator (Thermo Fisher, United States) supplemented with 5% CO2 at 37 °C.
Total RNA was extracted using RNAiso Plus (Takara, Japan) according to the manufacturer’s instructions. Reverse transcription was carried out using the PrimeScript RT Reagent Kit Perfect Real Time (Takara, Dalian, China) or the miScript II RT Kit (Qiagen, Germany). Quantitative polymerase chain reaction (qPCR) was used to detect the miRNA expression levels. The SYBR Green dye (Takara, Dalian, China) was used according to the manufacturer’s protocol. The following primers were used for analysis: TG737: forward primer, 5’-GTGCCAGTAGTAAAGGTG-3’ and reverse primer, 5’-GGTCGTTCTATTTGAGGG-3’; β-actin: forward primer, 5’-TCACCAACTGGGACGACA-3’ and reverse primer, 5’-ACAGCACCGCCTGGATAG-3’; miR-548a-5p: forward primer, 5’-AAAAGTAATTGCGAGTTTACC-3’ and reverse primer, Universal Primer (Qiagen, Germany). The CFX96 real-time PCR detection system (Bio-Rad, United States) was used to perform real-time PCR and the 2-ΔΔCT method was used to determine the relative gene expression. Mature miRNA was normalized to U6-snRNA.
MiR-548a-5p was obtained from Gene Pharma (Shanghai, China). The miR-548a-5p and 150 bp of flanking sequence was amplified with forward primer, 5’-CAGCTGGGTGCTCAGCCAGG-3’, reverse primer, 5’-GGCAACTTAATGTTTCTTGC-3’. A PCR fragment was inserted into the pENTRTM3C vector (Invitrogen, United States) using EcoR I and Xho I sites. The pLenti6.3-miR-548a-5p vector was constructed using Gateway LR Clonase II Enzyme Mix (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. The constructs were sequenced for verification. Overexpression and RNAi vectors were determined by Gene Pharma (Shanghai, China). The target sequence of sh-Tg737 was 5-’GTTACATACTTGAGACAAA-3’. Tg737 and Tg737-3’UTR overexpression vectors were also designed.
Proteins were extracted from cells with a RIPA buffer purchased from Beyotime with 1 mmol/L PMSF and a cocktail of protease inhibitors. The proper amount of loading buffer was added into cell lysis, followed by a BCA protein determination. SDS-PAGE was performed according to Genshare’s instructions (Genshare, Xi’an, China). The results were analyzed using Image J 5.0 software. Anti-Tg737 antibody (13967-1-AP) and anti-β-actin antibody (14395-1-AP) were purchased from Proteintech (Proteintech, Wuhan, China). Goat anti-rabbit IgG HL (HRP) (ab6721) was supplied by Abcam (United States).
Two hundred cells were seeded into a 6 cm dish and cultured in a complete medium for 10 d. Cells were fixed in methanol and stained with crystal violet, washed with PBS three times before fixing and staining. Colonies were washed with ddH2O after 30-min staining. The number and area of the colonies were calculated with Image J 5.0 software.
Cells were seeded in 96-well plates (2.0 × 103 cells/well). The cells were incubated for 0, 1, 2, 3 and 4 d using three replicates. Cell Counting Kit-8 (CCK-8) (7Sea Pharmatech, China) was used for cell proliferation analysis according to the manufacturer’s protocol. The Bio-Rad iMARKTM microplate reader was used to detect the optical density at 450 nm.
Cells were seeded in 6-well plates with 2.0 × 105 cells in each well. After 48 h, flow cytometry was performed to detect cell cycle analysis and the percentage of dying cells. The annexin V-FITC/PI apoptosis detection kit (Biovision, Palo Alto, CA, United States) was used according to the manufacturer’s protocol. Flow cytometry was performed with the Epics XL-MCL (BECKMAN coulter, United States). The results were analyzed using ModFit LT V3.1 (BECKMAN coulter).
HepG2 and MHCC97H cells were seeded in 48 well plates and allowed to be adherent for 24 h. The cells were then co-transfected with a mixture of 100 ng pGL3-Tg737-3’UTR, 20 μmol miR-548a-5p mimic or antisense-miR-548a-5p, and 5 ng pRL-TK using Lipofectamine 2000 reagent and performed in three independent experiments. Firefly and Renilla luciferase activities were detected using the Dual-Glo luciferase assay system (Promega, United States) following the manufacturer’s instructions.
HepG2 cells (2 × 106) stably overexpressing miR-548a-5p or miR-control were injected subcutaneously into the flank site of male Balb/c nude mice (Fourth Military Medical University, Xi’an, China). Tumor volume was measured with calipers every 5 d[26]. On day 30, mice were killed by cervical dislocation. All animal procedures were performed in accordance with a protocol approved by the Fourth Military Medical University Animal Care and Usage Committee.
Sample capacity for each experiment was adjusted according to the variance obtained. All experiments were performed in triplicate. In graphs, all data are presented as mean ± SD and were evaluated by the Student’s t-test. Statistical analyses were carried out using SPSS 16.0 software. Differences were considered significant at P < 0.05.
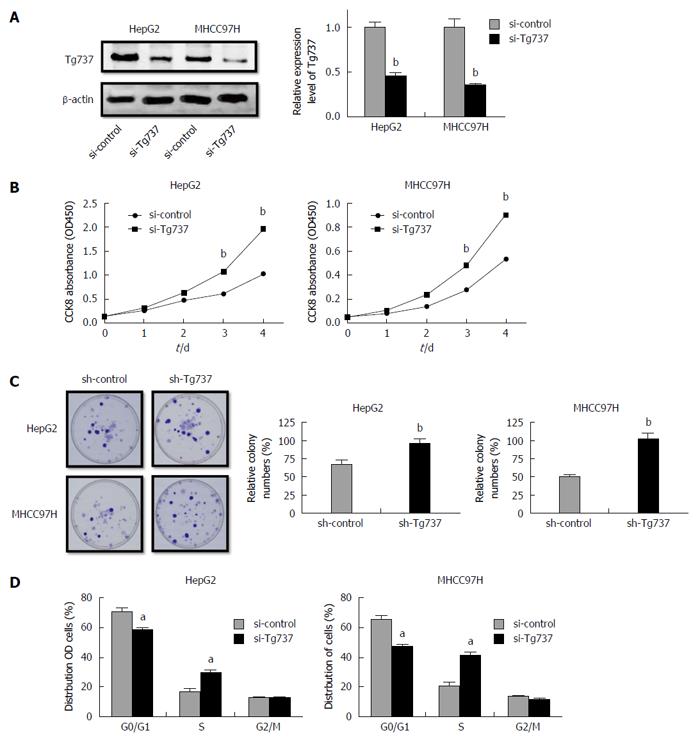
To illuminate the role of Tg737 in HCC cell proliferation, HCC cell lines HepG2 and MHCC97H were transfected with a si-Tg737 sequence or negative control (Figure 1A). Down-regulation of Tg737 significantly promoted the proliferation of HepG2 and MHCC97H cells and enhanced colony forming capability (Figure 1B and C). The distribution of HepG2 and MHCC97H cell cycles showed that the percentage of cells in G0/G1 phase significantly decreased in Tg737 down-regulated cells compared with their counterparts, while the cells in S phase increased sharply (Figure 1D). In all cases, down-regulation of Tg737 promoted cell proliferation and inhibited G0/G1 phase arrest in HCC cells.
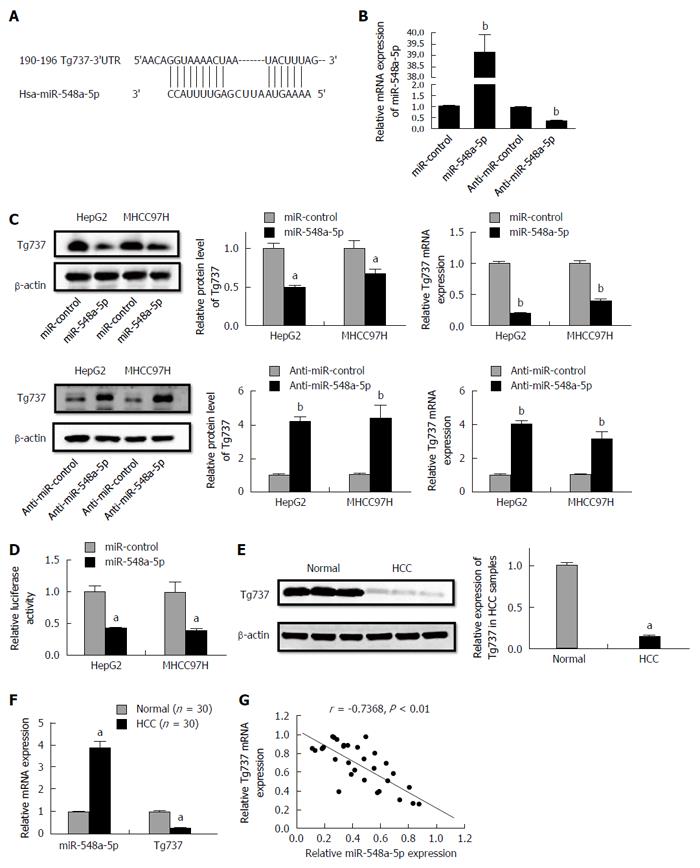
A bio-informatics assay with TargetScan demonstrated that Tg737 is a potential target of miR-548a-5p (Figure 2A). To illuminate whether miR-548a-5p acts on Tg737, we transfected miR-548a-5p as well as anti-miR-548a-5p to HCC cells. Relative miR-548a-5p levels are shown in Figure 2B. MiR-548a-5p overexpression decreased mRNA and protein levels of Tg737, while miR-548a-5p inhibition increased mRNA and protein levels of Tg737 in HepG2 and MHCC97H cells (Figure 2C). To confirm whether Tg737 acts as a molecular target regulated by miR-548a-5p, we constructed luciferase reporter vectors containing Tg737-3’UTR. The reporter vectors were co-transfected into HepG2 and MHCC97H cells. Up-regulation of miR-548a-5p expression significantly decreased the luciferase activity of Tg737 containing 3’UTR (Figure 2D). Further, to illuminate whether miR-548a-5p inhibits Tg737 in patients diagnosed with HCC, we detected the expression of miR-548a-5p and Tg737 in 30 HCC specimens as well as 30 normal ones. Compared with normal liver tissues, the HCC specimens showed higher miR-548a-5p and lower Tg737 expression (Figure 2E and F). In addition, a statistically significant correlation was revealed by Spearman’s correlation analysis between mRNA levels of miR-548a-5p and Tg737 (r = -0.7368, P < 0.01; Figure 2G). Together, these data suggest that Tg737 is a target of miR-548a-5p in HCC.
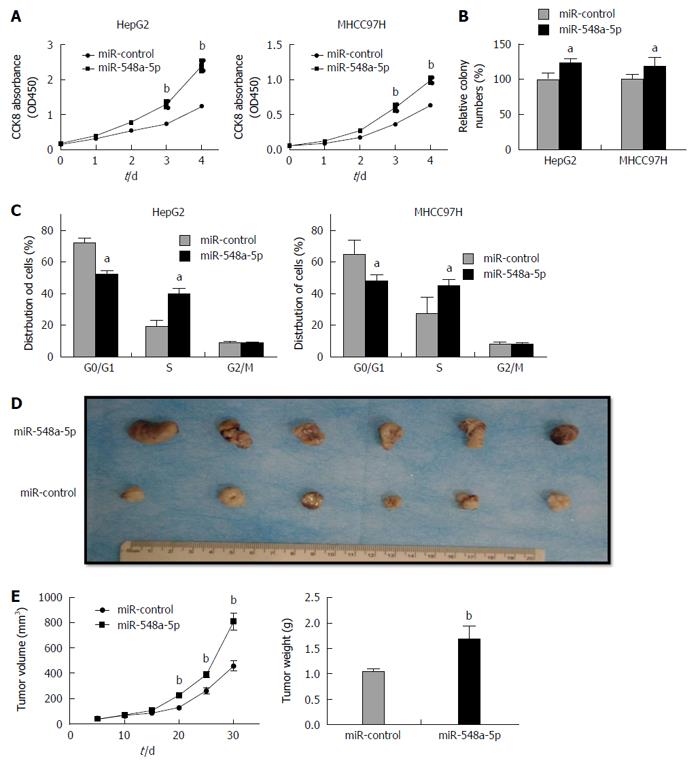
Continuing our analysis, we detected the impact of miR-548a-5p on HCC cell proliferation. Similar to the effect of Tg737 knockdown, miR-548a-5p overexpression in HepG2 and MHCC97H cells significantly accelerated cell proliferation, promoted colony forming capacity, and inhibited cell arrest in G0/G1 phase (Figure 3A-C). To investigate the relationship between miR-548a-5p and its tumor promoting characteristics in vivo, HepG2 cells exhibiting sustained expression of miR-548a-5p or control cells were injected into the subcutaneous skin layer of nude mice. Tumor size was detected every 5 d from the injection. The mice were killed 30 d after implantation. As shown in Figure 3D, tumors in those specimens with miR-548a-5p overexpression were larger significantly than those in the control group. The average size and weight of miR-548a-5p overexpressing tumors were increased significantly (Figure 3D and E). Collectively, miR-548a-5p may have a vigorous ability to enhance HCC cell proliferation in vivo and in vitro.
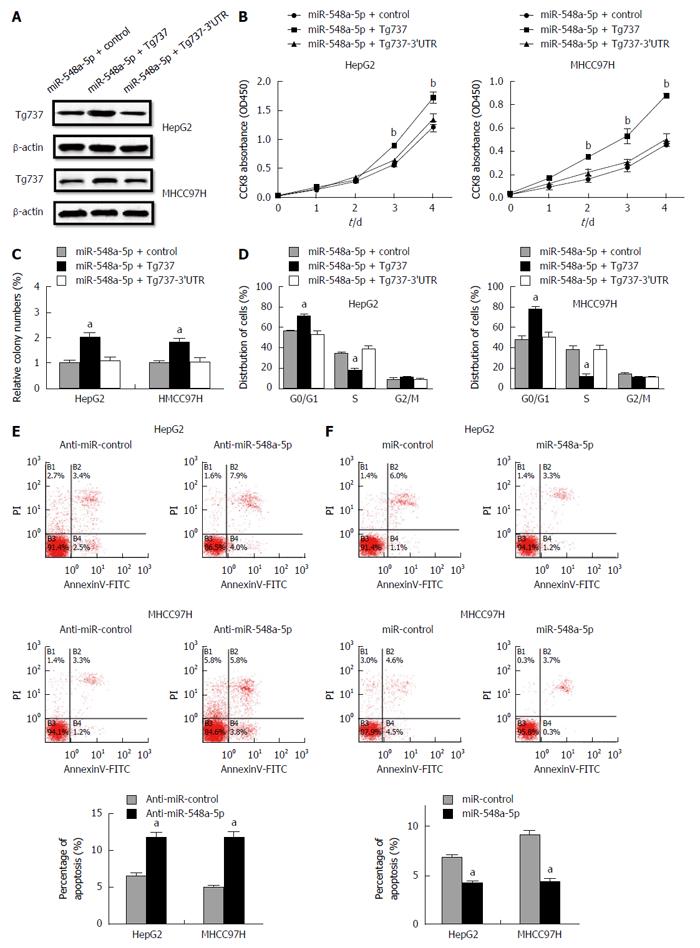
We further traced whether the restoration of Tg737 could arrest the HCC cell proliferation induced by miR-548a-5p overexpression in HepG2 and MHCC97H cells. A Western blot technique was performed 48 h after transfection of control, Tg737 and Tg737-3’UTR overexpression vectors into miR-548a-5p overexpressing HepG2 and MHCC97H cells (Figure 4A). Tg737 up-regulation significantly decreased the capacity of cell proliferation and colony formation (Figure 4B). Up-regulation of Tg737 rescued miR-548a-5p induced inhibition in cell cycle arrest (Figure 4B-D). However, overexpression of Tg737-3’UTR in miR-548a-5p overexpressing HepG2 and MHCC97H cells had no significance (Figure 4B-D). These results again illustrate that Tg737 is a functional target of miR-548a-5p. We evaluated the impact of miR-548a-5p on apoptosis by flow cytometry. Cell apoptosis increased in anti-miR-548a-5p transfected HCC cells (Figure 4E). Meanwhile, the cell apoptosis ratio decreased in miR-548a-5p transfected HCC cells (Figure 4F).
To our knowledge, this is the first report describing the effects of miR-548a-5p on HCC cell proliferation and apoptosis and the relationship with the Tg737 protein. In this study, we showed that Tg737 was the key regulator of the proliferation of human HCC cell lines. Overexpression of miR-548a-5p observably increased oncogenesis in nude mice. Moreover, when the expression of Tg737 was inhibited through interaction with its 3’UTR, HCC cells grew quickly when induced by miR-548a-5p. These findings suggest that miR-548a-5p acts as a novel tumor promoter in HCC cells and contributes to the tumor behavior by targeting the Tg737 gene.
MiR-548 was identified as a large human gene family with 69 members, which are mainly expressed in primates and play a key role in multiple biological processes[22]. The miR-548 family possesses the most abundant primate-specific miRNA common seed (5’-AAAGUA-3’) detected by computational characterization[27] and the signatures were identified to be down-regulated in peripheral blood mononuclear cells from heart failure patients. This information suggests that miR-548 level could be used as a potential biomarker specific for cardiac dysfunction[28]. In addition, endogenous miR-548 levels were suppressed during viral infections. MiR-548 shows significant identity to three viruses (HCV, HBV and HIV-1)[29], and regulated the host antiviral response via direct targeting interferon[30]. The marked up-regulation of miR-548ah-5p suggests that it might be related to the transition from the immune tolerance phase to the immune activation phase in chronic hepatitis B[31]. Such evidence makes miRNA a valuable therapeutic agent for patients diagnosed with multiple virus infection. Regarding the relationship of miR-548 with tumors, an analysis of the expression profiles and functional affinities of 3500 putative target genes of miR-548 suggested its cancer-related regulatory role[32]. A recent study found that miR-548d-3p, another miR-548 family member, is usually overexpressed in numerous types of cancer and affects tumorigenesis[33]. Overexpression of the miR-548 family, especially miR-548a-5p and miRNA-548d-5p, could play a complementary role in supporting the oncogenicity in cervical carcinogenesis[34]. Similar results were obtained in our study. MiR-548a-5p overexpression accelerated HepG2 and MHCC97H cell proliferation in vitro and increased the tumor size and weight in vivo, which indicates that miR-548a-5p promotes hepatic neoplasia.
Tg737 has attracted much attention because its functional inactivation by regulation of its expression is relevant to the formation of many solid tumors, such as those in the liver, kidney, and pancreas[4-5]. As a liver tumor suppressor gene, it has been implicated to be important for cell proliferation and apoptosis[35], because it is found deficient in HCC samples from chemical-induced rats and clinical patients[7,36]. In our previous research, Tg737 was found to be remarkably decreased in gene expression in side population cells of HCC, and the loss of heterozygosity of Tg737 was markedly associated with a poor prognosis in patients with HCC after curative liver resection[37]. In the present study, we confirmed these results and found that a loss of Tg737 function showed growth promotion in HepG2 and MHCC97H cells, which corresponds to the results of miR-548a-5p overexpression. Furthermore, miR-548a-5p knockdown rescued the effects caused by Tg737. There was a significant inverse correlation between miR-548a-5p expression and Tg737 mRNA and protein levels in hepatocellular carcinoma tissue and cell specimens. Thus, all these results support the hypothesis that miR-548a-5p functions in HCC by targeting the 3’UTR of Tg737.
In summary, our observations provide novel evidence that miR-548a-5p negatively regulates the tumor inhibitor gene Tg737 and promotes tumorigenesis in vitro and in vivo, adding to our understanding of the biological function of miR-548a-5p, as well as its relationship with Tg737. Therapeutic strategies to inhibit miR-548a-5p therefore potentially may be useful in limiting HCC growth and metastasis. A practical application of our findings could be the use of miR-548a-5p expression as a potential predictor of tumors that may be more likely to respond to Tg737-targeting therapies.
Primary hepatocellular carcinoma (HCC) is one of the most common and lethal malignancies. Despite advances in the surgical therapies, the reason of frequent recurrence remains mostly unclear. Several studies show that some genes may have good roles in cell proliferation and migration in HCC cells. The Tg737 gene is now identified as a tumor suppressor gene in multiple cancers, including those of the liver, kidney, and pancreas. In liver tissues from HCC patients, a 59% down-regulation of the Tg737 was observed. This gene may participate in alterations in a series of human or rodent liver tumors and tumorigenic cell lines. Nevertheless, the regulatory factor for Tg737 is not fully clear.
In recent years, microRNAs (miRNAs) emerged as a group of important endogenous modulators of gene function at the posttranscriptional level. It has been revealed that many miRNAs are underscored by the promotion of tumorigenesis and cancer progression. More importantly, in HCC, several miRNAs have been demonstrated to contribute to the tumor regulation, including, but not limited to, migration, invasion, and proliferation. Multiple miRNAs have been identified as down-regulated tumor-suppressing genes involved in cellular processes, as oncogenic miRNAs, showed up-regulated expressions which potentially targeted many tumor-suppressive genes. MiR-548 is a large and poorly conserved primate-specific miRNA family. The authors previous studies have found high expression of miR-548c-3p and miR-548c-5p in side population cells from HCC. However, like the homologous gene, the exact effect of miR-548a-5p on HCC is still unknown. The direct aim of this study was to investigate whether Tg737 is regulated by miR-548a-5p, and correlates with HCC cell proliferation and apoptosis.
MiR-548a-5p negatively regulates the tumor inhibitor gene Tg737 and promotes tumorigenesis in vitro and in vivo. The novelty of the research represents the idea that the inhibition of miR-548a-5p may limit HCC growth, and miR-548a-5p expression may be the potential predictor of tumors that respond to Tg737-targeting therapies.
In summary, this observations provide novel evidence that miR-548a-5p negatively regulates the tumor inhibitor gene Tg737, and promotes tumorigenesis in vitro and in vivo, which adds to the authors understanding of the biological function of miR-548a-5p, as well as the relationship with Tg737. Therapeutic strategies to inhibit miR-548a-5p therefore potentially may be useful to limit HCC growth and metastasis. A practical application of the authors findings could be the use of miR-548a-5p expression as a potential predictor of tumors that may be more likely to respond to Tg737-targeting therapies.
The study by Zhao Ge et al on microRNA-548a-5p and its role in hepatocellular carcinoma via Tg737 is very interesting. The authors show that miR-548a-5p regulates negatively the tumor suppressor gene Tg737 and promotes tumorigenesis in vitro and in vivo. The authors suggest that therapeutic strategies to inhibit miR-548a-5p in the future may prove useful in limiting HCC growth and metastasis.
P- Reviewer: Liaskou E, Peltec A, Sirin G, Wang GY S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3595] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 2. | Sarkar N, Chakravarty R. Hepatitis B Virus Infection, MicroRNAs and Liver Disease. Int J Mol Sci. 2015;16:17746-17762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999;144:385-388. [PubMed] |
| 4. | Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn. 2003;227:78-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709-718. [PubMed] |
| 6. | Chen CF, Yeh SH, Chen DS, Chen PJ, Jou YS. Molecular genetic evidence supporting a novel human hepatocellular carcinoma tumor suppressor locus at 13q12.11. Genes Chromosomes Cancer. 2005;44:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Isfort RJ, Cody DB, Doersen CJ, Richards WG, Yoder BK, Wilkinson JE, Kier LD, Jirtle RL, Isenberg JS, Klounig JE. The tetratricopeptide repeat containing Tg737 gene is a liver neoplasia tumor suppressor gene. Oncogene. 1997;15:1797-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | You N, Liu W, Tang L, Zhong X, Ji R, Zhang N, Wang D, He Y, Dou K, Tao K. Tg737 signaling is required for hypoxia-enhanced invasion and migration of hepatoma cells. J Exp Clin Cancer Res. 2012;31:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | You N, Liu W, Zhong X, Ji R, Zhang M, You H, Dou K, Tao K. Tg737 inhibition results in malignant transformation in fetal liver stem/progenitor cells by promoting cell-cycle progression and differentiation arrest. Mol Carcinog. 2012;51:659-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1837] [Cited by in RCA: 1796] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 11. | Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065-7070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2930] [Cited by in RCA: 3052] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 12. | Liu YX, Long XD, Xi ZF, Ma Y, Huang XY, Yao JG, Wang C, Xing TY, Xia Q. MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. Biomed Res Int. 2014;2014:482926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Gori M, Arciello M, Balsano C. MicroRNAs in nonalcoholic fatty liver disease: novel biomarkers and prognostic tools during the transition from steatosis to hepatocarcinoma. Biomed Res Int. 2014;2014:741465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Cheng J, Zhou L, Xie QF, Xie HY, Wei XY, Gao F, Xing CY, Xu X, Li LJ, Zheng SS. The impact of miR-34a on protein output in hepatocellular carcinoma HepG2 cells. Proteomics. 2010;10:1557-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 16. | Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184-5193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2843] [Cited by in RCA: 3083] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 18. | Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2191] [Cited by in RCA: 2184] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 19. | Yuan K, Lian Z, Sun B, Clayton MM, Ng IO, Feitelson MA. Role of miR-148a in hepatitis B associated hepatocellular carcinoma. PLoS One. 2012;7:e35331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 548] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 21. | Hu B, Ying X, Wang J, Piriyapongsa J, Jordan IK, Sheng J, Yu F, Zhao P, Li Y, Wang H. Identification of a tumor-suppressive human-specific microRNA within the FHIT tumor-suppressor gene. Cancer Res. 2014;74:2283-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Liang T, Guo L, Liu C. Genome-wide analysis of mir-548 gene family reveals evolutionary and functional implications. J Biomed Biotechnol. 2012;2012:679563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Rane JK, Scaravilli M, Ylipää A, Pellacani D, Mann VM, Simms MS, Nykter M, Collins AT, Visakorpi T, Maitland NJ. MicroRNA expression profile of primary prostate cancer stem cells as a source of biomarkers and therapeutic targets. Eur Urol. 2015;67:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Chang H, Kim N, Park JH, Nam RH, Choi YJ, Lee HS, Yoon H, Shin CM, Park YS, Kim JM. Different microRNA expression levels in gastric cancer depending on Helicobacter pylori infection. Gut Liver. 2015;9:188-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Liu WH, Tao KS, You N, Liu ZC, Zhang HT, Dou KF. Differences in the properties and mirna expression profiles between side populations from hepatic cancer cells and normal liver cells. PLoS One. 2011;6:e23311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Cheng Y, Li Y, Liu D, Zhang R, Zhang J. miR-137 effects on gastric carcinogenesis are mediated by targeting Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett. 2014;588:3274-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Lin S, Cheung WK, Chen S, Lu G, Wang Z, Xie D, Li K, Lin MC, Kung HF. Computational identification and characterization of primate-specific microRNAs in human genome. Comput Biol Chem. 2010;34:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Gupta MK, Halley C, Duan ZH, Lappe J, Viterna J, Jana S, Augoff K, Mohan ML, Vasudevan NT, Na J. miRNA-548c: a specific signature in circulating PBMCs from dilated cardiomyopathy patients. J Mol Cell Cardiol. 2013;62:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Khokhar A, Noorali S, Sheraz M, Mahalingham K, Pace DG, Khanani MR, Bagasra O. Computational analysis to predict functional role of hsa-miR-3065-3p as an antiviral therapeutic agent for treatment of triple infections: HCV, HIV-1, and HBV. Libyan J Med. 2012;7:19774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Li Y, Xie J, Xu X, Wang J, Ao F, Wan Y, Zhu Y. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1. Protein Cell. 2013;4:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Xing TJ, Xu HT, Yu WQ, Wang B, Zhang J. MiRNA-548ah, a potential molecule associated with transition from immune tolerance to immune activation of chronic hepatitis B. Int J Mol Sci. 2014;15:14411-14426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Piriyapongsa J, Jordan IK. A family of human microRNA genes from miniature inverted-repeat transposable elements. PLoS One. 2007;2:e203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Chen H, Sun JG, Cao XW, Ma XG, Xu JP, Luo FK, Chen ZT. Preliminary validation of ERBB2 expression regulated by miR-548d-3p and miR-559. Biochem Biophys Res Commun. 2009;385:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Mandal P, Bhattacharjee B, Das Ghosh D, Mondal NR, Roy Chowdhury R, Roy S, Sengupta S. Differential expression of HPV16 L2 gene in cervical cancers harboring episomal HPV16 genomes: influence of synonymous and non-coding region variations. PLoS One. 2013;8:e65647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Robert A, Margall-Ducos G, Guidotti JE, Brégerie O, Celati C, Bréchot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci. 2007;120:628-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Bonura C, Paterlini-Brechot P, Brechot C. Structure and expression of Tg737, a putative tumor suppressor gene, in human hepatocellular carcinomas. Hepatology. 1999;30:677-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Song Z, Li R, You N, Tao K, Dou K. Loss of heterozygosity of the tumor suppressor gene Tg737 in the side population cells of hepatocellular carcinomas is associated with poor prognosis. Mol Biol Rep. 2010;37:4091-4101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |