Published online Jan 7, 2016. doi: 10.3748/wjg.v22.i1.338
Peer-review started: May 8, 2015
First decision: July 14, 2015
Revised: August 15, 2015
Accepted: December 1, 2015
Article in press: December 1, 2015
Published online: January 7, 2016
Processing time: 244 Days and 18.3 Hours
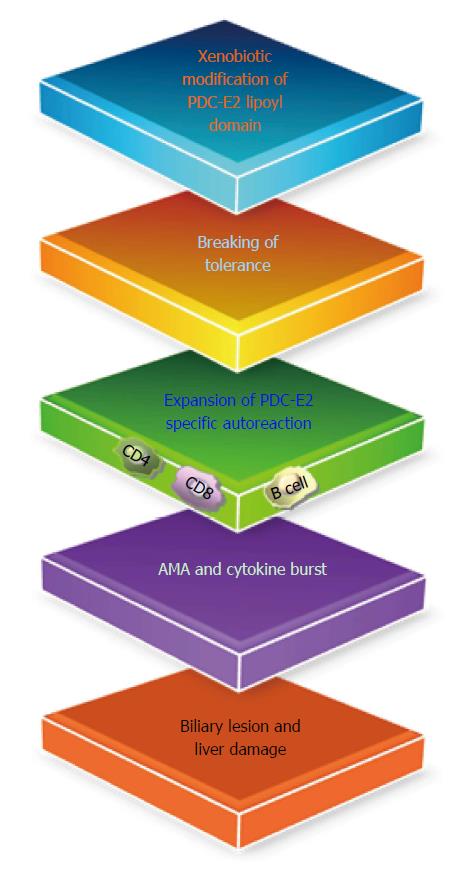
Data from genome wide association studies and geoepidemiological studies established that a combination of genetic predisposition and environmental stimulation is required for the loss of tolerance in primary biliary cholangitis (PBC). The serologic hallmark of PBC are the presence of high titer anti-mitochondrial autoantibodies (AMA) that recognize the lipoyl domain of the mitochondrial pyruvate dehydrogenase E2 (PDC-E2) subunit. Extensive efforts have been directed to investigate the molecular basis of AMA. Recently, experimental data has pointed to the thesis that the breaking of tolerance to PDC-E2 is a pivotal event in the initial etiology of PBC, including environmental xenobiotics including those commonly found in cosmetics and food additives, suggesting that chemical modification of the PDC-E2 epitope may render its vulnerable to become a neo-antigen and trigger an immune response in genetically susceptible hosts. Here, we will discuss the natural history, genetics and immunobiology of PBC and structural constraints of PDC-E2 in AMA recognition which makes it vulnerable to chemical modification.
Core tip: Environment influences immune functions. In this paper, we examine how environmental chemicals can trigger autoimmunity in an organ specific autoimmune disease, primary biliary cholangitis (PBC). PBC is liver specific autoimmune disease characterized by high titer of anti-mitochondrial autoantibodies directed against the E2 subunit of pyruvate dehydrogenase (PDC-E2) lipoyl domain. Here, we present experimental evidence from quantitative structure-activity relationship and animal models that xenobiotic modification of the PDC-E2 lipoyl domain could lead to loss of self-tolerance and is a pivotal event in the initial etiology of PBC in genetically susceptible hosts.
- Citation: Wang J, Yang G, Dubrovsky AM, Choi J, Leung PS. Xenobiotics and loss of tolerance in primary biliary cholangitis. World J Gastroenterol 2016; 22(1): 338-348
- URL: https://www.wjgnet.com/1007-9327/full/v22/i1/338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i1.338
The loss of tolerance is a central theme in autoimmunity and genetics and geoepidemiological studies have reflected that environmental factors contribute to this breach of tolerance[1-10]. This thesis is exemplified in primary biliary cholangitis (PBC), a prototype organ specific autoimmune disease[11]. The mechanism of how immunological tolerance is broken in PBC is still enigmatic[12]. Importantly, the autoantigen recognized by AMA was first cloned in 1987 and subsequently identified as the E2 subunit of pyruvate dehydrogenase (PDC-E2)[13,14]. The epitopes of AMA have been mapped to the highly conserved lipoic acid binding domain of the 2-oxo acid dehydrogenases including PDC-E2, branched chain 2-oxo-acid dehydrogenases (BCOADC-E2), oxoglutarate dehydrogenase (OGDC-E2) and the E3 binding protein (E3BP)[13-16]. Extensive efforts in defining the target mitochondrial autoantigens, T and B cell epitopes, the innate and adaptive immune responses, the immunobiology of the biliary epithelium, and the pathology of biliary duct epithelial cell destruction have greatly advanced our knowledge of the molecular mechanisms in tissue damage[13,17-29]. This focus of this review is to provide a comprehensive view of our current understanding on the natural history, genetics and immunobiology of PBC with emphasis on experimental data that illustrate the loss of tolerance to PDC-E2 is a pivotal event in the etiology of PBC[25,30-32].
Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis[33] is a female predominant liver-specific autoimmune disease with middle-age onset. It has an average incidence of 2.7 cases per 100000[34], but epidemiological studies suggest that the incidence of PBC is increasing[35]. There is variation in the prevalence of disease between geographic locations[35,36]; PBC is more prevalent in Northern Europe, North America and Latin America and less common in Eastern Asia, Africa, and Australia[37,38]. Clinically, PBC is characterized by the presence of high titer AMA and immune-mediated progressive destruction of biliary epithelial cells (BECs) within small bile ducts, eventually leading to cholestasis, fibrosis, and, potentially, liver cirrhosis[12]. Approximately 50%-60% of patients are asymptomatic at diagnosis. The disease has a long latency period[39,40], followed by the development of symptoms that may include fatigue, pruritus, cutaneous pigmentation and, later, bleeding varices, edema, or ascites[41]. The prognosis of patients diagnosed with PBC has improved significantly over the past two decades, perhaps because patients are being diagnosed earlier. PBC is a “model” autoimmune disease with significant literature on genetics, environment and animal models[17,25,33,42-51].
The female predominance among individuals with PBC suggests that there are significant genetic components in this disease, supported by the high frequency of X chromosome monosomy in patients with PBC[52,53] and Y chromosome loss in male patients with PBC[54]. Reports from recent genetic studies demonstrate that in addition to the MHC, several loci are associated with susceptibility to PBC, including interleukin (IL) 12-related pathways, SPIB, IRF5-TNPO3, and 17q12-2. The candidate genes identified by genome wide association studies include STAT4, DENND1B, CD80, IL7R, CXCR5, TNFRSF1A, CLEC16A, and NFKB1[55-58]. Data on familial clustering of PBC demonstrates that first-degree relatives of PBC patients have an increased risk of developing disease and most often these clusters involve mother-daughter pairs, consistent with the female preponderance of the disease[59-61]. Furthermore, twin studies have demonstrated a high concordance for PBC in monozygotic twins[62]. These studies provide evidence for a genetic basis underlying PBC. Genome analysis of DNA methylation, copy number variation and gene expression of monozygotic twins and sisters discordant for PBC have also indicated a contribution of epigenetic events[63]. However, environmental factors also play a role in the development of the disease[64], and multiple environmental components including chemicals[30,65-67] and bacteria[68-71] have been implicated.
AMA are present in over 95% of patients with PBC and are diagnostic of PBC[23]. The autoantigens of AMA have been identified as the E2 subunits of the 2-oxo-acid dehydrogenase complexes (2OADC-E2), including the E2 subunits of the pyruvate dehydrogenase complex (PDC-E2), branched chain 2-oxo acid dehydrogenase complex (BCOADC-E2) and 2-oxo-glutarate dehydrogenase complex (OGDC-E2) within the inner mitochondrial matrix[13,15,16,72]. The E2 enzymes have a common structure consisting of an N-terminal domain containing a single or multiple lipoyl groups. Previous studies have demonstrated that the dominant epitopes recognized by AMA are all within the lipoyl domains of these target antigens[73].
In patients with PBC, Both CD4+ and CD8+ T cells are present in portal tracts, around damaged bile ducts, strongly suggesting the participation of cellular immune mechanisms in biliary damage[12]. PDC-E2 autoreactive CD4 T cells are present in peripheral blood and liver; there is a specific 100-150 fold increase in the number of PDC-E2-specific CD4 T cells in the hilar lymph nodes and liver versus peripheral blood in patients with PBC[27]. The PDC-E2 autoepitope for both CD4 and HLA class I restricted CD8 T cells, overlaps with the B cell epitope, which spans the lipoyl domain[74]. Similar to CD4 autoreactive T cells, there is a 10-fold higher frequency of PDC-E2 specific CD8 T cells within the liver versus peripheral blood. Moreover, the precursor frequency of PDC-E2-specific autoreactive CD8 T cells is significantly higher in early rather than late stage of the disease[74]. Recent reports also substantiate the significance of innate immunity, including monocytes, toll like receptors and natural killer cells in the development of PBC[75,76]. The multi-lineage response to the PDC-E2, the immunodominant mitochondrial autoantigen in PBC, points to the thesis that loss of tolerance to PDC-E2 is the initiating event that leads to the subsequent development of clinical biliary pathology[12].
Epidemiological and mechanistic studies on autoimmunity have strongly demonstrated the etiologic contribution of environment[77], likely through molecular mimicry. Although microorganisms are possible candidates for the induction of autoimmune disease by molecular mimicry[78-84], there are other potential environmental factors, including chemical compounds foreign to a living organism. Examples include drugs, pesticides or other organic molecules that have the potential to modify host proteins and render them more immunogenic[77].
Halothane hepatitis is a xenobiotic-induced liver disease that occurs when susceptible individuals develop an immune response against trifluoroacetylated (TFA)-adduct protein. Exposure to TFA-conjugated self proteins results in antibody responses against such TFA-self proteins. Interestingly, anti-TFA also recognizes the lipoylated domain of PDC-E2[14,85]. The immunological cross-reactivity of anti-TFA antibodies with the immunodominant epitope in PBC prompted us to examine in depth molecular mimicry.
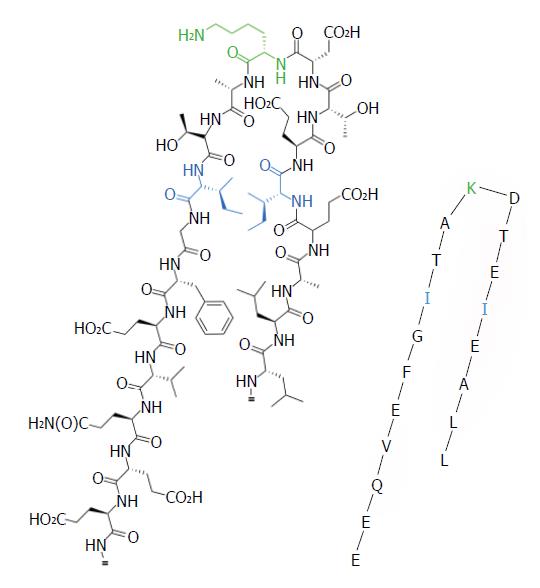
Site-directed mutagenesis of the PDC-E2 lipoyl domain demonstrated that AMA recognition is constrained by respective amino acid sequence in epitope (Figure 1, Table 1)[86,87]. The uniqueness of epitope specificity of AMA within the lipoyl domains of the 2OADC-E2 enzymes in patients with PBC[87-89] suggests that the lipoic acid domain is likely a lynchpin to the etiology of PBC. High resolution structural analysis and modeling studies of the PDC-E2 lipoyl domains from both prokaryotes and eukaryotes demonstrates that lipoic acid is covalently attached to the ε group of lysine (K) via an amide bond and is prominently displayed on the outer surface of PDC-E2. More importantly, the ability of lipoic acid to rotate by means of its “swinging arms” with respect to the bulk of the entire PDC-E2 molecule allows accessibility of its dithiolane ring for reduction acylation[90,91]. Although the change in conformation and the existence of multiple conformations of the lipoyl domain during reductive acetylation are important in catalyzing acyl transfer[90], it also renders PDC-E2 susceptible to aberrant chemical modifications.
| Mutant No. | Amino acid sequence | PBC sera2 | Purified PBC IgG3 | ||
| IgG | IgM | ||||
| PDC-E2 wild type | LLAEIETDKATIGFEVQEE | 1 | 1 | 1 | |
| Mutant 3 | LLAEAETDKATIGFEVQEE | 0.476 ± 0.029 | 0.504 ± 0.043 | 0.408 ± 0.052 | |
| Mutant 9 | LLAEIETDKATAGFEVQEE | 0.706 ± 0.029 | 0.781 ± 0.054 | 0.552 ± 0.065 | |
| Mutant 12 | LLAEIETDKATIGFAVQEE | 0.659 ± 0.034 | 0.768 ± 0.096 | 0.482 ± 0.074 | |
| Double amino acid substitution | Mutant 1 | ALAEIETDKATAGFEVQEE | 0.334 ± 0.029 | 0.253 ± 0.034 | 0.075 ± 0.023 |
| Mutant 2 | ALAEIETDKATIGFAVQEE | 0.461 ± 0.031 | 0.435 ± 0.045 | 0.663 ± 0.069 | |
| Mutant 3 | LLAEAETDKATAGFEVQEE | 0.066 ± 0.009 | 0.093 ± 0.016 | 0.024 ± 0.007 | |
| Mutant 4 | LLAEAETDKATIGFAVQEE | 0.111 ± 0.017 | 0.095 ± 0.016 | 0.043 ± 0.016 | |
| Triple amino acid substitution | Mutant 1 | ALAEAETDKATAGFEVQEE | 0.017 ± 0.004 | 0.044 ± 0.009 | 0.038 ± 0.017 |
| Mutant 2 | LLAEAETDKATAGFAVQEE | 0.019 ± 0.003 | 0.054 ± 0.012 | 0.050 ± 0.007 | |
| Quadruple amino acid substitution | ALAEAETDKATAGFAVQEE | 0.024 ± 0.005 | 0.066 ± 0.012 | 0.075 ± 0.031 | |
Accumulating evidence implicates that the loss of tolerance to PDC-E2 is pivotal in the initiation event of PBC and that AMA specificities reflect aspects of the induction phase of the disease[11,25,31,39]. Indeed the role of environment is well-known in many autoimmune diseases[30,92-98].
We hypothesized that xenobiotic modification of the native lipoyl moiety of the major mitochondrial autoantigen PDC-E2, may lead to loss of self-tolerance and eventually biliary lesions (Figure 2)[99]. This thesis is based on the findings of (1) readily detectable levels of immunoreactivity of PBC sera against comprehensive panels of protein microarrays, which mimic the inner lipoyl domain of PDC-E2; and (2) subsequent quantitative structure-activity relationships. Data from quantitative structure-activity relationship (QSAR) analysis demonstrated that AMA-positive PBC sera, but not controls, reacted to a number of xenobiotic-modified PDC-E2 structures[66,100] with a particularly striking level of reactivity against 6,8-bis(acetylthio) octanoic acid (SAc)-PDC-E2[101]. Recent data further suggest that chemical modification of PDC-E2 lipoic acid, via an electrophilic attack on the lipoic acid disulfide bond, triggers loss of tolerance to PDC-E2[30,101,102]. Such modifications could substantially affect the conformation of the PDC-E2 lipoyl domain and its immunogenicity in genetically susceptible hosts. Importantly, one of these chemical compounds is 2-octynoic acid (2-OA), a chemical commonly found in cosmetics and food additives[66].
Interestingly, immunization of C57BL/6 mice and NOD.1101 (NOD.B6 Idd10 Idd18r2) mice with 2-OA coupled to BSA, but not BSA alone, induced high titer AMAs, portal inflammation, and autoimmune cholangitis similar to human PBC[103,104]. These models provide a persuasive argument in favor of an environmental origin for human PBC[81,103,105,106]. We further investigated the role of IL-12-Th1/IL-23-Th17 pathways in the development of autoimmune cholangitis in this PBC model by using specific cytokine knockout mice (Table 2)[18]. In particular, we constructed several unique gene-deleted mice, including C57BL/6 mice deleted in both Th1 and Th17 (IL-12p40), Th1 cytokine (IL-12p35, IFN-γ) or Th17 cytokine (IL-23p19, IL-17A, IL-17F or IL-22). We immunized each of these cytokine-deficient mice with 2-OA-BSA and followed the natural history of their immunopathology. Our data indicate that while both IL-12/Th1 and IL-23/Th17 are involved in cholangitis, it is the IL-12/Th1 signaling pathway that elicits liver pathology in this xenobiotic induction disease model of PBC. In fact, deletion of IFN-γ prevents disease and suppresses autoantibodies. Importantly, deletion of the Th17 cytokines IL-17A and IL-22, but not IL-17F, reduces biliary damage; IL-17A-knockout mice have also reduced levels of AMAs. We further demonstrated that the production of IFN-γ is significantly decreased in livers of IL-23p19-/-, IL-17A-/- and IL-22-/- mice compared with controls. However, the ability of T cells to produce IFN-γ was not affected in Th17 cytokine-deficient mice. Thus, in the 2-OA-BSA immunized mice model: (1) Both IL-12/Th1 and IL-23/Th17 are involved in cholangitis; (2) IL-12/Th1 signaling pathway is critical in eliciting liver pathology; and (3) IL-23/Th17 pathway is involved in perpetuating the IL-12/IFN-γ mediated pathology. We also investigated the role of B cells in the pathogenesis of PBC by depleting B cells using two different monoclonal antibodies, CD20 and CD79. B cell depletion led to exacerbated cholangitis, with higher T cell infiltrates and inflammatory cytokines, indicating a protective role of B cells in PBC[107].
| Pathway | Cytokine k/o | Liver pathology |
| Th1 | IL-12p35-/- | Reduced liver infiltrates, reduced bile duct damage |
| Th1 | IFN-γ-/- | Marked reduction in liver infiltrates, bile duct normal |
| Th1/Th17 | IL-12/IL-23p40-/- | Abolish autoimmune cholangitis |
| Th17 | IL-23p19-/- | Reduced liver infiltrates, reduced bile duct damage |
| Th17 | IL-17A-/- | Reduced liver infiltrates, reduced bile duct damage |
| Th17 | IL-17F-/- | Similar to positive control |
| Th17 | IL-22-/- | Reduced liver infiltrates, reduced bile duct damage |
2OA-BSA immunized C57BL/6 mice were also studied for the potential of CTLA4-based therapy on cholangitis by using CTLA4-Ig. CTLA4-Ig is a soluble recombinant human fusion protein comprised of the extracellular domain of human CTLA4 linked to a modified portion of the Fc domain of human IgG[108,109]. In mice treated begun one day before 2-OA-BSA immunization, CTLA4-Ig completely inhibits the manifestations of cholangitis, including AMA production, intra-hepatic T cell infiltrates and bile duct damage. However, treatment with CTLA-4 Ig initiated after the development of autoimmune cholangitis in 2OA-BSA immunized mice, reduced intra-hepatic T cell infiltrates and biliary cell damage, although AMA levels were not altered[110].
We also investigated the role of innate immunity and natural killer T (NKT) cells on modulating disease activity in this xenobiotic-induced mouse model. Briefly, we immunized mice with and without the addition of α-galactosylceramide (α-GalCer), an invariant natural killer T cell activator. 2-OA-BSA-immunized mice exposed to α-GalCer developed a profound exacerbation of their autoimmune cholangitis, including significant increases in CD8+ T cell infiltrates, portal inflammation, granuloma formation, and bile duct damage. Moreover, these mice produced increased levels of AMAs and evidence of fibrosis[111]. CD4 and CD8 knock-out mice immunized with either 2-OA-BSA/PBS or 2-OA-BSA/α-GalCer develop AMAs and portal infiltrates. However, 2-OA-BSA/α-GalCer treated mice also develop fibrosis. Indeed, our data suggest that innate immunity is critical for immunopathology and that the pathology is exacerbated in the presence of α-GalCer[50]. More recently, we also reported that 2-OA-BSA-immunized mice administered with a Th2-biasing agonist (2s,3s,4r)-1-O-(a-D-galactopyranosyl)-N-tetracosanoyl-2-amino-1,3,4-nonanetriol (OCH), developed portal inflammation and hepatic fibrosis similar to mice treated with α-GalCer[75]. However, inflammatory portal cell infiltrates and AMA responses are reduced in iNKT cell deficient CD1d knockout mice treated with OCH. These results suggest that activation iNKT cells can occur via overlapping and/or promiscuous pathways and further highlight the role of innate immunity in the natural history of PBC.
Our data also provides clues to the mechanisms by which autoimmune diseases could be perpetuated in humans and also helps explain recurrence of PBC following liver transplantation in the absence of major histocompatibility complex (MHC) compatibility matching. Thus, in the absence of MHC restriction, disease reoccurrence would depend on a non MHC restricted cellular mechanisms, suggesting that biliary epithelial cells are simply an innocent victim of an immune attack. Thus, they attract immune attack by virtue of their unique biochemical mechanisms by which they process PDC-E2 during apoptosis[20]. Bile duct cells may have a direct effector role in immune-mediated cholangiopathies and fibrosis through their own cellular senescence pathway[112]. This also explains the suggested success of ursodiol in PBC, a drug that appears to have anti-apoptotic properties and also may modulate innate responses. Our data would also explain the relative failure of immunosuppressive drugs to alter PBC, because such agents are relatively ineffective against innate mechanisms. Finally, the induction of fibrosis in 2-OA-BSA-immunized mice exposed to α-GalCer permits not only dissection of its induction, but also has the potential to be useful in studies of intervention.
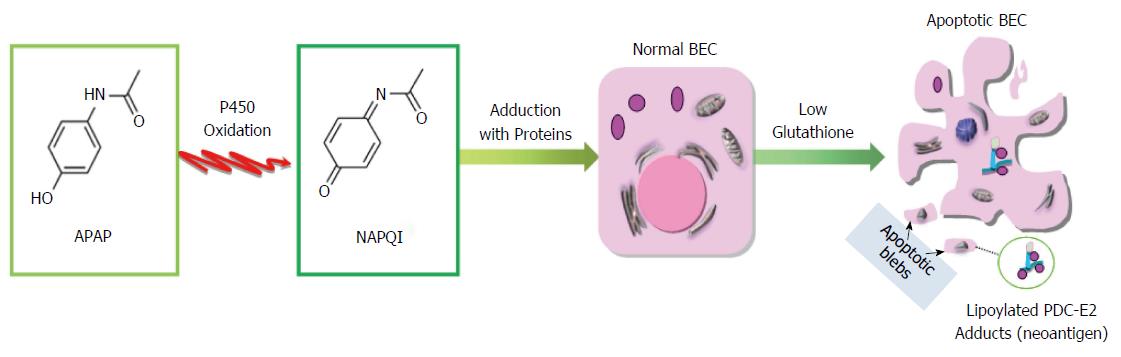
Although it is not clear how xenobiotics or the modified cellular proteins initiate autoimmunity in PBC, analysis of serum samples from subjects with acute liver failure indicate that a severe liver oxidant injury could lead to AMA production[113]. Specifically, 217 serum samples from 69 patients with acute liver failure (ALF) collected up to 24 mo post-ALF were compared with controls, for titer and reactivity with 2OADC-E2. AMA were detected in 28/69 (40.6%) ALF patients with reactivity found against all of the major mitochondrial autoantigens. The strikingly high frequency of AMAs in patients with ALF supports the thesis that oxidative stress-induced liver damage may lead to AMA induction. In particular, we note that AMA with the same antigen and epitope specificity as in patients with PBC was found in almost 35% of the acetaminophen (or APAP, chemically named N-acetyl-p-aminophenol) poisoning subjects, suggesting that the PDC-E2 lipoyl domain is likely a target of APAP induced reactive oxygen species. This finding is of significance as toxic doses of APAP produces reactive oxygen and nitrogen species and reactive metabolites[114-117] that could result in mitochondrial damage and liver injury as evidenced by the elevation of serum alanine amino transferase and P450 dependent centrilobular damage[118,119].
APAP is the most widely used non-prescription drug in the United States. Using the recommended therapeutic dosage (1000 mg per single dose and up to 4000 mg per day for adults), 85% of acetaminophen is metabolized in liver to non-toxic compounds via the conjugation of the aromatic ring to sulfate or glucoronic acid. The remaining 15% is converted into a highly-electrophilic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI) through isozymes of microsomal cytochrome P450[120]. In the presence of the reduced form of glutathione (GSH), NAPQI can either be covalently linked to GSH via Michael’s addition to the aromatic ring or reduced back to APAP[121]. The predominant method of NAPQI detoxification occurs through the former mechanism, resulting in depletion of the intracellular glutathione pool[122]. However, in the presence of excess APAP or when microsomal P450 is increased, hepatic GSH is depleted more extensively and cannot compete efficiently with the increased NAPQI. The resulting decrease in cellular glutathione further allows the accumulation of reactive NAPQI, which then reacts with nucleophilic sites such as cysteine and lysine residues on cellular proteins and related cofactors[123].
Previous data[124] have suggested that glutathiolation decreases the antigenicity of PDC-E2. Due to cellular depletion of glutathione, very little glutathione would be available for such covalent protection of PDC-E2. The depletion of glutathione could lead to neo-antigens through modification of native PDC-E2 by high levels of reactive NAPQI or other electrophilic agents. We reason that in PBC such electrophilic modification on lipoic-acid-conjugated PDC-E2 will inhibit the physiological function of PDC-E2 and subsequently lead to disruption of ATP synthesis, cell death and the release of either unmasked PDC-E2 or neoantigens formed by xenobiotics-modified PDC-E2. Microarray studies on APAP toxicity also revealed consistent altered transcriptome expression in oxidative phosphorylation, protein post-translational modification in liver and blood samples[125,126]. The exposure of this chemical modified self-protein to the immune system of genetically susceptible individuals could lead to the breakdown of self-tolerance to native PDC-E2 itself by molecular mimicry and epitope spreading mechanism. Thus, in genetically susceptible individuals, the prolonged exposure to electrophilic agents, such as acetaminophen may initiate and/or enhance the breakdown of self-tolerance to PDC-E2 and eventually lead to PBC (Figure 3).
The etiological mechanism of the immunological specificity of the 2-OADC-E2 enzymes lipoyl domain in PBC remains an enigma. Recent quantitative structure-activity relationship (QSAR) studies suggest that disruption of the lipoyl ring S-S linkage renders the lipoic acid “activated” and receptive for xenobiotic modification and subsequent AMA recognition[101]. Data from immunological characterization of antigen and Ig isotype specificities against one such lipoyl acid mimic SAc and rPDC-E2 strongly support a xenobiotic etiology in PBC. This observation is of significance in light of the high frequency of AMAs in patients with ALF. In particular, AMA was found in almost 35% of APAP poisoning subjects in a cohort of ALF patients[113]. Highly reactive electrophilic metabolites of APAP such as NAPQI can deplete the intracellular glutathione pool and render PDC-E2 vulnerable to further modification by electrophiles. Such mechanisms of in vivo generation of xenobiotic modified self proteins could lead to the breaking of tolerance to native proteins through molecular mimicry and antigen spreading in genetically susceptible individuals[102]. Finally, the recapitulation of AMA and PBC-like biliary lesions in 2OA-BSA immunized mice further support our working hypothesis on xenobiotic etiology of PBC[103]. Future work is directed at examining the biochemical and immunological mechanisms underlying the breach of tolerance in autoimmunity in PBC by environmental chemicals. Knowledge gained from this model may have significant preventive and therapeutic implications in the clinical management of PBC.
P- Reviewer: Lakatos PL S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Catrina AI, Deane KD, Scher JU. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford). 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | El-Fawal HA. Neuroantibody biomarkers: links and challenges in environmental neurodegeneration and autoimmunity. Autoimmune Dis. 2014;2014:340875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ellis JA, Kemp AS, Ponsonby AL. Gene-environment interaction in autoimmune disease. Expert Rev Mol Med. 2014;16:e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Somers EC, Richardson BC. Environmental exposures, epigenetic changes and the risk of lupus. Lupus. 2014;23:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124:4673-4677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Magid-Bernstein J, Mahajan K, Lincoln J, Ming X, Rohowsky-Kochan C. Case report: cytokine and CD4+ T-cell profiles of monozygotic twins with autism and divergent comorbidities and drug treatment. J Child Neurol. 2015;30:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Garetto S, Trovato AE, Lleo A, Sala F, Martini E, Betz AG, Norata GD, Invernizzi P, Kallikourdis M. Peak inflammation in atherosclerosis, primary biliary cirrhosis and autoimmune arthritis is counter-intuitively associated with regulatory T cell enrichment. Immunobiology. 2015;220:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Pollard KM. Environment, autoantibodies, and autoimmunity. Front Immunol. 2015;6:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Ma HD, Wang YH, Chang C, Gershwin ME, Lian ZX. The intestinal microbiota and microenvironment in liver. Autoimmun Rev. 2015;14:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Tang R, Chen H, Miao Q, Bian Z, Ma W, Feng X, Seldin MF, Invernizzi P, Gershwin ME, Liao W. The cumulative effects of known susceptibility variants to predict primary biliary cirrhosis risk. Genes Immun. 2015;16:193-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hirschfield GM, Gershwin ME. The immunobiology and pathophysiology of primary biliary cirrhosis. Annu Rev Pathol. 2013;8:303-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791-1799. [PubMed] |
| 14. | Christen U, Quinn J, Yeaman SJ, Kenna JG, Clarke JB, Gandolfi AJ, Gut J. Identification of the dihydrolipoamide acetyltransferase subunit of the human pyruvate dehydrogenase complex as an autoantigen in halothane hepatitis. Molecular mimicry of trifluoroacetyl-lysine by lipoic acid. Eur J Biochem. 1994;223:1035-1047. [PubMed] |
| 15. | Leung PS, Chuang DT, Wynn RM, Cha S, Danner DJ, Ansari A, Coppel RL, Gershwin ME. Autoantibodies to BCOADC-E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatology. 1995;22:505-513. [PubMed] |
| 16. | Moteki S, Leung PS, Dickson ER, Van Thiel DH, Galperin C, Buch T, Alarcon-Segovia D, Kershenobich D, Kawano K, Coppel RL. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2-oxoglutarate dehydrogenase complex. Hepatology. 1996;23:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Huang W, Kachapati K, Adams D, Wu Y, Leung PS, Yang GX, Zhang W, Ansari AA, Flavell RA, Gershwin ME. Murine autoimmune cholangitis requires two hits: cytotoxic KLRG1(+) CD8 effector cells and defective T regulatory cells. J Autoimmun. 2014;50:123-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Kawata K, Tsuda M, Yang GX, Zhang W, Tanaka H, Tsuneyama K, Leung P, He XS, Knechtle S, Ansari AA. Identification of potential cytokine pathways for therapeutic intervention in murine primary biliary cirrhosis. PLoS One. 2013;8:e74225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Kawata K, Yang GX, Ando Y, Tanaka H, Zhang W, Kobayashi Y, Tsuneyama K, Leung PS, Lian ZX, Ridgway WM. Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGFβRII mice. Hepatology. 2013;58:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Lleo A, Zhang W, McDonald WH, Seeley EH, Leung PS, Coppel RL, Ansari AA, Adams DH, Afford S, Invernizzi P. Shotgun proteomics: identification of unique protein profiles of apoptotic bodies from biliary epithelial cells. Hepatology. 2014;60:1314-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Norman GL, Yang CY, Ostendorff HP, Shums Z, Lim MJ, Wang J, Awad A, Hirschfield GM, Milkiewicz P, Bloch DB. Anti-kelch-like 12 and anti-hexokinase 1: novel autoantibodies in primary biliary cirrhosis. Liver Int. 2015;35:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Gershwin ME. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Yang CY, Ma X, Tsuneyama K, Huang S, Takahashi T, Chalasani NP, Bowlus CL, Yang GX, Leung PS, Ansari AA. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Yao Y, Yang W, Yang YQ, Ma HD, Lu FT, Li L, Tao YY, Tsuneyama K, Zhang W, Friedman S. Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Rα(-/-) mice. J Autoimmun. 2014;51:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Rong G, Zhong R, Lleo A, Leung PS, Bowlus CL, Yang GX, Yang CY, Coppel RL, Ansari AA, Cuebas DA. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Tanaka H, Yang GX, Iwakoshi N, Knechtle SJ, Kawata K, Tsuneyama K, Leung P, Coppel RL, Ansari AA, Joh T. Anti-CD40 ligand monoclonal antibody delays the progression of murine autoimmune cholangitis. Clin Exp Immunol. 2013;174:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Tanaka H, Zhang W, Yang GX, Ando Y, Tomiyama T, Tsuneyama K, Leung P, Coppel RL, Ansari AA, Lian ZX. Successful immunotherapy of autoimmune cholangitis by adoptive transfer of forkhead box protein 3(+) regulatory T cells. Clin Exp Immunol. 2014;178:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Leung PS, Wang J, Naiyanetr P, Kenny TP, Lam KS, Kurth MJ, Gershwin ME. Environment and primary biliary cirrhosis: electrophilic drugs and the induction of AMA. J Autoimmun. 2013;41:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Wang J, Yang GX, Tsuneyama K, Gershwin ME, Ridgway WM, Leung PS. Animal models of primary biliary cirrhosis. Semin Liver Dis. 2014;34:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Wang L, Wang FS, Chang C, Gershwin ME. Breach of tolerance: primary biliary cirrhosis. Semin Liver Dis. 2014;34:297-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Hepatology. 2015;62:1620-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Kim WR, Lindor KD, Locke GR, Therneau TM, Homburger HA, Batts KP, Yawn BP, Petz JL, Melton LJ, Dickson ER. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631-1636. [PubMed] |
| 35. | Myers RP, Shaheen AA, Fong A, Burak KW, Wan A, Swain MG, Hilsden RJ, Sutherland L, Quan H. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology. 2009;50:1884-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Rautiainen H, Salomaa V, Niemelå S, Karvonen AL, Nurmi H, Isoniemi H, Färkkilä M. Prevalence and incidence of primary biliary cirrhosis are increasing in Finland. Scand J Gastroenterol. 2007;42:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Farrell GC. Primary biliary cirrhosis in Asians: less common than in Europeans, but just as depressing. J Gastroenterol Hepatol. 2008;23:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Invernizzi P. Geoepidemiology of autoimmune liver diseases. J Autoimmun. 2010;34:J300-J306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial analysis of antimitochondrial antibody in patients with primary biliary cirrhosis. Clin Dev Immunol. 2004;11:129-133. [PubMed] |
| 40. | Mayo MJ. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2008;12:277-288; viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865-870. [PubMed] |
| 42. | Dong M, Li J, Tang R, Zhu P, Qiu F, Wang C, Qiu J, Wang L, Dai Y, Xu P. Multiple genetic variants associated with primary biliary cirrhosis in a Han Chinese population. Clin Rev Allergy Immunol. 2015;48:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Katsumi T, Tomita K, Leung PS, Yang GX, Gershwin ME, Ueno Y. Animal models of primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Rong G, Wang H, Bowlus CL, Wang C, Lu Y, Zeng Z, Qu J, Lou M, Chen Y, An L. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. J Autoimmun. 2015;57:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 46. | Mousa HS, Lleo A, Invernizzi P, Bowlus CL, Gershwin ME. Advances in pharmacotherapy for primary biliary cirrhosis. Expert Opin Pharmacother. 2015;16:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Floreani A, Franceschet I, Cazzagon N, Spinazzè A, Buja A, Furlan P, Baldo V, Gershwin ME. Extrahepatic autoimmune conditions associated with primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 48. | Liaskou E, Hirschfield GM, Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol. 2014;36:553-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Kurth MJ, Yokoi T, Gershwin ME. Halothane-induced hepatitis: paradigm or paradox for drug-induced liver injury. Hepatology. 2014;60:1473-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Chang CH, Chen YC, Yu YH, Tao MH, Leung PS, Ansari AA, Gershwin ME, Chuang YH. Innate immunity drives xenobiotic-induced murine autoimmune cholangitis. Clin Exp Immunol. 2014;177:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Hudspeth K, Pontarini E, Tentorio P, Cimino M, Donadon M, Torzilli G, Lugli E, Della Bella S, Gershwin ME, Mavilio D. The role of natural killer cells in autoimmune liver disease: a comprehensive review. J Autoimmun. 2013;46:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, Selmi C, Watnik M, Gershwin ME, Podda M. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187-J192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 54. | Lleo A, Oertelt-Prigione S, Bianchi I, Caliari L, Finelli P, Miozzo M, Lazzari R, Floreani A, Donato F, Colombo M. Y chromosome loss in male patients with primary biliary cirrhosis. J Autoimmun. 2013;41:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, Podda M, Xu C, Xie G, Macciardi F. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 56. | Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 57. | Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, Lu Y, Chen W, Juran BD, Coltescu C. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 58. | Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, Gu X, Walker EJ, Jing K, Juran BD. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544-2555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 60. | Lazaridis KN, Juran BD, Boe GM, Slusser JP, de Andrade M, Homburger HA, Ghosh K, Dickson ER, Lindor KD, Petersen GM. Increased prevalence of antimitochondrial antibodies in first-degree relatives of patients with primary biliary cirrhosis. Hepatology. 2007;46:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Yanagisawa M, Takagi H, Takahashi H, Uehara M, Otsuka T, Yuasa K, Hosonuma K, Mori M. Familial clustering and genetic background of primary biliary cirrhosis in Japan. Dig Dis Sci. 2010;55:2651-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485-492. [PubMed] |
| 63. | Selmi C, Cavaciocchi F, Lleo A, Cheroni C, De Francesco R, Lombardi SA, De Santis M, Meda F, Raimondo MG, Crotti C. Genome-wide analysis of DNA methylation, copy number variation, and gene expression in monozygotic twins discordant for primary biliary cirrhosis. Front Immunol. 2014;5:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Smyk D, Cholongitas E, Kriese S, Rigopoulou EI, Bogdanos DP. Primary biliary cirrhosis: family stories. Autoimmune Dis. 2011;2011:189585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, Odin JA, Bach N. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874-5883. [PubMed] |
| 67. | Smyk D, Mytilinaiou MG, Rigopoulou EI, Bogdanos DP. PBC triggers in water reservoirs, coal mining areas and waste disposal sites: from Newcastle to New York. Dis Markers. 2010;29:337-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 68. | Leung PS, Park O, Matsumura S, Ansari AA, Coppel RL, Gershwin ME. Is there a relation between Chlamydia infection and primary biliary cirrhosis? Clin Dev Immunol. 2003;10:227-233. [PubMed] |
| 69. | Liang Y, Yang Z, Zhong R. Smoking, family history and urinary tract infection are associated with primary biliary cirrhosis: A meta-analysis. Hepatol Res. 2011;41:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 71. | Wang JJ, Yang GX, Zhang WC, Lu L, Tsuneyama K, Kronenberg M, Véla JL, Lopez-Hoyos M, He XS, Ridgway WM. Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (NOD).B6 (Idd10/Idd18) mice. Clin Exp Immunol. 2014;175:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525-3531. [PubMed] |
| 73. | Moteki S, Leung PS, Coppel RL, Dickson ER, Kaplan MM, Munoz S, Gershwin ME. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Matsumura S, Kita H, He XS, Ansari AA, Lian ZX, Van De Water J, Yamamoto K, Tsuji T, Coppel RL, Kaplan M. Comprehensive mapping of HLA-A0201-restricted CD8 T-cell epitopes on PDC-E2 in primary biliary cirrhosis. Hepatology. 2002;36:1125-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Chang CH, Chen YC, Zhang W, Leung PS, Gershwin ME, Chuang YH. Innate immunity drives the initiation of a murine model of primary biliary cirrhosis. PLoS One. 2015;10:e0121320. [PubMed] |
| 76. | Schrumpf E, Tan C, Karlsen TH, Sponheim J, Björkström NK, Sundnes O, Alfsnes K, Kaser A, Jefferson DM, Ueno Y. The biliary epithelium presents antigens to and activates natural killer T cells. Hepatology. 2015;62:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 77. | Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, De Roos AJ. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 78. | Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol. 2014;33:314-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 79. | Ehser J, Holdener M, Christen S, Bayer M, Pfeilschifter JM, Hintermann E, Bogdanos D, Christen U. Molecular mimicry rather than identity breaks T-cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. J Autoimmun. 2013;42:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Gowthaman U, Eswarakumar VP. Molecular mimicry: good artists copy, great artists steal. Virulence. 2013;4:433-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Selmi C, Leung PS, Sherr DH, Diaz M, Nyland JF, Monestier M, Rose NR, Gershwin ME. Mechanisms of environmental influence on human autoimmunity: a National Institute of Environmental Health Sciences expert panel workshop. J Autoimmun. 2012;39:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 82. | Tchernev G, Wollina U. Bacterial antigens and molecular mimicry: the bridging common problematic link in the pathogenesis of sarcoidosis and sarcoid-like reactions: Isn’t it time to wake up? Wien Med Wochenschr. 2014;164:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Vojdani A. Molecular mimicry as a mechanism for food immune reactivities and autoimmunity. Altern Ther Health Med. 2015;21 Suppl 1:34-45. [PubMed] |
| 84. | Yusung S, Braun J. Molecular mimicry, inflammatory bowel disease, and the vaccine safety debate. BMC Med. 2014;12:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Gut J, Christen U, Huwyler J, Bürgin M, Kenna JG. Molecular mimicry of trifluoroacetylated human liver protein adducts by constitutive proteins and immunochemical evidence for its impairment in halothane hepatitis. Eur J Biochem. 1992;210:569-576. [PubMed] |
| 86. | Leung PS, Iwayama T, Coppel RL, Gershwin ME. Site-directed mutagenesis of lysine within the immunodominant autoepitope of PDC-E2. Hepatology. 1990;12:1321-1328. [PubMed] |
| 87. | Wang J, Budamagunta MS, Voss JC, Kurth MJ, Lam KS, Lu L, Kenny TP, Bowlus C, Kikuchi K, Coppel RL. Antimitochondrial antibody recognition and structural integrity of the inner lipoyl domain of the E2 subunit of pyruvate dehydrogenase complex. J Immunol. 2013;191:2126-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, Ishibashi H. Mimicry peptides of human PDC-E2 163-176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Jones DD, Stott KM, Howard MJ, Perham RN. Restricted motion of the lipoyl-lysine swinging arm in the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 2000;39:8448-8459. [PubMed] |
| 91. | Vijayakrishnan S, Kelly SM, Gilbert RJ, Callow P, Bhella D, Forsyth T, Lindsay JG, Byron O. Solution structure and characterisation of the human pyruvate dehydrogenase complex core assembly. J Mol Biol. 2010;399:71-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Adutler-Lieber S, Zaretsky I, Platzman I, Deeg J, Friedman N, Spatz JP, Geiger B. Engineering of synthetic cellular microenvironments: implications for immunity. J Autoimmun. 2014;54:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Berrih-Aknin S. Myasthenia Gravis: paradox versus paradigm in autoimmunity. J Autoimmun. 2014;52:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 94. | Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 95. | Kurkó J, Besenyei T, Laki J, Glant TT, Mikecz K, Szekanecz Z. Genetics of rheumatoid arthritis - a comprehensive review. Clin Rev Allergy Immunol. 2013;45:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 96. | Liu Y, Li H, Xiao T, Lu Q. Epigenetics in immune-mediated pulmonary diseases. Clin Rev Allergy Immunol. 2013;45:314-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon-Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J Autoimmun. 2013;47:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 98. | Zhang Y, Zhao M, Sawalha AH, Richardson B, Lu Q. Impaired DNA methylation and its mechanisms in CD4(+)T cells of systemic lupus erythematosus. J Autoimmun. 2013;41:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 99. | Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, Colvin ME, Lam KS, Coppel RL, Ansari A. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956-2963. [PubMed] |
| 100. | Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, Ansari AA, Coppel RL, Mackay IR. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7-16. [PubMed] |
| 101. | Naiyanetr P, Butler JD, Meng L, Pfeiff J, Kenny TP, Guggenheim KG, Reiger R, Lam K, Kurth MJ, Ansari AA. Electrophile-modified lipoic derivatives of PDC-E2 elicits anti-mitochondrial antibody reactivity. J Autoimmun. 2011;37:209-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Leung PS, Lam K, Kurth MJ, Coppel RL, Gershwin ME. Xenobiotics and autoimmunity: does acetaminophen cause primary biliary cirrhosis? Trends Mol Med. 2012;18:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, Yoshida K, Yang GX, Hibi T. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 104. | Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, Lian ZX, Hibi T, Ansari AA, Wicker LS. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38:J156-J169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 106. | Shoenfeld Y, Tincani A, Gershwin ME. Sex gender and autoimmunity. J Autoimmun. 2012;38:J71-J73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Dhirapong A, Lleo A, Yang GX, Tsuneyama K, Dunn R, Kehry M, Packard TA, Cambier JC, Liu FT, Lindor K. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53:527-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Rozelle AL, Genovese MC. Efficacy results from pivotal clinical trials with abatacept. Clin Exp Rheumatol. 2007;25:S30-S34. [PubMed] |
| 109. | Davis PM, Abraham R, Xu L, Nadler SG, Suchard SJ. Abatacept binds to the Fc receptor CD64 but does not mediate complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. J Rheumatol. 2007;34:2204-2210. [PubMed] |
| 110. | Dhirapong A, Yang GX, Nadler S, Zhang W, Tsuneyama K, Leung P, Knechtle S, Ansari AA, Coppel RL, Liu FT. Therapeutic effect of cytotoxic T lymphocyte antigen 4/immunoglobulin on a murine model of primary biliary cirrhosis. Hepatology. 2013;57:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 111. | Wu SJ, Yang YH, Tsuneyama K, Leung PS, Illarionov P, Gershwin ME, Chuang YH. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 112. | Nakanuma Y, Sasaki M, Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2015;62:934-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, Kikuchi K, Miyakawa H, Norman GL, Lee W, Gershwin ME. Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology. 2007;46:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Miettinen TP, Björklund M. NQO2 is a reactive oxygen species generating off-target for acetaminophen. Mol Pharm. 2014;11:4395-4404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Noh JR, Kim YH, Hwang JH, Choi DH, Kim KS, Oh WK, Lee CH. Sulforaphane protects against acetaminophen-induced hepatotoxicity. Food Chem Toxicol. 2015;80:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 116. | Shuhendler AJ, Pu K, Cui L, Uetrecht JP, Rao J. Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol. 2014;32:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 466] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 117. | Ferret PJ, Hammoud R, Tulliez M, Tran A, Trébéden H, Jaffray P, Malassagne B, Calmus Y, Weill B, Batteux F. Detoxification of reactive oxygen species by a nonpeptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001;33:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 118. | Hinson JA, Pohl LR, Monks TJ, Gillette JR. Acetaminophen-induced hepatotoxicity. Life Sci. 1981;29:107-116. [PubMed] |
| 119. | Hinson JA, Roberts DW, Benson RW, Dalhoff K, Loft S, Poulsen HE. Mechanism of paracetamol toxicity. Lancet. 1990;335:732. [PubMed] |
| 120. | Harvison PJ, Guengerich FP, Rashed MS, Nelson SD. Cytochrome P-450 isozyme selectivity in the oxidation of acetaminophen. Chem Res Toxicol. 1988;1:47-52. [PubMed] |
| 121. | Moldéus P. Paracetamol metabolism and toxicity in isolated hepatocytes from rat and mouse. Biochem Pharmacol. 1978;27:2859-2863. [PubMed] |
| 122. | David Josephy P. The molecular toxicology of acetaminophen. Drug Metab Rev. 2005;37:581-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525-548, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 124. | Mao TK, Davis PA, Odin JA, Coppel RL, Gershwin ME. Sidechain biology and the immunogenicity of PDC-E2, the major autoantigen of primary biliary cirrhosis. Hepatology. 2004;40:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 125. | Toska E, Zagorsky R, Figler B, Cheng F. Transcriptomic studies on liver toxicity of acetaminophen. Drug Dev Res. 2014;75:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Jetten MJ, Gaj S, Ruiz-Aracama A, de Kok TM, van Delft JH, Lommen A, van Someren EP, Jennen DG, Claessen SM, Peijnenburg AA. ’Omics analysis of low dose acetaminophen intake demonstrates novel response pathways in humans. Toxicol Appl Pharmacol. 2012;259:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |