Published online Jun 21, 2016. doi: 10.3748/wjg.v22.i23.5332
Peer-review started: March 22, 2016
First decision: April 14, 2016
Revised: May 2, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: June 21, 2016
Processing time: 83 Days and 23.7 Hours
Although monoclonal antibodies (mAbs) against epidermal growth factor receptor (EGFR) have largely enriched the available therapeutic choices for colorectal cancer (CRC), the understanding and management of their associated clinical toxicities are limited. In addition, the combined strategies of administering EGFR mAbs and traditional cytotoxic agents, such as 5-fluorouracil, oxaliplatin and irinotecan, have resulted in a more complicated management of CRC treatment-related side effects compared with EGFR mAb monotherapy. We believe that a thorough recognition of the toxicities of EGFR mAb drugs is essential for physicians to increase the therapeutic index in the treatment of CRC. This review aims to summarize the existing information regarding the treatment dilemmas of cetuximab combined with chemotherapy in the management of metastatic CRC.
Core tip: The advent of epidermal growth factor receptor monoclonal antibodies (EGFR mAbs), especially cetuximab, has provided a meaningful transformation in the available treatment options for advanced colorectal cancer (CRC). Nevertheless, their efficacy is accompanied by some undesired complications. Additionally, combination treatments comprising EGFR mAbs and traditional cytotoxic agents have resulted in a more complex management of CRC treatment-related side effects. Therefore, it is imperative to understand and appropriately address the treatment dilemmas of cetuximab combined with chemotherapy for the management of metastatic CRC.
- Citation: Wen F, Li Q. Treatment dilemmas of cetuximab combined with chemotherapy for metastatic colorectal cancer. World J Gastroenterol 2016; 22(23): 5332-5341
- URL: https://www.wjgnet.com/1007-9327/full/v22/i23/5332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i23.5332
Colorectal cancer (CRC), one of the most commonly diagnosed malignancies, is the third leading cause of cancer-related deaths, with more than 600000 worldwide[1]. It is predicted that nearly 123 million new cases are diagnosed yearly, although the incidence has declined in long-term records[1]. Nevertheless, approximately 50% of patients have metastatic disease when first diagnosed, and among the 60% of patients with an initial curative intent, approximately 25%-40% will suffer from disease recurrence or progression[2,3]. Hence, the treatment of metastatic disease plays a key role in the management of advanced CRC, the cost of which accounts for an appreciable global healthcare burden.
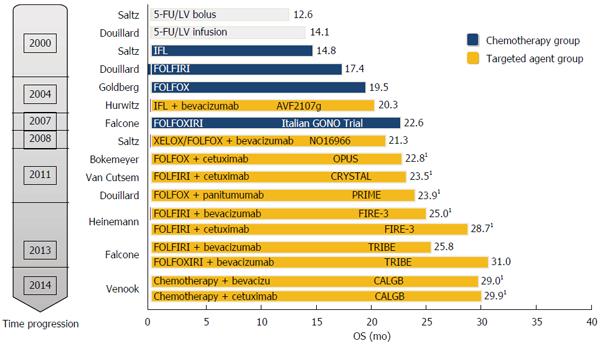
Notably, with the advent of new biologic drugs during the last decade, an unprecedented surge of new treatment strategies for the management of advanced CRC has been witnessed. Consequently, the overall survival of patients with advanced CRC has been extended and their quality of life has improved significantly. According to reviews of institutional databases since 2004, which is when novel therapeutic drugs became available, the median overall survival of patients with advanced CRC has recently increased from 18 mo (95%CI: 15.8-20.2 mo) to almost 29.2 mo (95%CI: 24.3-34.2 mo)[4,5]. Moreover, the 5-year relative survival rates have changed significantly from 51% of patients diagnosed during 1975-1977 to 65% of patients treated from 2004 to 2010[1]. Therefore, considerable pharmacological advancements in recent years have transformed CRC from a disease that is rapidly lethal to one that can be managed chronically for 2-3 years (Figure 1).
Most of these new biologics should work effectively in combination with at least one of the chemotherapy regimens. The combination of 5-fluorouracil (5-Fu) plus leucovorin (LV) with the addition of irinotecan (iri) or oxaliplatin (oxa) is recommended as the primary backbone chemotherapy for advanced CRC[6,7]. Despite extensive chemotherapy treatments, even including the new biological agents, the clinical outcomes in CRC remain limited, and increasing severity of toxicity is often observed. Therefore, there is an urgent need to improve the accurate selection of treatment strategies for individuals based on the expected clinical outcomes and accompanied toxicities. Ultimately, advanced knowledge of molecular medicine might guide clinicians to select the right treatment regimen for individual patients[8]. This review aims to summarize the existing information regarding the treatment dilemmas of cetuximab (Cmab), one of the epidermal growth factor receptor (EGFR) monoclonal antibodie (mAb), combined with chemotherapy, including 5-Fu, oxa and iri, for the management of metastatic CRC.
Targeting EGFR and its ligands’ pathways is a promising treatment strategy because it is reported that approximately 25%-77% of CRC cases exhibited overexpression of EGFR as well as its ligands, including EGF and transforming growth factor α (TGF-α)[9,10]. Notably, mAbs targeting EGFR have had a profound beneficial effect in the treatment of CRC since the clinical application of Cmab was approved by the United States Food and Drug Administration in 2004 followed by the authorization of panitumumab (Pmab) two years later[11]. Significant improvement was achieved in the CALGB80405 trial, which showed that the OS of CRC patients reached 29.93 mo with Cmab treatment combined with chemotherapy; in particular, the OS was 30.1 mo for the Cmab and mFOLFOX6 (oxaliplatin/5-FU/leucovorin) combination[12].
Cetuximab is a human/mouse recombinant immunoglobulin G1 mAb that has a higher affinity for the extracellular domain of EGFR than other ligands, such as EGF and TGF-α. The binding of Cmab to EGFR prevents intracellular ligand-mediated receptor-related tyrosine kinase phosphorylation, resulting in the inhibition of downstream signaling pathways, including the RAS-RAF-MAPK and PI3K-Akt/mTOR pathways[13]. Consequently, the antitumor effects of Cmab are due to multiple mechanisms: (1) cell proliferation suppression: Cmab arrests the cell cycle in G1 phase and, consequently, the number of S-phase cells is decreased. This effect is a result of the increased expression of p27KIP1, a CDK2 inhibitor, and the over phosphorylation of Rb protein[14]. Then, apoptosis-associated proteins are activated, including the induction of BAX, the release of Smac and the activation of caspase 8. As a result, the number of cells arrested in G1 declines; (2) antiangiogenesis: the production of angiogenic factors is reduced by the inhibition of EGFR pathways; for example, the production of vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and interleukin-8 (IL-8) is decreased, which contributes to a decline in microvessel density and enhanced endothelial cell apoptosis[15]; (3) antibody-dependent cellular cytotoxicity might be induced[16]; and (4) cancer metastasis-related matrix metalloproteinases are decreased by the inactivated EGFR. Hence, cell adhesion is reduced and metastasis is further down-regulated. The anti-tumor effect has been demonstrated in EGFR-expressing CRC cells and nude mice in vivo. Additionally, the combination of Cmab and chemotherapy drugs or radiotherapy exhibits significant tumor inhibition in nude mice bearing CRC cell xenografts[17].
Notably, Cmab has achieved demonstrable clinical anti-cancer efficacy, and awareness of the underlying mechanism is essential in the management of advanced CRC. Cmab is a chimeric mAb against EGFR, namely, the immunoglobulin’s constant region is derived from humans, but the variable domain is derived from mice[18]. Hence, the nonhuman nature of these early antibodies leads to inflammatory reactions with repeated administrations, which might be related to the increasing immunogenicity of recipients[19,20].
According to Lee et al[21], to better understand of the toxicity-associated mechanism, adverse events should be distinguished into two categories: target-related (on target) and agent-related (off-target) toxicities. Generally, on-target adverse events cannot be avoided because of the specific target the agent inhibited, and they should be managed proactively. By contrast, off-target toxicities are the result of the cross-inhibition of unintended targets or cross-interaction with undesired pathways, and they are related to the specificity of the targeted agents. In addition, the pharmacokinetics are closely related to the toxicities and are determined by the inter-individual variations of drug absorption, distribution and metabolism. Consequently, ABC drug transporter polymorphisms and the cytochrome P450 genotype of the patient could be pharmacogenetic contributors to adverse events[22,23]. Importantly, it has been suggested that the mAbs could induce immune activity indirectly in a known non-allergic, cytokine-associated process of infusion reaction[24]. Chung et al[25] found that a high frequency of infusion reactions was significantly related to elevated circulating anti-cetuximab IgE levels pretreatment.
With the increasing emergence of targeted agents, optimization of therapeutic drugs has received widespread attention, especially the choice of chemotherapy backbone and mAbs or antiangiogenic regimens. Until now, the combination of 5-Fu or oral capecitabine (cap) with either oxa (FOLFOX), XELOX, iri (FOLFIRI ) or XELIRI (cap and iri) has been recommended as the standard treatment combined with targeted agents for patients with advanced CRC[7]. Hence, there is an urgent need to determine how best to integrate these regimens to achieve clinical outcomes with a high efficacy but low toxicity. To better understand the reciprocal interactions between chemotherapy regimens and targeted agents, the current study reviewed clinical trials from January 2002 and March 2015 collected from the PubMed, American Society of Clinical Oncology annual meeting, gastrointestinal cancer symposium and European Society for Medical Oncology databases; the clinical trials were phase 3 or multicenter, randomized phase 2 trials studying the FDA recommended target drugs combined with cytotoxicity chemotherapies for the first-line treatment of CRC. Research studies involving adjuvant, neo-adjuvant and maintenance regimens of CRC were excluded. Moreover, incomplete studies without safety results were also excluded.
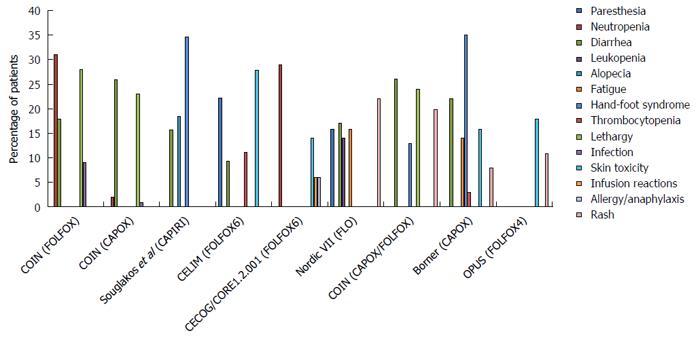
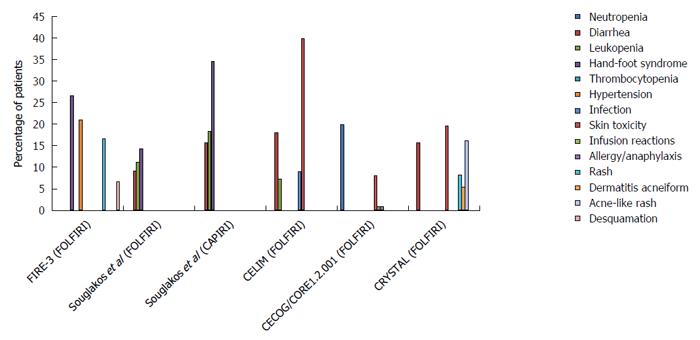
It must be noted that Cmab has contributed greatly to improving the clinical outcome of CRC, with a prolonged OS of more than 30 mo (Figures 2 and 3, and Table 1). Of the multi-chemotherapies, FOLFIRI and FOLFOX are the most commonly used. Nevertheless, some unpredictable toxicity related to the combination occurred (Figures 2 and 3). An overview of the figures showing the adverse events of Cmab and chemotherapies reveals that the incidence of grade 3/4 hand-foot syndrome was 13%-35%, followed by skin toxicity (8%-40%); diarrhea, neutropenia and lethargy were also common.
| Name of study | Clinical trial phase | n | Chemotherapy | OS (mo) | PFS (mo) |
| FIRE-3[55-57] | 3 | 171 | Cmab + FOLFIRI | 33.1 | 10.2 |
| CALGB80405[12] | 3 | 547 | Cmab + CT | 29.93 | 10.5 |
| COIN[58] | 3 | 279 | Cmab + FOLFOX | 14.9 | 8.5 |
| COIN | 3 | 523 | Cmab + CAPOX | 15.0 | 7.4 |
| Souglakos et al[59] | 2 | 167 | Cmab + FOLFIRI | 25.7 | 10.0 |
| Souglakos et al[59] | 2 | 166 | Cmab + CAPIRI | 27.5 | 8.9 |
| CELIM[60,61] | 3 | 56 | Cmab + FOLFOX6 | 35.7 | 11.2 |
| CELIM | 3 | 55 | Cmab + FOLFIRI | 29.0 | 10.5 |
| CECOG/CORE1.2.001[62] | 2 | 74 | Cmab + FOLFOX6 | 17.4 | 8.6 |
| CECOG/CORE1.2.001 | 2 | 77 | Cmab + FOLFIRI | 18.9 | 8.3 |
| CRYSTAL[63,64] | 3 | 316 (WT) | Cmab + FOLFIRI | 23.5 | 9.9 |
| Nordic VII[65] | 3 | 97 (WT) | Cmab + Nordic FLOX(bolus) | 20.1 | 7.9 |
| COIN[66] | 3 | 362 (WT) | Cmab + CAPOX/FOLFOX | 17.0 | 8.6 |
| Borner[67] | 2 | 37 | Cmab + CAPOX | 20.5 | 7.2 |
| OPUS[68,69] | 2 | 82 (WT) | Cmab + FOLFOX4 | 18.3 | 7.2 |
The efficacy and toxicity mechanism of Cmab and oxa-based chemotherapies is perplexing. In 2001, oxa was recommended as a cytotoxicity backbone for the treatment of adjuvant and advanced chemotherapy settings by the United States Food and Drug Administration[26,27]. As a conventional partner of Cmab, the regular regimens were FOLFOX in the PRIME, OPUS, and COIN trials; FLOX in the NORDIC VII trial; and XELOX in the COIN trial. However, the outcomes were distinctly different. The outcomes become more confusing when considering the results of CALGB80405, which added another layer to the already puzzling situation.
The interaction of Cmab and oxa is a double-edged sword - the interaction has shown both synergistic and antagonistic effects in vitro. First, the combined administration enhanced cell cycle arrest and induced cell apoptosis by elevating pro-apoptotic proteins, such as Bax and Caspase 8; meanwhile, it reduced the expression of anti-apoptotic proteins, for example, Bcl-2, and NF-κB was also decreased[28]. Second, the level of AKT phosphorylation, a target of the EGFR downstream pathway, was increased after the administration of oxa, which was apparently inhibited by Cmab[28]. Third, Cmab promoted the oxa anti-tumor efficacy by suppressing the DNA repair system, which involves increasing platinum-DNA adducts; inducing apurinic or apyrimidinic sites; and decreasing Claspin, CDC45 and CDC6 levels expressed at the beginning of DNA replication[29]. Prewett et al[30] found that Cmab decreased the phosphorylation of ERK1/2 and AKT, resulting in the inhibition of ERCC1 and XPF. Additionally, Balin-Gauthier et al[29] reported that the mRNA level and protein expression of ERCC1 and XRCC1 declined after treatment with Cmab[31]. Last, EGFR expression in CRC cells increased when stimulated by oxa, which sensitized the treatment with Cmab[32,33]. By contrast, Cmab inhibited NOX1 expression, which assisted NADPH, as a coenzyme, to produce ROS. When the levels of ROS produced by the cell was reduced, the anti-tumor effect induced by oxa was also reduced[34,35].
It is known that oxa alone has little efficacy, and its activation requires fluoropyrimidine as a partner. Moreover, the toxicity of oxa-based chemotherapy and Cmab combination was different (Figure 2), varying with the mode of administration of fluoropyrimidine (infusional 5-Fu, bolus 5-Fu, and cap). Preclinical research studies addressing the question of the optimal administration method of 5-Fu are rare. One study showed that the longer the infusion time, the more significant the suppression of thymidylate synthase (TS)[11,36]. In that study, three different 5-Fu-sensitive human cancer cell lines, gastric cancer, colorectal cancer and breast cancer, were exposed to 5-Fu for either one hour or 24 h repeatedly. The 5-Fu concentration was fixed, and the two treatments had equivalent effective doses. The results showed that cells exposed to one-hour of 5-Fu developed resistance more rapidly than those exposed to 24 h of 5-Fu. Additionally, only a small fraction of one-hour exposed cells was cross-resistant to a 24-h treatment, whereas obvious cross-resistance was seen for 24-h exposed cells to a one-hour schedule. Moreover, increasing TS expression was observed in all of the 24-h exposed cells, but in only one cell line treated with one-hour 5-Fu. Hence, the author concluded that the effect of 5-Fu was determined by the mode of application because the inhibition of TS was more significant with a prolonged infusion time. Although the pre-clinical data are limited, the efficacy of 5-Fu infusion application has been demonstrated by clinicians. Aschele et al[37] suggested that the application schedule and biochemical modulators of 5-Fu-based chemotherapy determine the relationship between intratumoral TS levels and clinical outcomes.
Meanwhile, Cmab promotes 5-Fu activity by inhibiting TS[36,38]. Skvortsov et al[39] illustrated that in EGFR-overexpressed CRC cell lines, such as Caco-2, HRT-18, HT-29, WiDr and SW-480, TS expression were suppressed, whereas in the EGFR-negative cell line SW-620, inhibition disappeared. Additionally, the combined treatment of 5-Fu and Cmab was related to a synergistic activation of the MAPK pathway. The in vitro results were consistent with a meta-analysis showing that the efficacy of oxa and Cmab combination was optimized by infusional 5-Fu[40].
The efficacy and toxicity mechanisms of Cmab and iri-based chemotherapies are quite clear compared with those of the oxa and Cmab combination. According to clinical trial results, iri is the only cytotoxic agent combined with all targeted drugs that is recommended in the first-line treatment of CRC. The reciprocal interactions of Cmab and iri result in reduced DNA damage repair, increased SN-38 plasma concentration and enhanced suppression of the EGFR signaling pathway. Chu et al[41] found that the EGFR inhibitor could reduce SN-38 excretion by suppressing ABB1 in vivo. The researcher studied the influence of Cmab on the iri concentration and its effective metabolite SN-38 in mice via HPLC analysis. Human CRC xenografted nude mice were generated and treated with oral iri alone or with iri following pre-treatment with Cmab. They found that the AUC of SN-38 in the plasma and tumors of mice given the combined treatment was nearly 1.7-fold higher than that in mice treated with iri alone, which demonstrated that Cmab was associated with the distribution of iri into tissues. In addition, Yashiro et al[42] suggested that EGFR inhibitors decreased the expression of uridinediphosphoglucuronate glucuronosyltransferase 1A1 (UGT1A1) and ABCG2 to prolong the active ingredient concentration. However, the improved efficacy did not occur without toxicity. The common adverse events of the combination treatments include hand-foot syndrome, which occurs at a rate as high as 34.6%; diarrhea, which occurs at a rate of approximately 15%; and skin toxicity (Figure 3).
Of particular note, dermatologic toxicities have received considerable attention in clinical practice because of their prognostic role in Cmab treatment[23,43,44]. As the most common side effect related to anti-EGFR therapy, the incidence of all grades of rash is as high as 45%-95%, of which 5%-18% are grades 3 or above[43]. Papulopustular eruption, also known as acneiform rash, is the most common dermatologic adverse event induced by EGFR inhibitor treatment. In addition, nail changes, ocular changes, hair changes, pruritis, photosensitivity, xerosis and erythema also appear during Cmab treatment[44]. Usually, the rash occurs within two to three days following initiation of Cmab treatment, and it worsens within one to three weeks. Although not life threatening, the dermatologic toxicities are significantly related with impaired quality of life, especially in younger patients because of the discomfort and detriment in some obvious locations, such as the face[45,46].
Indeed, oral minocycline or doxycycline is suggested as a prophylactic treatment during Cmab treatment. In addition, broad-spectrum sunscreen should be applied to reduce sunshine exposure, and alcohol-containing skin products should be avoided. For dry skin, emollients and mild topical steroids, such as 1% hydrocortisone cream twice or three times a day, are suggested. For papulopustular eruptions, topical antibiotics should be administered. For moderate pruritus or tender skin rashes, 0.1% triamcinolone or 2.5% hydrocortisone cream is recommended. The Cmab treatment should be adjusted once a grade 3 rash appears, and oral corticosteroids or even oral antibiotics are administered to these patients.
Gastrointestinal toxicities are common adverse events for traditional chemotherapy regimens and are also a common toxic effect of targeted therapies. The frequency of diarrhea and colitis of all grades is 20%-66%, and it is 2%-16% for grade 3 or above. In addition, 38%-43% of patients exhibit elevated transaminase elevation and 7% to 32% from mucositis/stomatitis[43]. The appearance of diarrhea is due to widespread mucosal inflammation, from oropharyngolaryngeal inflammation to frank stomatitis. It is reported that the mechanism of this diarrhea is associated with Notch signaling pathway inhibition, which results from the transformation of proliferative undifferentiated intestinal crypt cells into secretory goblet cells[47-49]. Regarding the elevated transaminase levels, this increase might be associated with the inhibition of UGT1A1, the polymorphic variants of which contribute to isolated hyperbilirubinemia in Gilbert’s syndrome[50,51].
To treat diarrhea and colitis, the cause of diarrhea should be determined along with the administration of anti-motility agents, for example, loperamide and diphenoxylate/atropine, especially for patients who have received chemotherapy combined therapy. Alcohol- or peroxide-based mouthwashes should be avoided in the management of mucositis and stomatitis, and anesthetic mouthwashes should be administered at the same time. If infection is found, antifungal agents should be applied. Liver function laboratory investigations should be taken at baseline and at least once monthly if transaminase is elevated during treatment. If the aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels are above 53 upper limits of normal (ULN), treatment should be withheld. If the AST or ALT levels are less than 33 ULN, treatment can be resumed at a reduced dose.
Hypomagnesemia often occurs as a metabolic abnormality during Cmab treatment, and the frequency of all grades is 11%-38%, of which 4%-5% is grade 3 or 4[43]. A positive association has been demonstrated between total treatment duration and defective renal magnesium reabsorption, and the age and baseline serum magnesium concentrations are negatively associated with hypomagnesemia[52]. The activity and distribution of the transepithelial magnesium channel TRPM6 is regulated by EGF, resulting in excretion of renal magnesium. In addition, Thebault et al[53] and Groenestege et al[54] discovered an EGFR gene point mutation that contributes to isolated hypomagnesemia.
The suggested management is the optimal management of diarrhea. Significant QT-prolongation potential medications should be avoided. Oral supplementation should be used if necessary. Patients with grade 2 hypomagnesemia should be given a weekly intravenous infusion of replacement magnesium. Treatment should be initiated for patients with grade 3/4 or symptomatic hypomagnesemia, and intravenous magnesium should be increased to every 2-3 d.
Corneal abnormalities (keratoconjunctivitis, corneal ulceration) and corneal epithelium are the direct ocular toxicity effects of Cmab, and Cmab indirectly affects the associated glands and appendages, resulting in indirect adverse effects (meibomitis, cicatricial ectropion, dry eye). The incidence of all-grade ocular toxicity is reported to be 4%-18%, of which less than 1% is above grade 3[43].
The advised management of ocular toxicity is to continue the treatment. Artificial tears are applied if necessary, and antibacterial ointment should be used if infection is confirmed. Ophthalmologic evaluation is recommended for patients with vision changes, persistent eye pain, photosensitivity or presence of other drug-induced ocular anomalies, such as trichiasis. For patients with grade 3 symptoms, treatment should be withheld.
The advent of EGFR mAbs, especially Cmab, has provided a meaningful transformation in the available treatment options for CRC. Nevertheless, although the application of these targeted drugs has yielded a tremendous benefit for patients with advanced CRC and although these drugs have even outperformed conventional chemotherapies, their efficacy is accompanied by some undesired complications. In addition, combination treatments comprising EGFR mAbs and traditional cytotoxic agents, such as 5-fluorouracil, oxa and iri, have resulted in a more complex management of CRC treatment-related side effects compared with EGFR mAb monotherapy. We believe that a thorough recognition of the toxicities from EGFR mAbs is essential for physicians to evaluate the potential risks into the correct context of clinical benefit. Therefore, it is imperative to understand and appropriately address the treatment dilemmas of cetuximab combined with chemotherapy for the management of metastatic CRC.
Currently, investigations of precise drug mechanisms are needed to refine existing drugs, to develop new targeted therapies and to optimize amenable therapeutic settings for personalized medicine. However, the existing research to precisely guide regimen and dose selection and to better reveal the mechanisms of interactions related to efficacy and toxicity is limited. Hence, more information is needed, especially for the reciprocal interactions between iri/oxa-based chemotherapies and mAbs. Additionally, a better understanding of the mechanisms of targeted agents regarding their activity, metabolism and resistance is also urgently needed to employ these combined therapies most effectively. Finally, an improved knowledge of robust markers of prognosis and toxicity is vital to implement treatment strategies and accurately select patients. Consequently, emerging molecular technologies will result in a definitive treatment for colorectal cancer.
Manuscript source: Invited manuscript
P- Reviewer: Braet F S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 2. | Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Scholefield JH, Steele RJ. Guidelines for follow up after resection of colorectal cancer. Gut. 2002;51 Suppl 5:V3-V5. [PubMed] |
| 4. | Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677-3683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 1032] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 5. | Kasi PM, Hubbard JM, Grothey A. Selection of biologics for patients with metastatic colorectal cancer: the role of predictive markers. Expert Rev Gastroenterol Hepatol. 2015;9:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1106] [Article Influence: 85.1] [Reference Citation Analysis (1)] |
| 7. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1720] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 8. | Chua W, Kho PS, Moore MM, Charles KA, Clarke SJ. Clinical, laboratory and molecular factors predicting chemotherapy efficacy and toxicity in colorectal cancer. Crit Rev Oncol Hematol. 2011;79:224-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Peeters M, Price T, Van Laethem JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: where are we today? Oncologist. 2009;14:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Capdevila J, Elez E, Macarulla T, Ramos FJ, Ruiz-Echarri M, Tabernero J. Anti-epidermal growth factor receptor monoclonal antibodies in cancer treatment. Cancer Treat Rev. 2009;35:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Grothey A, Lenz HJ. Explaining the unexplainable: EGFR antibodies in colorectal cancer. J Clin Oncol. 2012;30:1735-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Alan P. Venook DN, Heinz-Josef Lenz, Federico Innocenti, Michelle R. Mahoney, Bert H. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32:(suppl; abstr LBA3). |
| 13. | Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Fan Z, Shang BY, Lu Y, Chou JL, Mendelsohn J. Reciprocal changes in p27(Kip1) and p21(Cip1) in growth inhibition mediated by blockade or overstimulation of epidermal growth factor receptors. Clin Cancer Res. 1997;3:1943-1948. [PubMed] |
| 15. | Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257-265. [PubMed] |
| 16. | Herbst RS, Kim ES, Harari PM. IMC-C225, an anti-epidermal growth factor receptor monoclonal antibody, for treatment of head and neck cancer. Expert Opin Biol Ther. 2001;1:719-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311-1318. [PubMed] |
| 18. | Beck A, Wurch T, Corvaïa N. Therapeutic antibodies and derivatives: from the bench to the clinic. Curr Pharm Biotechnol. 2008;9:421-422. [PubMed] |
| 19. | Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. [PubMed] |
| 20. | Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 736] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 21. | Lee SJ, Kavanaugh A. Adverse reactions to biologic agents: focus on autoimmune disease therapies. J Allergy Clin Immunol. 2005;116:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Levêque D, Veal G, Astier A. Review of therapeutic drug monitoring of anticancer drugs part two--targeted therapies. Eur J Cancer. 2014;50:2020-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 23. | Gharwan H, Groninger H. Kinase inhibitors and monoclonal antibodies in oncology: clinical implications. Nat Rev Clin Oncol. 2016;13:209-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 24. | Dassonville O, Bozec A, Fischel JL, Milano G. EGFR targeting therapies: monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit Rev Oncol Hematol. 2007;62:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1264] [Cited by in RCA: 1084] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 26. | Kemeny N, Garay CA, Gurtler J, Hochster H, Kennedy P, Benson A, Brandt DS, Polikoff J, Wertheim M, Shumaker G. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J Clin Oncol. 2004;22:4753-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Grothey A, Sargent DJ. FOLFOX for stage II colon cancer? A commentary on the recent FDA approval of oxaliplatin for adjuvant therapy of stage III colon cancer. J Clin Oncol. 2005;23:3311-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60 Suppl 1:15-23; discussion 41-42. [PubMed] |
| 29. | Balin-Gauthier D, Delord JP, Pillaire MJ, Rochaix P, Hoffman JS, Bugat R, Cazaux C, Canal P, Allal BC. Cetuximab potentiates oxaliplatin cytotoxic effect through a defect in NER and DNA replication initiation. Br J Cancer. 2008;98:120-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Prewett M, Deevi DS, Bassi R, Fan F, Ellis LM, Hicklin DJ, Tonra JR. Tumors established with cell lines selected for oxaliplatin resistance respond to oxaliplatin if combined with cetuximab. Clin Cancer Res. 2007;13:7432-7440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Vidal AE, Boiteux S, Hickson ID, Radicella JP. XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions. EMBO J. 2001;20:6530-6539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 361] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Correale P, Marra M, Remondo C, Migali C, Misso G, Arcuri FP, Del Vecchio MT, Carducci A, Loiacono L, Tassone P. Cytotoxic drugs up-regulate epidermal growth factor receptor (EGFR) expression in colon cancer cells and enhance their susceptibility to EGFR-targeted antibody-dependent cell-mediated-cytotoxicity (ADCC). Eur J Cancer. 2010;46:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Ekblad L, Johnsson A. Cetuximab sensitivity associated with oxaliplatin resistance in colorectal cancer. Anticancer Res. 2012;32:783-786. [PubMed] |
| 34. | Dahan L, Sadok A, Formento JL, Seitz JF, Kovacic H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br J Pharmacol. 2009;158:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Morazzani M, de Carvalho DD, Kovacic H, Smida-Rezgui S, Briand C, Penel C. Monolayer versus aggregate balance in survival process for EGF-induced apoptosis in A431 carcinoma cells: Implication of ROS-P38 MAPK-integrin alpha2beta1 pathway. Int J Cancer. 2004;110:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Harstrick A, Gonzales A, Schleucher N, Vanhoefer U, Lu K, Formento JL, Milano G, Wilke H, Seeber S, Rustum Y. Comparison between short or long exposure to 5-fluorouracil in human gastric and colon cancer cell lines: biochemical mechanism of resistance. Anticancer Drugs. 1998;9:625-634. [PubMed] |
| 37. | Aschele C, Debernardis D, Bandelloni R, Cascinu S, Catalano V, Giordani P, Barni S, Turci D, Drudi G, Lonardi S. Thymidylate synthase protein expression in colorectal cancer metastases predicts for clinical outcome to leucovorin-modulated bolus or infusional 5-fluorouracil but not methotrexate-modulated bolus 5-fluorouracil. Ann Oncol. 2002;13:1882-1892. [PubMed] |
| 38. | Bijnsdorp IV, Kruyt FA, Fukushima M, Smid K, Gokoel S, Peters GJ. Molecular mechanism underlying the synergistic interaction between trifluorothymidine and the epidermal growth factor receptor inhibitor erlotinib in human colorectal cancer cell lines. Cancer Sci. 2010;101:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Skvortsov S, Sarg B, Lindner H, Lukas P, Hilbe W, Zwierzina H, Skvortsova I. Cetuximab inhibits thymidylate synthase in colorectal cells expressing epidermal growth factor receptor. Proteomics Clin Appl. 2008;2:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Wen F, Tang R, Sang Y, Li M, Hu Q, Du Z, Zhou Y, Zhang P, He X, Li Q. Which is false: oxaliplatin or fluoropyrimidine? An analysis of patients with KRAS wild-type metastatic colorectal cancer treated with first-line epidermal growth factor receptor monoclonal antibody. Cancer Sci. 2013;104:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Chu C, Abbara C, Tandia M, Polrot M, Gonin P, Farinotti R, Bonhomme-Faivre L. Cetuximab increases concentrations of irinotecan and of its active metabolite SN-38 in plasma and tumour of human colorectal carcinoma-bearing mice. Fundam Clin Pharmacol. 2014;28:652-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Yashiro M, Qiu H, Hasegawa T, Zhang X, Matsuzaki T, Hirakawa K. An EGFR inhibitor enhances the efficacy of SN38, an active metabolite of irinotecan, in SN38-refractory gastric carcinoma cells. Br J Cancer. 2011;105:1522-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63:249-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Abdullah SE, Haigentz M, Piperdi B. Dermatologic Toxicities from Monoclonal Antibodies and Tyrosine Kinase Inhibitors against EGFR: Pathophysiology and Management. Chemother Res Pract. 2012;2012:351210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Joshi SS, Ortiz S, Witherspoon JN, Rademaker A, West DP, Anderson R, Rosenbaum SE, Lacouture ME. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116:3916-3923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 46. | Jatoi A, Thrower A, Sloan JA, Flynn PJ, Wentworth-Hartung NL, Dakhil SR, Mattar BI, Nikcevich DA, Novotny P, Sekulic A. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, Patnaik A, Falchook GS, Dasari A, Shapiro GI. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 48. | Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 49. | van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Singer JB, Shou Y, Giles F, Kantarjian HM, Hsu Y, Robeva AS, Rae P, Weitzman A, Meyer JM, Dugan M. UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia. 2007;21:2311-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Xu CF, Reck BH, Xue Z, Huang L, Baker KL, Chen M, Chen EP, Ellens HE, Mooser VE, Cardon LR. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer. 2010;102:1371-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Tejpar S, Piessevaux H, Claes K, Piront P, Hoenderop JG, Verslype C, Van Cutsem E. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 54. | Groenestege WM, Thébault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117:2260-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 55. | Stintzing S, Fischer von Weikersthal L, Decker T, Vehling-Kaiser U, Jäger E, Heintges T, Stoll C, Giessen C, Modest DP, Neumann J. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 56. | Heinemann V, Decker T. Random-ized comparison of FOLFIRI plus cetuximab versus FOLFIRI plusbevacizumab as first-line treatment of KRAS wild-type metastaticcolorectal cancer: German AIO study KRK-0306 (FIRE-3). J Clin Oncol. 2013;Abstract LBA3506. |
| 57. | Modest DP, Stintzing S. FOLFIRIplus cetuximab versus FOLFIRI plus bevacizumab as first-line treat-ment of KRAS-wildtype metastatic colorectal cancer: German AIOstudy KRK-0306 (FIRE-3). Ann Oncol. 2013;24:Abstract O-0029. |
| 58. | Madi A, Fisher D, Wilson RH, Adams RA, Meade AM, Kenny SL, Nichols LL, Seymour MT, Wasan H, Kaplan R. Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial. Br J Cancer. 2012;107:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, Xynogalos S, Christophyllakis Ch, Kouroussis Ch, Vamvakas L. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br J Cancer. 2012;106:453-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 61. | Folprecht G, Bechstein W. Survival withcetuximab/FOLFOX or cetuximab/FOLFIRI of patients with nonre-sectable colorectal liver metastases in the CELIM study. J Clin Oncol. 2012;30:Abstract 540. |
| 62. | Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol. 2010;16:3133-3143. [PubMed] |
| 63. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3122] [Article Influence: 195.1] [Reference Citation Analysis (1)] |
| 64. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 65. | Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 420] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 66. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 67. | Borner M, Koeberle D, Von Moos R, Saletti P, Rauch D, Hess V, Trojan A, Helbling D, Pestalozzi B, Caspar C. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: a randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 69. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1240] [Article Influence: 72.9] [Reference Citation Analysis (0)] |