Published online Feb 14, 2015. doi: 10.3748/wjg.v21.i6.1827
Peer-review started: October 6, 2014
First decision: November 14, 2014
Revised: November 28, 2014
Accepted: December 19, 2014
Article in press: December 22, 2014
Published online: February 14, 2015
Processing time: 128 Days and 23.7 Hours
AIM: To investigate the efficacy of the digitally reinforced hematoxylin-eosin polarization (DRHEP) technique for detection of amyloidosis in rectal biopsies.
METHODS: One hundred hematoxylin-eosin (HE) stained rectal biopsies with Congo-red (CR)-positive amyloid depositions and 50 control cases with CR-negative amyloid-mimicking areas were scanned blinded to the CR results for amyloid depositions under both bright and polarized light, and digitally photographed using the DRHEP technique, to accentuate the faint birefringence observed in HE slides under polarization. The results of DRHEP and HE evaluation were statistically correlated with CR polarization results with respect to presence and localization of amyloid deposits as well as amyloid types.
RESULTS: Amyloid deposits showed yellowish-green birefringence by DRHEP, which allowed identification of amyloidosis in 41 HE-unsuspected cases (P = 0.016), 31 of which only had vascular deposits. True positivity was higher, and false negativity and positivity were lower by DRHEP, compared to evaluation by HE (69%, 31%, and 0.8% vs 33%, 67%, and 33%, respectively; P < 0.0001). The sensitivity, specificity, accuracy, and positive and negative predictive values for DRHEP were 69%, 98%, 78.6%, 98.5%, and 61.25%, respectively. Reasons for DRHEP false negativity were presence of extensive background birefringence in 12 cases, absence of CR birefringent vessel in 3 cases, and missing of the tiny deposits in 9 cases, which could be improved by experience, especially in the latter case. No correlation was found between age, gender, sites of deposits, or amyloid types.
CONCLUSION: The DRHEP technique improves diagnostic accuracy when used as an adjunct or a prior step to CR staining, especially for cases with limited tissues for further analysis.
Core tip: Amyloid fibrils show a faint birefringence with polarization microscopy even when they are stained with hematoxylin-eosin (HE), and this effect can be reinforced when digital images are captured. We researched the efficacy of this technique in rectal biopsies and observed that it allowed identification of unsuspected cases with HE. True positivity was higher, and false negativity and positivity were lower compared to evaluation by HE. Therefore it can be used as an adjunct or a prior step to Congo-red staining, especially for cases with limited tissues for further analysis as it is a fast and safe method.
- Citation: Doganavsargil B, Buberal GE, Toz H, Sarsik B, Pehlivanoglu B, Sezak M, Sen S. Digitally reinforced hematoxylin-eosin polarization technique in diagnosis of rectal amyloidosis. World J Gastroenterol 2015; 21(6): 1827-1837
- URL: https://www.wjgnet.com/1007-9327/full/v21/i6/1827.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i6.1827
Amyloidosis is a heterogeneous group of disorders characterized by excessive deposition of misfolded amyloid fibrils in tissues and organs. The diagnostic work-up of amyloidosis requires a multidisciplinary approach and a careful clinical evaluation; however definitive diagnosis still relies on microscopic examination of the tissues. Amyloid fibrils are seen as amorphous, eosinophilic deposits on routine hematoxylin-eosin (HE)-stained preparations, but sole HE staining is insufficient for diagnosis, as these can easily be confused with hyaline changes or sclerosis. Congo red (CR) stain and apple-green birefringence of CR-stained deposits under polarized light has long been considered as the gold standard for definitive diagnosis since it was first described by Kyle[1] in 1927.
However, despite the higher sensitivity and specificity of CR staining, false negative results can be seen due to the amount of the amyloid present in the tissue, age of amyloid deposits, thickness of the sections, fixation of the tissues, and even the staining technique itself[2]. Various methods have been investigated to increase the sensitivity of CR staining and more accurately diagnose early deposits, such as combining CR with immunohistochemistry[3] or CR fluorescence[4]. These methods are technically challenging and are not used widely for routine diagnostic purposes.
Digitally reinforced HE polarization (DRHEP) is a recently introduced technique, which was proposed as an adjunct to the diagnosis of amyloidosis in HE-stained sections in renal biopsies[5]. The technique is a combination of routine light microscopy and digital photography, and was inspired by the observation of amyloid fibrils showing a weak birefringence by polarization microscopy even in HE-stained slides[6]. This faint polarization effect is, however, more pronounced and readily identifiable in digitally captured images of the area. Therefore, utilization of digital enhancement may allow detection of birefringence that is not recognizable through the microscope objective.
Although this technique is currently being used in kidney biopsies, its utility in other specimens is not well characterized. Therefore, we investigated DRHEP in rectal biopsies, as the rectum is not only one of the most frequently biopsied sites for diagnosis of amyloidosis, but also is a target organ, as the gastrointestinal (GI) tract is one of the most commonly affected organ systems in systemic amyloidosis[7].
A retrospective data search was conducted in the Pathology database between 2000 and 2010 using the words “rectum” and “amyloid”. All cases with a clinical or histologic suspicion of amyloidosis and a Congo red stain were collected. Clinical data, including patient age and amyloid subtype, were collected from patient records within the Nephrology Department.
As reliable subtyping could not be done in all cases due to either inadequacy of tissue or technical reasons, amyloid subtypes were classified into three main groups as amyloid A (AA), amyloid light-chain (AL), or undetermined for statistical analyses.
The study set was arranged by one of the researchers who blinded other researchers to the case identities and CR results. The study group was composed of 100 rectal biopsies with CR-positive amyloid deposits, and the control group consistent of 50 CR-negative cases bearing amorphous eosinophilic amyloid-mimicking areas.
Four-micron thickness sections were obtained from paraffin-embedded tissue blocks. HE staining was carried following standard protocols. CR staining was performed using a modified stringent, alkaline, alcoholic Putchtler’s method[4].
All HE stained sections were evaluated at low power (4×) and areas representative/suspicious for amyloid deposition were identified and photographed under both 10× and 20× magnifications. The same microscopic fields were then consecutively photographed on a dark background during polarization. CR-stained sections of the biopsies were also similarly photographed both under visible bright light and under polarization. Care was taken to capture the same or similar area that was photographed in HE images.
Digital photography was conducted according to the details of the technique described in the related reference using an Olympus BX51 polarizing microscope (Olympus Corp., Tokyo, Japan) equipped with a DP21, SAL camera[5]. In total, each case had four images: HE, DRHEP, CR, and CR with polarization (CRP).
Both HE and DRHEP images were evaluated blind to clinical data and CR staining of the biopsies, which was regarded as the gold standard. The evaluation was done independently by two researchers. In cases of disagreement, a third opinion was obtained and a consensus diagnosis was reached with two-thirds majority.
First, plain HE and DRHEP images were consecutively evaluated for the presence of suspicious amyloid depositions. The deposits showing green or yellowish-green birefringence were regarded as positive[5]. However, extensive yellowish-green birefringence was regarded as nonspecific refraction. Later, CR-stained images, which were photographed under both bright visible light and polarized light, were evaluated in a similar fashion, with investigators blinded to results of HE and DRHEP evaluations. The site of amyloid deposits, such as vascular (vessel walls), interstitial (lamina propria) or muscular (muscularis mucosae), were also recorded. Last, the results between DRHEP and CR staining were compared and discrepant cases were re-evaluated.
The frequency analyses and statistical evaluation were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, United States). The agreement between researchers was analyzed by κ analysis. The results of HE, DRHEP, and CR evaluation and their relationships with clinicopathologic features were compared by nonparametric tests (χ2). The sensitivity, specificity, accuracy, and positive and negative predictive values for HE and DRHEP were additionally calculated.
The CR-positive study group was composed of samples from 40 women and 60 men with a mean age of 53.28 ± 14.9 years (range: 16-83 years). The mean age of the control group was 51.16 ± 15.8 years (range: 14-78 years). Patient age was not significantly different between study and control groups (P = 0.146). The three most common underlying etiologies were ankylosing spondylitis, familial Mediterranean fever, and rheumatoid arthritis. The subtype of amyloidosis was AA in 54 cases and AL in 9 cases. Clinical details and amyloid types of the cases are given in Table 1.
| Clinical history | Cases identified (n) | ||||||
| Total | By DRHEP | By DRHEP only | By both DRHEP and HE | By HE | By HE only | By neither technique | |
| AA-type amyloidosis | |||||||
| Ankylosing spondylitis | 13 | 8 | 6 | 2 | 5 | 2 | 3 |
| Familial mediterranean fever | 13 | 8 | 3 | 5 | 5 | 0 | 5 |
| Rheumatoid arthritis | 10 | 5 | 4 | 1 | 2 | 1 | 4 |
| Inflammatory bowel disease | 6 | 5 | 3 | 2 | 2 | 0 | 1 |
| Behcet’s disease | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Tuberculosis | 2 | 2 | 2 | 0 | 0 | 0 | 0 |
| Chronic obstructive lung disease | 2 | 2 | 1 | 1 | 1 | 0 | 0 |
| Still syndrome | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Reiter syndrome | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Psoriasis | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| TRAPS | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Chronic infection | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Total | 54 | 34 | 23 | 11 | 15 | 3 | 17 |
| AL-type amyloidosis | |||||||
| Multiple myeloma | 8 | 7 | 7 | 0 | 0 | 0 | 1 |
| Plasma cell dyscrasia | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| Total | 9 | 8 | 7 | 1 | 1 | 0 | 1 |
| Amyloidosis-type undetermined | |||||||
| Hereditary amyloidosis1 | 10 | 7 | 3 | 5 | 4 | 1 | 2 |
| Chronic renal failure | 5 | 3 | 1 | 2 | 2 | 0 | 2 |
| Plasma cell dyscrasia | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
| Malignancy | 4 | 4 | 2 | 2 | 2 | 0 | 0 |
| Unknown causes | 17 | 12 | 5 | 6 | 8 | 1 | 4 |
| Total | 37 | 27 | 11 | 16 | 17 | 2 | 8 |
| Overall total | 100 | 69 | 41 | 28 | 33 | 5 | 26 |
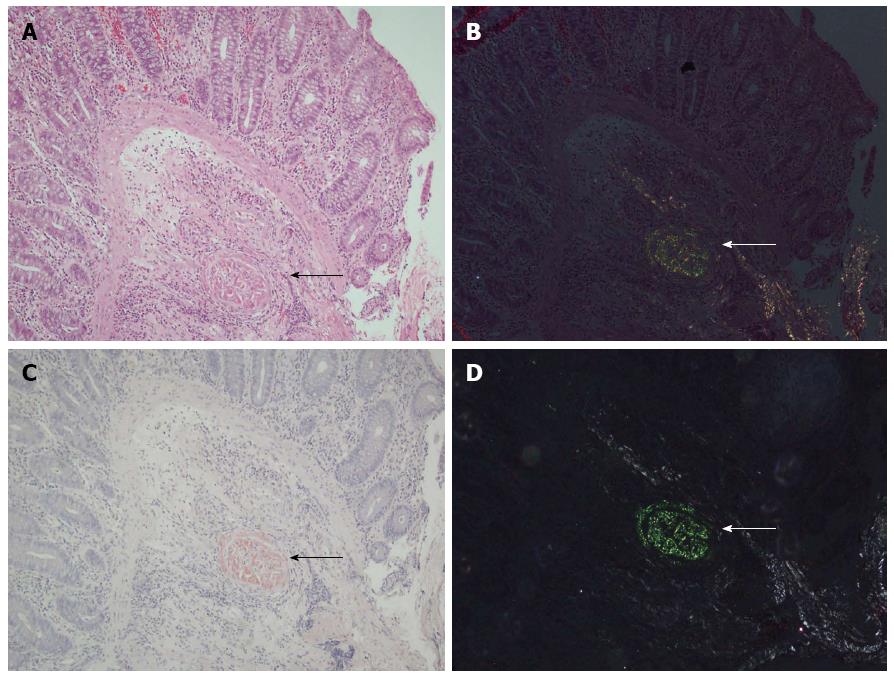
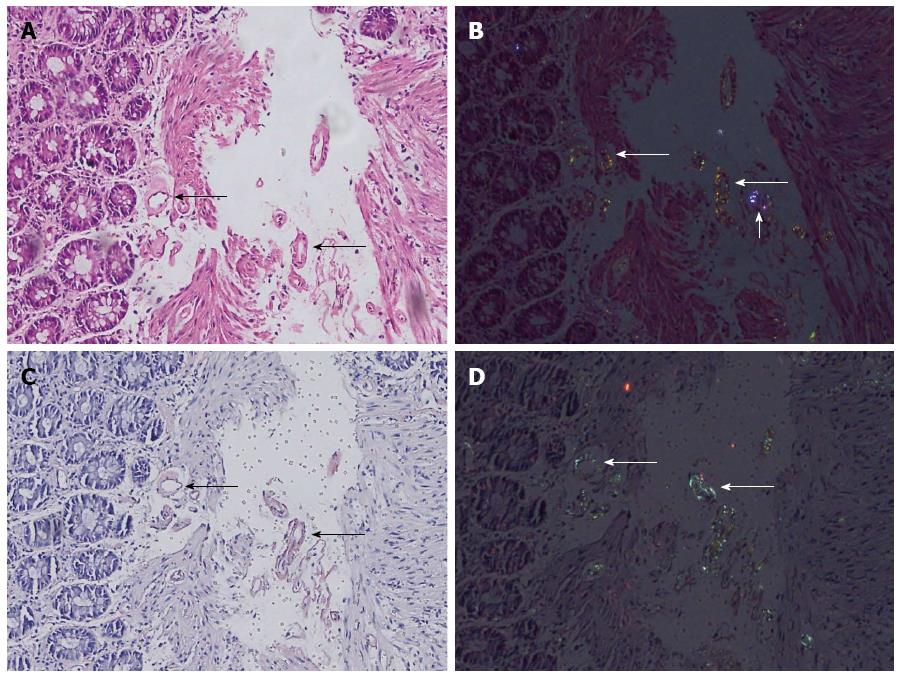
In 74.7% of the cases, the investigators agreed upon the amyloidosis status. Amyloidosis could readily be identified both in HE and DRHEP images of 28 CR-positive cases (28%) (Figure 1). Amyloid fibrils appeared as fluffy or condensed pink deposits on HE sections (Figure 1A) and showed yellowish-green birefringence on DRHEP images (Figure 1B). They stained salmon-pink/red by CR (Figure 1C) and showed apple-green birefringence by CRP (Figure 1D). However, DRHEP alone allowed identification of an additional 41 cases, which were not suspected by brightfield HE examination (P = 0.016), as even smaller deposits were easily identified by DRHEP because of their striking luminescence, which was comparable to CRP (Figure 2). The observed refraction pattern was specific to amyloidosis. Even though some of the foreign bodies also showed a variety of polarization patterns, they lacked the yellowish-green tint of the polarization observed in amyloid-deposited areas (Figure 2). Likewise, collagen bundles also showed a distinct white to silver polarization by DRHEP.
Sensitivity and specificity of DRHEP evaluation: The results of the HE and DRHEP image evaluation in study and control groups, as well as the sensitivity, specificity, accuracy, and positive and negative predictive values are given in Table 2.
| Variable | DRHEP | HE |
| Congo red positive (n = 100) | ||
| True positive | 69 (69) | 33 (33) |
| False negative | 31 (31) | 67 (67) |
| Congo red negative (n = 50) | ||
| True negative | 49 (98) | 16 (20) |
| False positive | 1 (4) | 34 (80) |
| Sensitivity | 69% | 34% |
| Specificity | 98% | 32% |
| Accuracy | 78.6% | 33.3% |
| Positive predictive value | 98.5 | 50.0 |
| Negative predictive value | 61.3 | 19.5 |
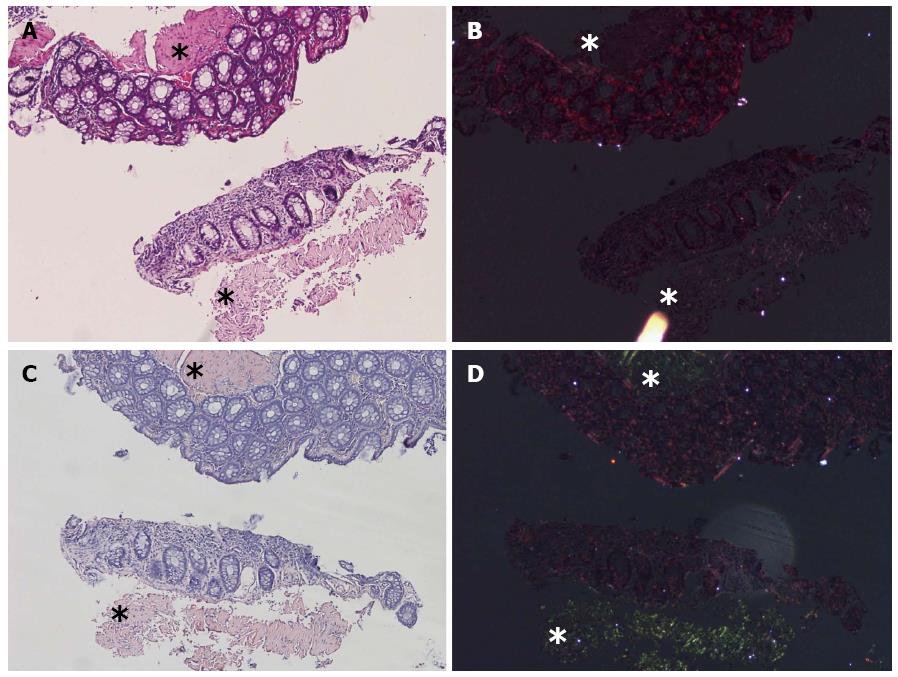
There were only five cases that were suspected as positive by HE evaluation but were negative by DRHEP technique (Figure 3). However, the overall ratio of false-negative cases was lower by DRHEP evaluation compared to HE image evaluation alone (31 cases vs 67 cases, P < 0.001).
In addition, the ratio of false-positive cases by DRHEP was also significantly lower, as there was only one (0.8%) false-positive case among the DRHEP-positive group (P < 0.001) in contrast to 34 false-positive cases by HE evaluation only. Whether this false-positive case had an undetermined birefringent foreign body or a small amyloid deposition, which could be lost on consecutive sections, remains unclear. Overall the sensitivity, specificity, accuracy, and positive and negative predictive values for DRHEP technique were calculated as 69%, 98%, 78.6%, 98.5% and 61.25% while they were 34%, 32%, 33.3%, 50%, and 19.5% for HE evaluation respectively (Table 2).
Effect of amyloid type and site of deposition: The sites of the amyloid depositions observed by DRHEP and the effect of the DRHEP technique in identification are summarized in Table 3. It was easier to identify or suspect mixed vascular, interstitial, and muscular deposits than only vascular ones both with HE and DRHEP. Although no statistically significant correlation was found between the underlying etiologies or amyloid subtypes, DRHEP identified more cases regardless of site of distribution (Table 3).
| Site of deposition | Confirmed by CRP | Identified by DRHEP | Identified by HE | ||||||||||
| Total | P value | HE-/DRHEP+ | P value | HE+/DRHEP+ | P value | Total | P value | HE+/DRHEP- | P value | HE-/DRHEP- | P value | ||
| Vascular deposits only | 74 | 48 | 0.24 | 31 | 0.982 | 17 | 0.147 | 21 | 0.381 | 4 | 0.823 | 22 | 0.407 |
| Mixed deposits | 26 | 21 | 0.24 | 10 | 0.982 | 11 | 0.147 | 12 | 0.381 | 1 | 0.823 | 4 | 0.407 |
| Vascular, muscular | 13 | 9 | 0.24 | 5 | 0.982 | 4 | 0.147 | 5 | 0.381 | 1 | 0.823 | 3 | 0.407 |
| Vascular, muscular, interstitial | 7 | 6 | 0.24 | 2 | 0.982 | 4 | 0.147 | 4 | 0.381 | 0 | 0.823 | 1 | 0.407 |
| Vascular, interstitial | 6 | 6 | 0.24 | 3 | 0.982 | 3 | 0.147 | 3 | 0.381 | 0 | 0.823 | 0 | 0.407 |
| Total number of cases | 100 | 69 | - | 41 | - | 28 | - | 33 | - | 5 | - | 26 | - |
Identification by DRHEP was not affected by the type of amyloid deposition (Table 1). However, it is noteworthy that some of the cases, such as myeloma and tuberculosis, were only evaluated as positive by DRHEP while they were not suspected by HE examination.
Although no significant correlation was found between age, gender, the sites of amyloid deposition, and amyloid type, the ratio of false positivity was higher in patients in the fifth decade (n = 15 cases; P = 0.049) if evaluated by HE alone in contrast to only one age-matched false-positive case (0.8%) in the DRHEP group.
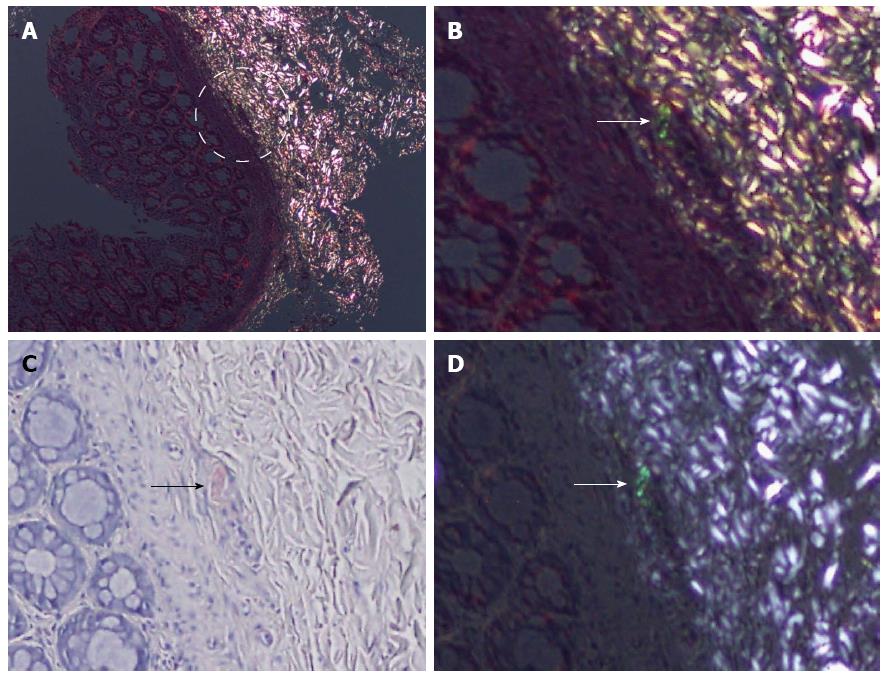
Thirty-one false-negative cases by DRHEP were re-evaluated in comparison with the CRP images. Nine (29%) cases showed positive birefringent deposits that were more easily identified on CRP images. In some of those cases, background refraction obscured the tiny deposits (Figure 4).
Apart from the nine missed cases, the most common cause of false negativity was the presence of extensive yellowish-green birefringence (38.7%), which was interpreted as nonspecific refraction. Loss of the CR positive birefringent vessel (foci) (9.7%) on the DRHEP image was the other common cause for false negativity.
Similarly, the positive foci on DRHEP images were more prominent in comparison to the two false-negative cases by CR staining. Table 4 summarizes the causes for the discrepancy and their relationship with amyloid subtypes and clinical etiologies.
| Cause of false negativity | Total number of cases, n (%) | Clinical history | n | Amyloid type |
| Extensive refraction | 12 (38.7) | Ankylosing spondylitis | 2 | AA |
| Familial Mediterranean fever | 1 | AA | ||
| Rheumatoid arthritis | 1 | AA | ||
| Behcet’s disease | 1 | AA | ||
| Psoriasis | 1 | AA | ||
| Multiple myeloma | 1 | AL | ||
| Chronic renal failure | 1 | Undetermined | ||
| Hereditary | 1 | Undetermined | ||
| Unknown causes | 3 | Undetermined | ||
| Tiny deposit ("Missed cases") | 9 (29.0) | Ankylosing spondylitis | 2 | AA |
| Familial Mediterranean fever | 1 | AA | ||
| Rheumatoid arthritis | 2 | AA | ||
| Inflammatory bowel disease | 1 | AA | ||
| Behcet’s disease | 1 | AA | ||
| Hereditary | 1 | Undetermined | ||
| Unknown causes | 1 | Undetermined | ||
| Absence of the positive vessel | 3 (9.7) | Familial Mediterranean fever | 1 | AA |
| Behcet’s disease | 1 | AA | ||
| Ankylosing spondylitis | 1 | AA | ||
| Perivascular illuminance | 2 (6.5) | Familial Mediterranean fever | 1 | AA |
| Rheumatoid arthritis | 1 | AA | ||
| Reason unidentified | 5 (16.1) | Familial Mediterranean fever | 1 | AA |
| (Real negative? cases) | Rheumatoid arthritis | 1 | AA | |
| Chronic renal failure | 1 | Undetermined | ||
| Unknown causes | 1 | Undetermined | ||
| Hereditary | 1 | ATTR | ||
| Total number of cases | 31 (100.0) | 31 |
In five of the false-negative cases, the deposits were inexplicably non-birefringent in DRHEP images, though they were all positive by CR (Figure 3).
Amyloidosis is still an etiopathogenetically mysterious disease, since its first description by Nicolaus Fontanus in 1639[1]. To date, 30 different human fibril proteins have been identified in localized or systemic forms of the disease[8]. Although amyloid fibril formation results from protein misfolding due to several factors such as mutations or proteolysis, it is not clear how these different proteins, with different amino acid sequences, three-dimensional structures, and biologic functions, convert to a fibrillary form, which has a considerably uniform morphology. Even though any protein deposits that stain with CR and exhibit green birefringence by polarization microscopy are considered as amyloid[9], we do not know how these different proteins with different physical orientations show similar birefringence under polarized light. Regardless of the mechanisms of this polarization, our results show that amyloid fibrils have a polarization property[6] and retain this polarization effect even after staining with HE, and can be detected easily if certain conditions are met. Within the scope of this study, this polarization effect was enhanced via a digital camera attached to the polarization microscope, which was set up according to the method described in the original literature[5].
Using the method of Sen et al[5], amyloid fibrils show a constant yellowish-green tint under polarization on HE-stained sections, and can be detected in rectal biopsies. Although the color was different than observed in polarization of CR-stained sections, it was easily identifiable in most of the cases. The physical basis of the constant yellowish-green color observed during HE polarization and the effect of digital enhancement on this polarization is beyond the scope of this article given the polarization mechanisms observed in CR-stained sections are not fully uncovered yet[10]. Of note, Howie et al[11] pointed to different colors diverging than the classically defined apple-green tint appearing during CR polarization. Therefore, the color we observed may vary in different camera settings. The physical basis of colors seen DRHEP requires further studies dedicated to this issue. Regardless of the mechanisms, this polarization enabled visualization of even the very tiny deposits and facilitated identification of amyloidosis with 69% sensitivity. The specificity of the technique was as high as 98%, and the observed yellowish-green color with polarization was almost unique to amyloidosis, which was comparable to CRP, as false positivity was not observed apart from one case with a tiny vascular and suspicious birefringence.
We must highlight that the aim of the study is neither asserting DRHEP as a substitute for CR nor for recommending rectal biopsies for scanning of amyloidosis in rectal biopsies. CR has long been regarded as the gold standard for diagnosis of amyloidosis with higher sensitivity and specificity ratios, and abdominal fat-pad aspiration biopsies have been favored for scanning of amyloidosis since the 1970s as a safe, simple, and fast method[12,13]. However, rectal biopsies are also still in use for diagnostic purposes in our routine practice[14]. Rectal biopsies are challenging because of the more collagenous and muscular tissue structure compared to kidney and subcutaneous fat tissue samples. The reported sensitivity of rectal biopsies in detection of amyloidosis varies within the literature ranging from 69% to 97%, and is largely affected by tissue sampling[15].
Identification of amyloidosis in rectum can also be valuable for prediction of renal involvement, as almost ninety percent of GI amyloidosis also have renal amyloid deposits[7,16]. Thus, GI biopsies can also be used with comparable convenience and sensitivity[17], and efforts to increase the diagnostic sensitivity of rectal biopsies is important. In this regard, DRHEP is valuable for distinguishing tiny amyloid deposits from sclerosis, hyalinization, or other red-pink colored structures, as they do not show the yellowish-green polarization observed in amyloidosis. Significant false positivity by HE evaluation in older patients (i.e., after the fifth decade) in our study group was meaningful in this respect, as it likely reflects hypertensive vascular changes that might have a similar morphologic appearance with amyloidosis and cause diagnostic difficulties. The higher specificity of DRHEP (96%) is therefore valuable.
The accurate diagnosis of amyloidosis in rectal biopsies is not only important for scanning purposes in clinically suspected cases, but also crucial in distinguishing it from its histologic mimics observed in the GI tract, such as collagenous colitis[18], ischemic bowel disease[19], and elastosis or elastofibromatous changes[20], which may cause important diagnostic challenges in inadequate biopsies. Moreover, many of the pathologies seen in the GI tract increase the risk of amyloidosis, such as inflammatory bowel disease[21,22], intestinal Behcet’s[23], or chronic infections as tuberculosis[24]. Therefore a concurrent case of amyloidosis can easily be overlooked, especially in clinically unsuspected cases. However, all these diagnostic and differential diagnostic considerations indeed rely on histologic suspicion of amyloidosis in HE-stained sections, particularly when the case is not suspected clinically. As we observed in our study design, blinded review of the cases caused false negativity in 67% of the cases by sole HE evaluation; the lower sensitivity and specificity ratios might result in either missing the case totally or application of excessive CR stains to all suspected tissue blocks. In this respect, we propose that DRHEP can be used as an adjunct to both HE and CR to increase their efficacy in detecting amyloidosis. It may be of help in selecting tissue blocks for further analysis and reduce time and extra costs.
Once the polarization microscope and attached digital camera are set up, this method is easy for scanning virtually all suspected cases during routine sign-out. In fact, in three of the study group cases, clinically unsuspected amyloidosis was initially diagnosed from rectal biopsies taken for other reasons, and DRHEP helped to pick up the cases as an initial step of evaluation. It was also valuable as an adjunct to CR stain in three more cases where the amyloid deposit was more extensive in HE-stained sections, and where the amyloid-bearing vessel was located at the edge of the biopsy and missed during serial cuts for CR staining. Multiple serial sections are needed to confirm the diagnosis by positivity in another vessel. Thus, we may benefit from this technique in routine practice, and with considerable safety, as the false-positivity ratio is significantly lower, comparable to CR staining. However, DRHEP should not be used as a replacement for routine CR staining, as there are still false-negative cases despite a significantly lower false-negativity ratio than with HE alone. The most common cause of false negativity was the presence of an extensive yellowish-green background birefringence (38.7%), which was suspicious for a nonspecific refraction. Although different from the silvery-white refraction of collagenous tissue, the visual complexity carried the risk of hiding true-positive tiny deposits, as we experienced in two of the cases. Loss of the CR-positive vessel in the HE section and nonspecific perivascular illumination were the other causes of false negativity. The importance of the perivascular birefringence is not known yet; whether it reflects a true deposit too tiny to be discriminated by light microscopy could not be ruled out within the scope of this study. However, this finding is worth mentioning as some of the sections also showed a sort of refraction in CRP images.
For the missed true-positive cases, we acknowledge that the recognition of the yellowish-green coloring upon polarization may require some experience and adjustment to the technique. After identifying the positive areas on CR, it was easier to comment on the observed refraction for discrepant cases. This troubleshooting or improved evaluation effect had a positive impact, particularly on the identification of tiny deposits. Thus, we believe that experience and increased practice may help to overcome some of these discrepancies.
The greater number of etiologically unknown cases, or cases in which the amyloid typing could not be further evaluated (other than AA or AL), is the major shortcoming of this study. Therefore further research is needed to uncover the effect of underlying etiology and amyloid types in relation to their visualization by DRHEP technique.
Today, many laboratories have adequate equipment (polarization attachment, polarization filter, and digital camera) for use with the DRHEP technique. Once the camera is attached and digital photography settings are made, it can be easily applied during routine biopsy evaluation with convincing safety. Laboratories can do their own optimizations. DRHEP may also be of help in reducing unnecessary CR staining in different tissue blocks of an individual case, which will save a considerable amount time, resources, and even tissue, in daily routine practice. It might even be valuable for the initial screening of cases of clinically unsuspected amyloidosis as a prior step or adjunct to CR evaluation[10,15].
The utility of a grading system as previously described in renal biopsies[25] and whether it can be applied to rectal biopsies should also be further evaluated. A standardized diagnostic approach and reporting, including the histologic structure of the deposits, should also be considered for all cases[26].
As mentioned above, the physical basis of this polarization, the effect of the CR-staining technique[27], or usage of a digital camera deserves further investigation. Dedicated research is also needed to reveal the relationship between the observed polarizations and the protein infrastructure of the deposits. The efficacy of the technique and its reproducibility in other tissues should also be investigated.
The data presented here will hopefully increase awareness about the DRHEP method and its efficacy as an adjunct to diagnosis in colorectal biopsies. To our knowledge, this is the first study dedicated to HE polarization in colorectal tissues.
Amyloidosis is a disparate set of disorders characterized by excessive deposition of amyloid fibrils in tissues and organs, rather than a single disease-entity with known causes and consequences. It may cause a wide range of clinical symptoms related with the involved target-organ dysfunction. Amyloid fibrils are composed of 30 different proteins, but have a considerably uniform histologic morphology. The gold standard for diagnosis is demonstration of amyloid fibril deposits in tissues, which appear as homogenous deposits that stain salmon pink with Congo red, and exhibit an apple-green birefringence when scanned with a polarization microscope. However, tissue diagnosis may be troublesome, especially in small biopsies when there is a paucity of amyloid deposits or when the tissue is too small for further staining.
In a previous study on renal amyloidosis, amyloid fibrils were shown to have a polarization property when scanned with a polarized microscope, even when they were stained with a standard hematoxylin-eosin stain that is used in conventional microscopic examination of tissues. However, this effect is more readily identifiable in digitally captured images as an unrevealed effect of the camera systems attached to the microscope. The utility of this technique, known as digitally reinforced hematoxylin-eosin polarization (DRHEP), has not been researched in other tissues. This is the first study to evaluate DRHEP for detecting amyloidosis in rectal biopsies.
The rectum is not only one of the most frequently biopsied sites for diagnosis of amyloidosis, but also is a target organ, as the gastrointestinal tract is one of the most commonly affected organ systems in systemic amyloidosis. Moreover, amyloidosis may complicate many primary pathologies of the gastrointestinal tract or can mimic them, requiring additional workups for either diagnosis of amyloidosis or for ruling it out. However, the rectal biopsies are often too small for further analysis. The results of the present study show that the striking yellowish-green birefringence of amyloid fibrils with DRHEP enables identification of even very tiny deposits, and that the true-positivity ratio of DRHEP is higher, while the false-negativity ratio is lower, than examination by hematoxylin-eosin without applying polarization. The extremely low false positivity is comparable to Congo-red staining results, rendering DRHEP a sensitive and specific method for diagnosis of amyloidosis, improving diagnostic accuracy. It can be used as an adjunct or a prior step to Congo-red staining, especially for cases with limited tissues for further analysis.
Today, many laboratories have adequate equipment (polarization attachment, polarization filter, and digital camera) for use with the DRHEP technique. Thus, it is a relatively easy method to scan virtually all suspected cases during routine sign-out.
Hematoxylin-eosin is the conventional stain used in pathology to evaluate tissues. Amyloid fibrils are seen as pinkish-red deposits under brightfield microscopy that cannot be differentiated from fibrosis, muscle tissue, or hyalinosis, with its sole appearance by this stain. Congo red, which is an aniline dye also used in the textile industry, is used for demonstrating amyloidosis in tissues. Congo-red staining is regarded as the gold standard for diagnosis of amyloidosis, and amyloid fibrils appear salmon pink with this stain. Polarized light microscopy is used to reveal polarized (light waves vibrating in only one plane) properties of tissues. Amyloid fibers show apple-green birefringence under polarized light. DRHEP is a novel technique to detect polarization of amyloid fibers under a polarization microscope without staining with Congo-red dye. The polarization effect of amyloid fibrils that are too faint to be seen with standard hematoxylin-eosin stain is more readily identifiable when digital images are captured, and amyloid fibers show yellowish-green birefringence in these images.
This manuscript is an interesting paper on a niche topic and is well documented.
P- Reviewer: Lakatos PL, Lorenzon L S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S
| 1. | Kyle RA. Amyloidosis: a convoluted story. Br J Haematol. 2001;114:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Puchtler H, Sweat F, Levine M. On the binding of congo red by amyloid. J Histochem Cytochem. 1962;10:355-364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 858] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 3. | Linke RP, Gärtner HV, Michels H. High-sensitivity diagnosis of AA amyloidosis using Congo red and immunohistochemistry detects missed amyloid deposits. J Histochem Cytochem. 1995;43:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Puchtler H, Sweat F. Congo red as a stain for fluorescence microscopy of amyloid. J Histochem Cytochem. 1965;13:693-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Sen S, Sarsik Kumbaraci B. Digitally reinforced polarization of hematoxylin-eosin in the diagnosis of renal amyloidosis. Turk Patoloji Derg. 2012;28:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Appel TR, Richter S, Linke RP, Makovitzky J. Histochemical and topo-optical investigations on tissue-isolated and in vitro amyloid fibrils. Amyloid. 2005;12:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103:776-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (2)] |
| 8. | Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P; Nomenclature Committee of the International Society of Amyloidosis. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Gertz MA. The classification and typing of amyloid deposits. Am J Clin Pathol. 2004;121:787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kröger M, Kovács BM, Appel TR, Gruys E, Makovitzky J. Polarization optical and histochemical analysis of amyloid samples from various animal species. The Proceedings of the XIIIth International Symposium on Amyloidosis, “From Misfolded Proteins to Well-Designed Treatment”; May 6-10, 2012. Groningen, the Netherlands: Groningen Unit for Amyloidosis Research and Development 2013; 32-35. |
| 11. | Howie AJ, Brewer DB, Howell D, Jones AP. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab Invest. 2008;88:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | van Gameren II, Hazenberg BP, Bijzet J, van Rijswijk MH. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Westermark P, Davey E, Lindbom K, Enqvist S. Subcutaneous fat tissue for diagnosis and studies of systemic amyloidosis. Acta Histochem. 2006;108:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ensari C, Ensari A, Tümer N, Ertug E. Clinicopathological and epidemiological analysis of amyloidosis in Turkish patients. Nephrol Dial Transplant. 2005;20:1721-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Bowen K, Shah N, Lewin M. AL-Amyloidosis Presenting with Negative Congo Red Staining in the Setting of High Clinical Suspicion: A Case Report. Case Rep Nephrol. 2012;2012:593460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yilmaz M, Unsal A, Sokmen M, Harmankaya O, Alkim C, Kabukcuoglu F, Ozagari A. Duodenal biopsy for diagnosis of renal involvement in amyloidosis. Clin Nephrol. 2012;77:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kuroda T, Tanabe N, Kobayashi D, Sato H, Wada Y, Murakami S, Nakano M, Narita I. Association between clinical parameters and amyloid-positive area in gastroduodenal biopsy in reactive amyloidosis associated with rheumatoid arthritis. Rheumatol Int. 2012;32:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | García-González R, Fernández FA, Garijo MF, Fernando Val-Bernal J. Amyloidosis of the rectum mimicking collagenous colitis. Pathol Res Pract. 1998;194:731-735. [PubMed] |
| 19. | Biggers JA, Remmers AR, Lindley JD, Folse DS, Sarles HE, Fish JC. Femoral neuropathy and ischemic colitis associated with amyloidosis in hemodialysis patients. Ann Surg. 1975;182:161-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Hobbs CM, Burch DM, Sobin LH. Elastosis and elastofibromatous change in the gastrointestinal tract: a clinicopathologic study of 13 cases and a review of the literature. Am J Clin Pathol. 2004;122:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Serra I, Oller B, Mañosa M, Naves JE, Zabana Y, Cabré E, Domènech E. Systemic amyloidosis in inflammatory bowel disease: retrospective study on its prevalence, clinical presentation, and outcome. J Crohns Colitis. 2010;4:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sattianayagam PT, Gillmore JD, Pinney JH, Gibbs SD, Wechalekar AD, Gilbertson JA, Rowczenio D, Hawkins PN, Lachmann HJ. Inflammatory bowel disease and systemic AA amyloidosis. Dig Dis Sci. 2013;58:1689-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Utku U, Dilek M, Akpolat I, Bedir A, Akpolat T. SAA1 alpha/alpha alleles in Behçet’s disease related amyloidosis. Clin Rheumatol. 2007;26:927-929. [PubMed] |
| 24. | Malhotra P, Agarwal R, Awasthi A, Jindal SK, Srinivasan R. How long does it take for tuberculosis to cause secondary amyloidosis? Eur J Intern Med. 2005;16:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Sen S, Sarsik B. A proposed histopathologic classification, scoring, and grading system for renal amyloidosis: standardization of renal amyloid biopsy report. Arch Pathol Lab Med. 2010;134:532-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Picken MM, Hazenberg BP, Westermark P, Eds . Hazenberg BPC, Bijzet J. Early detection of amyloid and its reporting: where are we and where are we heading? The Proceedings of the XIIIth International Symposium on Amyloidosis, “From Misfolded Proteins to Well-Designed Treatment”; May 6-10, 2012. Groningen, the Netherlands: Groningen Unit for Amyloidosis Research and Development 2013; 459-466. |
| 27. | Bély M, Makovitzky J. Sensitivity and specificity of Congo red staining according to Romhányi. Comparison with Puchtler’s or Bennhold’s methods. Acta Histochem. 2006;108:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |