Published online May 7, 2016. doi: 10.3748/wjg.v22.i17.4389
Peer-review started: December 4, 2015
First decision: December 31, 2015
Revised: February 15, 2016
Accepted: March 2, 2016
Article in press: March 2, 2016
Published online: May 7, 2016
Processing time: 148 Days and 23.2 Hours
AIM: To investigate efficacy and safety for granulocyte, monocyte apheresis in a population of pediatric patients with ulcerative colitis.
METHODS: The ADAPT study was a prospective, open-label, multicenter study in pediatric patients with moderate, active ulcerative colitis with pediatric ulcerative colitis activity index (PUCAI) of 35-64. Patients received one weekly apheresis with Adacolumn® granulocyte, monocyte/macrophage adsorptive (GMA) apheresis over 5 consecutive weeks, optionally followed by up to 3 additional apheresis treatments over 3 consecutive weeks. The primary endpoint was the change in mean PUCAI between baseline and week 12; the secondary endpoint was improvement in PUCAI categorized as (Significant Improvement, PUCAI decrease of ≥ 35), Moderate Improvement (PUCAI decrease of 20 < 35), Small Improvement (PUCAI decrease of 10 < 20) or No change (PUCAI decrease of < 10).
RESULTS: Twenty-five patients (mean age 13.5 years; mean weight 47.7 kg) were enrolled. In the intention-to-treat set (ITT), the mean value for PUCAI improvement was 22.3 [95%CI: 12.9-31.6; n = 21]. In the per-protocol (PP) set, the mean improvement was 36.3 [95%CI: 31.4-41.1; n = 8]. Significant Improvement was recorded for 9 out of 20 patients (45%); 5 out of 20 patients (25%) had Moderate Improvement and one patient (5%) had No Change in PUCAI score at week 12. In the PP set, six out of eight patients (75%) showed Significant Improvement; and in two out of eight patients (25%) Moderate Improvement was recorded. The endoscopic activity index (EAI) decreased by 3 points on average. Seven (7) out of 21 (33%) patients in ITT and 4 out of 8 (50%) patients in PP have used steroids during the clinical investigation. The mean steroid dosage for these patients in the ITT set decreased from a mean 12.4 mg to 10 mg daily on average from Baseline to week 12.
CONCLUSION: Adacolumn® GMA apheresis treatment was effective in pediatric patients with moderate active Ulcerative Colitis. No new safety signals were reported. The present data contribute to considering GMA apheresis as a therapeutic option in pediatric patients having failed first line therapy.
Core tip: For a considerable group of children with ulcerative colitis (UC), treatment options are limited especially after failure of conventional treatment. The ADAPT trial was designed to generate prospective cohort data on efficacy and safety levels in moderate active pediatric UC patients when treated with Adacolumn granulocyte, monocyte/macrophage adsorptive (GMA). The present data contribute to considering GMA apheresis as a therapeutic option in pediatric patients having failed first line therapy.
- Citation: Ruuska T, Küster P, Grahnquist L, Lindgren F, Wewer AV. Efficacy and safety of granulocyte, monocyte/macrophage adsorptive in pediatric ulcerative colitis. World J Gastroenterol 2016; 22(17): 4389-4396
- URL: https://www.wjgnet.com/1007-9327/full/v22/i17/4389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i17.4389
Active ulcerative colitis is associated with extravasation of large numbers of activated granulocytes and monocytes into the colonic mucosa. This infiltration is promoted by potent pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-8, leukotriene B4 and platelet-activating factor. Activated leukocytes can cause extensive mucosal tissue injury through the release of degradative proteases, reactive oxygen derivatives and pro-inflammatory cytokines[1]. While pharmacologic approaches target the inflammatory messengers, an alternative option reducing the activated cells and also reducing the associated circulating cytokines implicated in the pathogenesis of ulcerative colitis (UC) is selective granulocyte, monocyte/macrophage adsorptive (GMA) adsorptive using Adacolumn®, a medical device (JIMRO Co. Ltd., Takasaki-shi, Gunma, Japan). The selective adsorption of predominantly old and activated CD10+ neutrophils to Adacolumn carrier beads is governed by opsonins C3b/C3bi, Fcγ receptors and the leukocyte complement receptors, while lymphocytes are spared[2]. Flow cytometry analyses during apheresis sessions have shown an initial drop in peripheral neutrophils and the emergence of naïve CD10 neutrophils from the bone marrow, which represents a qualitative change within the circulating neutrophil population[3].
To date published meta-analyses and systematic reviews favor Adacolumn® GMA over control therapy for inducing remission and response at 12 wk in adult moderate active adult UC[4-7].
Pediatric onset UC is more complex, extensive and severe compared to adult UC, and has a high rate of steroid dependency[8,9]. As for the data on Adacolumn use in children to date, there are limited small scale investigations and case reports which point to good response especially in the treatment of corticosteroid-dependent and corticosteroid-resistant pediatric UC patients, with good treatment tolerance, mild side-effects, and a comparable rate of relapses as seen with drug treatments[10].
For a considerable group of children with UC, treatment options are limited especially after failure of conventional treatment. On the background of a retrospective on clinical results in children treated with GMA apheresis[11], the ADAPT trial was designed to generate prospective cohort data in order to report efficacy and safety levels in moderate active pediatric UC patients when treated with Adacolumn.
Enrolled patients underwent apheresis treatment using the Adacolumn® GMA apheresis device (JIMRO, Japan; EU Authorized Representative: Otsuka Pharmaceuticals Europe Ltd, UK), which is approved for clinical use in EU (CE-marked) and in Japan. Adacolumn is an adsorptive type, single-use column filled with cellulose acetate beads of 2 mm in diameter. The carriers adsorb leukocytes, mainly activated granulocytes and monocytes from peripheral venous blood, passing from one antecubital vein through the column at a flow rate of 30 mL⁄min and returned to the contralateral antecubital vein. One apheresis treatment usually lasted 60 min, during which a total of 1.8 L blood was exposed to the carriers[12]. In the present trial, all patients received one weekly Adacolumn® apheresis over 5 consecutive weeks. The investigator could decide to add up to three additional treatments in weekly intervals at his discretion.
This study used the PUCAI score[13] which encompasses abdominal pain, rectal bleeding, stool consistency, number of stools per 24 h, nocturnal stools and activity level; and the endoscopy activity index acc. to Rachmilewitz (EAI) comprising granulation, vascular pattern, vulnerability of mucosa and mucosal damage. Key inclusion criteria are listed in Table 1.
| Children and adolescents < 18 yr and with a body weight ≥ 30 kg |
| Ulcerative colitis documented by clinical symptoms, endoscopic findings and histology since at least 3 mo prior to inclusion |
| Moderate active ulcerative colitis at baseline, defined as a PUCAI score between 35 and 64 |
| Pancolitis or left-sided colitis |
| Receiving or having received one or more of the following medicinal products before screening: |
| Sulfasalazine, mesalamine and other 5-aminosalicylic acid agents for 4 wk or more with a stable dose for the last 2 wk |
| 0.5 mg/kg per body weight with a maximum of 20 mg per day of prednisone with a stable dose for the last 2 wk, or |
| 6-mercaptopurine or azathioprine for 12 wk or more with a stable dose for the last 4 wk |
Steroid-resistance or -dependency, defined as inability to completely withdraw steroids without inducing a relapse or flare-up of the disease, was an exclusion criterion. The patients were not to have received a re-treatment of UC with drugs other than 5-ASA and derivatives, azathioprine and/or corticosteroids, e.g., immunosuppressants and biologicals; or topical therapy for ulcerative colitis within the last 2 wk, or previous Adacolumn treatment.
At baseline, PUCAI was evaluated; flexible endoscopy (colonoscopy or sigmoidoscopy) for determination of EAI was performed, hematology and clinical chemistry tests were completed; vital signs, concomitant medication, and adverse events were recorded. During their treatment phase, patients were evaluated every week. Flexible endoscopy (colonoscopy or sigmoidoscopy) was performed at post screening unless there was one available from within 6 wk before and 5 d after screening. Endoscopy was optional at the evaluation visit in week 12 (Table 2).
| Visit | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 |
| Day | -07 | 00 | 07 | 14 | 21 | 28 | ||||
| Week | -1 | 0 | 1 | 2 | 3 | 4 | 6 | 7 | 8 | 12 |
| Apheresis | ▲ | ▲ | ▲ | ▲ | ▲ | (▲) | (▲) | (▲) | ||
| Physical examination | ● | ● | ||||||||
| Endoscopy/EAI | ●4 | (●)5 | ||||||||
| PUCAI | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Clin. Chemistry | ● | ● | ● | ● | ●2 | ●2 | ●2 | ● | ||
| ESR | ● | ● | ● | ● | ●2 | ●2 | ●2 | ● | ||
| Urinalysis | ● | ● | ● | ● | ●2 | ●2 | ●2 | ● | ||
| Fecal sample | ● | |||||||||
| Coagulation | ● | ● | ●2 | ●2 | ●2 | ● | ||||
| Vital signs | ● | ● | ● | ● | ● | ● | ●1 | ●1 | ●1 | ● |
| Concomitant medication | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Adverse events | ●3 | ● | ● | ● | ● | ● | ● | ● | ● | ● |
The primary response variable was defined as the improvement in disease activity index (PUCAI) at week 12 (Visit 10) compared to Baseline (Visit 02). Key Secondary response variables were the proportion of significant improvement, moderate improvement, small improvement and no change as per PUCAI categories at week 12 (Table 3), the proportion of patients without disease activity (PUCAI < 10) at week 12, and the difference in EAI between week 12 and Baseline for those patients with pre- and post-treatment phase endoscopies available. Safety was assessed during the course of the clinical investigation by monitoring adverse events (AE), assessment of vital signs and collection of laboratory parameters.
| Significant improvement | ≥ 35 |
| Moderate improvement | ≥ 20 |
| Small improvement | ≥ 10 |
| No change | < 10 |
This investigation was conducted in accordance with the Good Clinical Practice Guidelines according to CPMP/ICH/135/95, with the Declaration of Helsinki, ISO 14155:2003, and all relevant national guidelines. The Clinical Investigation Plan (CIP) was submitted to the structured Institutional Review Boards (ethics committees) of each investigational center and a positive vote was obtained prior to start of the enrolment, in accordance with local law. Hence, written informed consent was obtained from all patients and/or their legal guardians or representatives prior to participation in the clinical investigation.
Based on the assumption that the standard deviation of the primary response variable (change in PUCAI) is 20, we aimed at including a sample of 50 patients to ensure that the precision of the estimated mean change in PUCAI is ± 5 at P > 0.95. Where appropriate, data are presented as the average (mean ± SD) values. For efficacy response variables, 95% confidence intervals were provided. If the confidence interval was above 0 (i.e., the lower limit of the confidence interval was greater than 0), then Improvement in PUCAI was considered as statistically significant. For efficacy analyses based on the ITT set, last observation carried forward (LOCF imputational method) was used in case of missing data. For all other analyses, no imputation was done. All statistical analyses were carried out using SAS® Version 9.2 under Windows® Server 2008. Statistical review of the study was performed by a biomedical statistician.
Datasets analyzed in this investigation were the Safety set, the intent-to-treat (ITT) and the per-protocol (PP) datasets. The Safety set included all enrolled patients, in whom at least one treatment was initiated. The primary efficacy endpoint was based on the ITT population, which was defined as all enrolled patients who received at least one treatment and for whom there was at least one valid post-baseline PUCAI measurement. The PP analysis set was defined as the subset of the ITT population who received the full course of assigned treatment and for whom there were valid efficacy values at week 12. All results are presented for the ITT population unless otherwise stated.
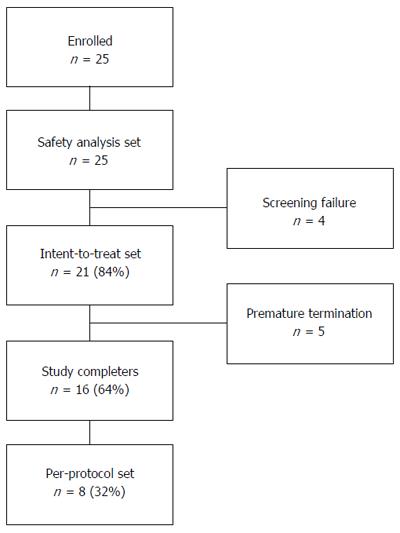
Twenty five children and adolescents with ulcerative colitis were enrolled (Figure 1, Table 4). All patients had at least one episode of active disease in the last 12 mo prior to enrollment in the clinical investigation.
| Characteristic | mean ± SD [range] | |
| Age (yr) | 13.5 ± 2.6 [8.1-17.8] | |
| Weight (kg) | 47.7 ± 11.3 [31.0-72.2] | |
| Height (cm) | 157.3 ± 12.3 [132.0-175.0] | |
| Duration of disease | 3.1 ± 3.2 [0.2-14] | |
| Smoking status | No patient ever smoked | |
| Male | n = 13 | 52.0% |
| Female | n = 12 | 48.0% |
| Caucasian | n = 22 | 88.0% |
| Oriental (near East) | n = 2 | 8.0% |
| Other | n = 1 | 4.0% |
| Pancolitis | n = 18 | 72% |
| Left sided | n = 7 | 28% |
| Entry PUCAI score | mean = 42.6 | median = 40 |
| Entry EAI score (median) | mean = 7.0 | median = 7.5 |
A total of 25 patients with moderate active UC (PUCAI score between 15 and 60) were screened and enrolled in the clinical investigation at 6 investigational centers. Twenty-five screened and enrolled patients entered the Safety Analysis set. There were four screening failures, one was due to detection of Clostridium Difficile, one was due to a diagnose change to Crohn’s disease, and two patients with too low PUCAI scores were excluded from the trial. Twenty-one (84%) patients entered the ITT analysis set. Out of these, five patients prematurely terminated the clinical investigation due to adverse events (n = 2), intake of not permitted medication or physician’s decision (n = 3). Sixteen patients (64%) completed the trial and 8 (32%) patients entered the Per-Protocol (PP) analysis set (Figure 1).
The most frequent used concomitant medication was Mesalazine, prescribed to 19 out of 25 patients (76%). Seven (7) of the 21 (33%) patients in ITT and 4 of the 8 (50%) patients in PP have used steroids during the clinical investigation. Fifteen out of 25 (60%) patients have been prescribed immunosuppressants, 13 received Azathioprine, one patient Mercaptopurine and one patient Methotrexate.
Nineteen out of 21 treated patients underwent at least 5 apheresis sessions, 17 patients received 6 treatments, 15 patients had 7 treatments, and 12 patients were treated with 8 Adacolumn aphereses.
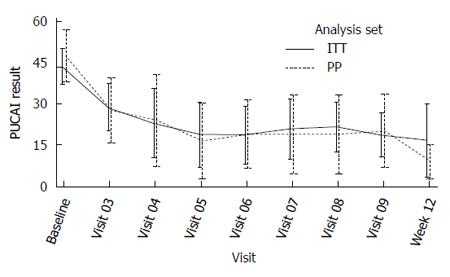
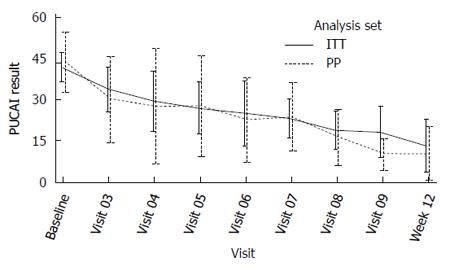
Primary efficacy endpoint: The mean PUCAI improvement at week 12 was 22.3 (CI: 12.9-31.6) in the ITT population and 36.3 (CI 31.4-41.1) in PP analysis set (Table 5). Eight out of 15 patients (53%) in ITT and 4 out of 8 patients (50%) in PP were not on steroids. For these efficacy subsets, the PUCAI scores over time are depicted separately below (Figures 2 and 3).
| Analysis set | n | mean | SD | 95%CI |
| ITT | 20 | 22.3 | 19.9 | 12.9-31.6 |
| PP | 8 | 36.3 | 5.8 | 31.4-41.1 |
Categorized PUCAI improvement: Defined as “significant improvement”, “moderate improvement”, “small improvement” and “no change” as per PUCAI categories at week 12 (Table 3), 70% of subjects had Significant improvement or moderate improvement; whereas a cumulated 30% of the patients experienced small improvement or no change comparing Visit 10 (week 12) vs baseline (Table 6).
| Category | n | % |
| Significant improvement | 9 | 45.0 |
| Moderate improvement | 5 | 25.0 |
| Small improvement | 1 | 5.0 |
| No change | 5 | 25.0 |
EAI: At entry, the median EAI score was 7.5. Ten patients in the ITT dataset and six patients in the PP dataset had both endoscopies, at entry and at week 12. Calculated as per the available data, the mean change in EAI score was -3.0 for the ITT and -2.8 for the PP analysis set. Upper confidence intervals for both analysis sets were below zero (-1.2 for ITT and -0.2 for PP), indicating that EAI meaningfully decreased at week 12 compared to the screening visit (Table 7).
| Analysis set | mean | SD | 95%CI | n |
| ITT | -3.0 | 2.5 | -4.8-(-1.2) | 10 |
| PP | -2.8 | 2.5 | -5.4-(-0.2) | 6 |
Disease activity: At Visit 03, Ulcerative Colitis disease activity of all patients (100%) was classified as “active”. At week 12, 70% of the ITT and 50% of the PP analysis group were classified as active, reflecting a decrease in disease activity of 30% and 50% respectively.
High sensitive C - reactive protein (hsCRP): The mean change in hsCRP at Visit 10 was 0.6 for the ITT and -2.5 for the PP analysis set. The changes in hsCRP - levels were not significantly different between a specific visit and baseline visit in any direction. Similarly, no significant change was observed in hsCRP between baseline and the end of the follow-up phase in both ITT and PP analysis sets (mean changes were 0.9 and 0.5 mg/L respectively).
Further secondary endpoints: Seven (7) of the 21 (33%) patients in ITT and 4 of the 8 (50%) patients in PP have used steroids during the clinical investigation. The mean steroid dosage for steroid users in the ITT set from Baseline to Termination visit significantly decreased by 10.0 mg (P < 0.04)
Clinical chemistry and hsCRP levels did not show any significant differences. Hematology, physical examination and vital signs did also not show any clinically significant changes throughout the clinical investigation.
Treatment safety and feasibility: During this clinical trial, up to week 12, no serious adverse event (SAE) occurred. Twenty one possibly or definitely related Adverse Events (AEs) were reported in 8 out of 25 (32%) patients, none of which was severe, 6 AEs were moderate, and 15 AEs were mild. Forty two unrelated AEs were furthermore recorded in 12 out of 25 (48%) patients. Unrelated mild transient headache, recorded for 6 patients, and procedural headache, recorded in 5 patients were the most prominent adverse events (Table 8).
| Relation | Severity | AEs; |
| patients | ||
| (safety set) | ||
| None | Mild | 35; 12 (48%) |
| Moderate | 7; 4 (16%) | |
| Severe | 0; 0 (0%) | |
| Total | 42; 13 (52%) | |
| Possibly or definitely related | Mild | 15; 6 (24%) |
| Moderate | 6; 5 (20%) | |
| Severe | 0; 0 (0%) | |
| Total | 21; 8 (32%) |
Despite the additional challenges of venous access in pediatric patients, no more than 3 patients per visit experienced blood access problems, perfusions were stopped in no more than 4 patients per visit, and the flow rate was adjusted in no more than 4 patients per visit.
Therapeutic options in inflammatory bowel disease (IBD) continue to evolve. The Joint ECCO and ESPGHAN Evidence-based Consensus Guidelines aimed to develop guidelines for managing UC in children based on a systematic review (SR) of the literature and a robust consensus process of an international working group of specialists, also considering series on Adacolumn[10,11,14,15]. The overall number of pediatric UC patients in the literature is nevertheless still low and results confirm a persistent unmet medical need[16-19].
Infliximab is currently the only anti-TNFα approved in EU for pediatric UC patients for reducing signs and symptoms and inducing and maintaining clinical remission in moderately to severely active disease with a prior inadequate response to “conventional” therapy. In an overall pediatric UC cohort of 31 patients, the ratio of primary non-response to IFX was reported as 29% (9 out of 31 patients), and further 29% discontinued IFX after a median duration of treatment of 12.7 mo[17]. Data also lack for maintenance schemes with immunosuppressants alone or in combination with anti-TNFα[19].
The ADAPT trial endpoints and outcome parameters are in line with the recommendations published in the practical statement paper of the pediatric ECCO committee[8]. A limitation of our study is the low number of patients enrolled (n = 25): When designing the trial, the Standard Deviation estimate of the Primary Endpoint (mean change in PUCAI) was 20 points; hence a sample size of 50 subjects would have ensured that the precision of the estimated mean change in PUCAI is ± 5 (P > 0.95). ADAPT inclusion and exclusion criteria defined eligible patients to be on the one hand not treatment-naïve, and had on the other hand not (yet) steroid-resistant or steroid-dependent. Practically, this allowed only cases with ongoing steroid medication but not yet at the edge of treatment escalation or surgery. While this profile is not uncommon in adult UC, it turned out to be difficult to enroll pediatric patients, as there were fewer such patients than originally assumed, and their therapy is faster escalated nowadays.
Comparing the Safety to published results in pediatric and adult UC patients, there are two meta-analyses[4,5] and one systematic review[6]. The results all favor GMA apheresis over control therapy at week 12. Other groups (Tanaka et al[15]) communicated results from a series of 17 steroid-naïve consecutive pediatric UC patients over 5 years from a single center in Japan, having received Adacolumn treatment as monotherapy or in combination with low dose prednisolone after failure of first-line medication (sulphasalazine or mesalazine dosed at 2-4 g per day), and with a short duration of disease (median 6.5 mo). The group had used the adult CAI score. With 12 out of 17 patients responding to Adacolumn monotherapy in the Tanaka cohort, this reminds to some extent the subgroup of patients not having received steroids from our trial, and points to the favorable use of Adacolumn early in the course of the disease. The safety signals were transient mild headache in 8 patients, nausea and lightheadedness in 6 patients (35.3%), vomiting in 4 patients (23.5%). This compares quite well to the present ADAPT safety results; both as per profile and per occurrence rate. Looking to retrospective adult UC data as described in a large post-marketing surveillance study on GMA apheresis in 656 adult UC patients an overall positive outcome (remission or clinical response) was achieved in 77.3% of patients. The proportion of adverse effects in the adult population was only 2.3% (all mild and not requiring premature interruption of the procedure)[20].
The most common adverse event with Adacolumn GMA apheresis is headache, which is possibly due to transitory blood volume shifts while on extracorporeal circulation amounting to ca. 210 mL blood. The higher rate of transitory AEs like headache in the pediatric samples could hence be due to relatively higher volume shifts and proportion of blood out in the extracorporeal system in pediatric patients, given their lower overall blood volume. On this background, it appears that the overall occurrence rate of adverse events is numerically less important in adult than in the pediatric patients of our cohort, but equal in nature and as mild and transient.
As for all induction treatments, transition to maintenance treatment and related compliance are a topic. Loss of response with or without antibody development seem to occur at least as often in pediatric patients as in adults, which is not the case with GMA maintenance schemes as published so far for adult UC patients: On-demand treatment with Adacolumn led to recurring remission, trend wise lasting longer than the prior remission phases[21].
Hematology and clinical chemistry tests did not show any treatment-related clinically significant changes throughout this clinical investigation, although the Adacolumn carriers deplete predominantly activated granulocytes and monocytes from the blood. The levels of these cells in the peripheral circulation are known to not be significantly lower after an apheresis session, which is due to a reactive influx of CD10 negative neutrophils from the bone marrow into the circulation (“pooling”) within the first 20 min into an apheresis session[22].
Within the confidence interval boundaries calculated for the ADAPT trial at 25 patients, the outcomes in efficacy and safety levels at week 12 allow the assumption that Adacolumn treatment in a pediatric UC population yields comparable profiles of efficacy and safety as documented to date in adult UC treatment looking back on a decade of clinical experience.
In conclusion, GMA apheresis with Adacolumn® was safe and effective in pediatric patients with moderate active Ulcerative Colitis. The present data contribute to considering GMA apheresis as a therapeutic option in pediatric patients having failed first line therapy.
Obituary: Sadly, Dr. Lena Grahnquist, Stockholm, passed away in January 2015 after long sickness. With the clinician’s sharp eye, the researcher’s intellect and her compassionate approach Lena Grahnquist combined the very best of what Pediatrics is about. We have lost a great person and colleague.
For a considerable group of children with ulcerative colitis (UC), treatment options are limited especially after failure of conventional treatment. The ADAPT trial was designed to generate prospective cohort data on efficacy and safety levels in moderate active pediatric UC patients when treated with Adacolumn granulocyte, monocyte/macrophage adsorptive (GMA) apheresis.
Few therapeutic concepts in inflammatory bowel disease have a registered pediatric indication, and conducting clinical trials in children is particularly challenging.
The investigators report that the outcomes in efficacy and safety levels at week 12 allow the assumption that Adacolumn treatment in a pediatric UC population yields comparable profiles of efficacy and safety as documented to date in adult UC treatment looking back on a decade of clinical experience.
The present data contribute to considering GMA apheresis as a therapeutic option in pediatric patients having failed first line therapy.
GMA apheresis is an extracorporeal, veno venous apheresis which selectively depletes neutrophils (granulocytes, monocytes) to adsorptive carriers in a single-use, sterile column. Adsorption to the carriers is governed by C3b/C3bi, FcgRs and the leukocyte complement receptors.
The authors aimed to investigate efficacy and safety of GMA prospectively in a population of pediatric patients with UC. In this study, significant improvement was detected in half of the patients who were treated. In adult patients with UC, surgery or anti TNF treatment might be considered. The present study suggests that Adacolumn treatment may be a useful option for pediatric patients in whom first line therapy has failed. Considering that GMA apheresis was well tolerated, this study provides useful new information.
P- Reviewer: Sarna S, Yuksel I S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Dignass AU, Eriksson A, Kilander A, Pukitis A, Rhodes JM, Vavricka S. Clinical trial: five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment Pharmacol Ther. 2010;31:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, Winqvist O, Lofberg R. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Ramlow W, Emmrich J, Ahrenholz P, Sparmann G, Kashiwagi N, Franz M, Yokoyama T, Yoshikawa T. In vitro and in vivo evaluation of Adacolumn cytapheresis in healthy subjects. J Clin Apher. 2005;20:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the Adacolumn device in ulcerative colitis. Dig Dis Sci. 2010;55:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Zhu M, Xu X, Nie F, Tong J, Xiao S, Ran Z. The efficacy and safety of selective leukocytapheresis in the treatment of ulcerative colitis: a meta-analysis. Int J Colorectal Dis. 2011;26:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32:1297-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Adlbrecht C, Breyer E, Gartlehner G. Selektive Zelladsorption bei entzündlichen Darmerkrankungen. Systematischer Review. In: Ludwig Boltzmann Institut Health Technology Assessment, Decision Support Document 2008; . |
| 8. | Ruemmele FM, Hyams JS, Otley A, Griffiths A, Kolho KL, Dias JA, Levine A, Escher JC, Taminiau J, Veres G. Outcome measures for clinical trials in paediatric IBD: an evidence-based, expert-driven practical statement paper of the paediatric ECCO committee. Gut. 2015;64:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Wewer V, Riis L, Vind I, Husby S, Munkholm P, Paerregaard A. Infliximab dependency in a national cohort of children with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2006;42:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Ruuska T, Lähdeaho ML, Sutas Y, Ashorn M, Grönlund J. Leucocyte apheresis in the treatment of paediatric ulcerative colitis. Scand J Gastroenterol. 2007;42:1390-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Martín de Carpi J, Vilar P, Prieto G, García Novo MD, Ribes C, Varea V. Safety and efficacy of granulocyte and monocyte adsorption apheresis in paediatric inflammatory bowel disease: a prospective pilot study. J Pediatr Gastroenterol Nutr. 2008;46:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Japan Immunoresearch Laboratories Co. Ltd. Adacolumn, a Granulocyte and Monocyte ⁄ Macrophage Adsorption Apheresis Device. Instructions for use. London, UK: Authorized Representative, Otsuka Pharmaceutical Europe Ltd 2010; . |
| 13. | Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 814] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 14. | Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, Bronsky J, Braegger CP, Cucchiara S. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 15. | Tanaka T, Sugiyama S, Goishi H, Kajihara T, Akagi M, Miura T. Treatment of children and adolescents with ulcerative colitis by adsorptive depletion of myeloid lineage leucocytes as monotherapy or in combination with low dose prednisolone after failure of first-line medications. BMC Gastroenterol. 2013;13:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yang LS, Alex G, Catto-Smith AG. The use of biologic agents in pediatric inflammatory bowel disease. Curr Opin Pediatr. 2012;24:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-year, single tertiary care center experience on the durability of infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | McGinnis JK, Murray KF. Infliximab for ulcerative colitis in children and adolescents. J Clin Gastroenterol. 2008;42:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Cucchiara S, Romeo E, Viola F, Cottone M, Fontana M, Lombardi G, Rutigliano V, de’Angelis GL, Federici T. Infliximab for pediatric ulcerative colitis: a retrospective Italian multicenter study. Dig Liver Dis. 2008;40 Suppl 2:S260-S264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Hibi T, Sameshima Y, Sekiguchi Y, Hisatome Y, Maruyama F, Moriwaki K, Shima C, Saniabadi AR, Matsumoto T. Treating ulcerative colitis by Adacolumn therapeutic leucocytapheresis: clinical efficacy and safety based on surveillance of 656 patients in 53 centres in Japan. Dig Liver Dis. 2009;41:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Lindberg A, Eberhardson M, Karlsson M, Karlén P. Long-term follow-up with Granulocyte and Monocyte Apheresis re-treatment in patients with chronically active inflammatory bowel disease. BMC Gastroenterol. 2010;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, Bjarnason I, Lofberg R. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |