Published online Feb 7, 2015. doi: 10.3748/wjg.v21.i5.1510
Peer-review started: July 13, 2014
First decision: August 15, 2014
Revised: October 10, 2014
Accepted: October 21, 2014
Article in press: October 21, 2014
Published online: February 7, 2015
Processing time: 211 Days and 6 Hours
AIM: To investigate the antibacterial effects of a crude extract of maggots against Escherichia coli (E. coli) and the underlying mechanisms, and to separate and purify the crude extract of maggots to assess the antibacterial effects of the active ingredients in the crude extract.
METHODS: Different concentrations of the crude extract of maggots were incubated with E. coli (O157:H7) and cultured. The optical density (OD) was measured at different time points to plot the OD-T curve. The effects of different concentrations of the crude extract on bacterial membrane permeability were determined by fluorescence probe technique. The effects of different concentrations of the crude extract on plasmid DNA replication were determined by agarose gel electrophoresis. DEAE-Sepharose ion exchange chromatography and Sephacryls-200HR gel filtration chromatography were used to separate and purify the crude extract of maggots. The molecular weight of proteins in the purified crude extract was determined by SDS-PAGD electrophoresis, and its antibacterial effects were determined by turbidimetric method.
RESULTS: The antibacterial effects of the crude extract of maggots at concentrations > 0.5 mg/mL were significant. The antibacterial effects of the crude extract at concentrations of 1.0, 1.5 and 2.0 mg/mL did not differ significantly. Fluorescence probe analysis showed that the rate of membrane permeability change was 1223.1% in bacteria incubated with 2 mg/mL of the crude extract, and 1300.0% in those incubated with 80 mg/mL of the crude extract. Plasmid DNA was undetectable in E. coli incubated with 2 and 80 mg/mL of the crude extract. A low molecular weight protein band (about 15 kDa) was detected in the crude extract of maggots and eluent, but not in eluant, from DEAE-Sepharose ion exchange chromatography. The antibacterial effects of the crude extract of maggots and eluent were superior to those of eluant, with the antibacterial effects of eluents being better than those of the crude extract of maggots. Of 24 tubes of filtrates, the antibacterial effects of filtrates in tubes 4, 5 and 11 were significantly higher than those of the control. The molecular weight of the protein in filtrates in tubes 4, 5 and 11 was about 15 kDa.
CONCLUSION: The crude extract of maggots exhibits obvious, dose-dependent antibacterial effects. The crude extract exerts antibacterial effects by changing the bacterial membrane permeability and inhibiting plasmid DNA replication. The protein that has antibacterial effects in the crude extract of maggots has a molecular weight of about 15 kDa.
Core tip: Previous studies have found the antibacterial activity of the maggots, but studies on the underlying mechanisms are still lacking. In this study, we separated and purified the crude extract of maggots using DEAE-Sepharose ion exchange chromatography and Sephacryls-200HR gel filtration chromatography. Agarose gel electrophoresis and fluorescence probe technique were used to investigate the antibacterial mechanism of the crude extract of maggots. We found that the protein of maggots weighing around 15 KD had the antibacterial effect and the crude extracts played the antibacterial role by destroying the cell membrane of Escherichia coli and disturbing the DNA replication of plasmids.
-
Citation: Ge QS, Zhang HM, Liu X, Wang SY, Lv DC, Li XD. Crude extract of maggots: Antibacterial effects against
Escherichia coli , underlying mechanisms, separation and purification. World J Gastroenterol 2015; 21(5): 1510-1517 - URL: https://www.wjgnet.com/1007-9327/full/v21/i5/1510.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i5.1510
Maggots are the larvae of Lucilia sericata, Chrysomya megacephala and their relatives, belonging to the Calliphoridae family[1,2]. According to the records in “Bencao Biandu” and “Bencao Qiuyuan”, maggots are nontoxic and cold-natured. After being dried and ground to a powder, they can be externally applied to treat ecthyma and lip pustules[3-5]. In 1929, the American doctor Baer[6] reported, for the first time, the successful treatment of more than 100 cases of chronic osteomyelitis with live maggots in vivo. In recent years, the abuse of antibiotics has led to the emergence of a large number of drug-resistant bacterial strains, and therefore, there is an urgent need to develop a new generation of antimicrobial drugs. Studies have found that active antimicrobial substances secreted by maggots can effectively control wound infection[7-10], and they have become the new hot spot of antimicrobial drug research.
Current research on antimicrobial substances of maggots is focused on small molecular weight antimicrobial peptides. Antimicrobial peptides not only have non-specific anti-bacterial, anti-fungal, anti-viral and anti-parasitic effects[11,12] but also can exert anti-tumor effects[13], thus having broad application prospects. Their main site of action is located on the membrane, and they can change the structure of the bacterial membrane and kill bacteria, with advantages of fast action and not easy to produce drug resistance[14]. However, their exact mechanisms of action remain to be studied. Wang et al[15,16] have purified a single antimicrobial peptide from maggot secretions by ion exchange chromatography and gel filtration chromatography. However, there is still a lack of experimental studies on antibacterial mechanisms of maggots.
This study investigated the antibacterial effects of a crude extract of maggots and preliminarily explored the antibacterial mechanisms of the crude extract using agarose gel electrophoresis and fluorescent probe technique. In addition, we separated and purified the crude extract of maggots by ion exchange chromatography and gel filtration chromatography and detected its antibacterial effects. The results obtained will provide a theoretical basis for the development of new biological antibiotics for treatment of infections.
Maggots used in the present study were the third instar larva of Lucilia sericata that were raised under sterile conditions. Maggots were cultured as previously described by Wang et al[17]. Escherichia coli (E. coli) strain (O157:H7) was provided by the Department of Microecology, Dalian Medical University. The bacteria were inoculated into 200 mL of broth, cultured with shaking at 37 °C for 12 h and centrifuged at 8000 rpm for 10 min. The pellet was re-suspended in broth and diluted to 108-1010 CFU/mL.
One hundred maggots were selected and 10 mL of TNE buffer (5 mmol/L EDTA, 10 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.5) precooled at 4 °C was added. After sufficient homogenization, the homogenate was centrifuged at 12000 r/min for 10 min at 4 °C. The supernatant was transferred to a new tube, and three volumes of TNE buffer were added. The mixture was boiled for 2 min, cooled and centrifuged at 12000 r/min at 4 °C for 10 min. The resulting supernatant was a crude extract of maggots. The crude extract was filtered through a membrane with a pore size of 0.22 μm, and the filtrate was frozen at -80 °C. The freeze-dried powder was stored in a refrigerator at -20 °C for further use.
The protein content of the crude extract was measured using the Coomassie brilliant blue assay system and adjusted to 2 mg/mL.
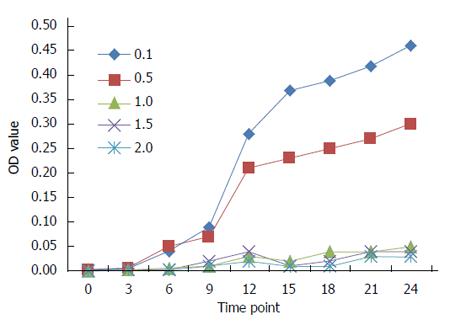
Two hundred microliters of the crude extract of maggots at a protein concentration of 0.1, 0.5, 1.0, 1.5 or 2.0 mg/mL were mixed with 22 μL of 10% sterile tryptone, respectively. The mixture (150 μL) was added into a sterile 96 well plate, followed by the addition of 30 μL of the bacterial suspension. The plate was shaken to mix the liquid evenly and then incubated at 37 °C. The optical density (OD) at 589 nm was measured with an automated microplate reader at 0, 3, 6, 9, 12, 15, 18, 21 and 24 h after zeroing the absorbance with a blank solution. The OD-T curve was plotted with time on X-axis and OD value on Y-axis.
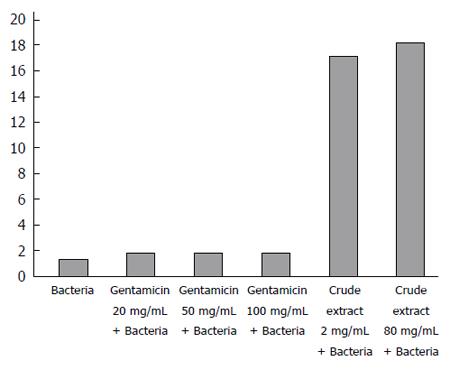
The co-cultured broth of the crude extract of maggots and E. coli was centrifuged, washed with sterile normal saline, re-suspended with 1 mmol/L of Fluo-3/AM, and adjusted to a final concentration of 3 μmol/L. After incubation and centrifugation (10000 g, 5 min), the supernatant was discarded and the pellet was washed and re-suspended in 3 mL of normal saline. The sample was scanned between 380 and 430 nm using 405 nm as the excitation wavelength, and an absorption peak was detected at 408 nm. Then, changes in intracellular calcium were monitored with the excitation wavelength set at 408 nm. The rate of membrane permeability change was calculated as [(sample fluorescence intensity - blank fluorescence intensity)/fluorescence intensity of blank] × 100%. Gentamicin was used as a control.
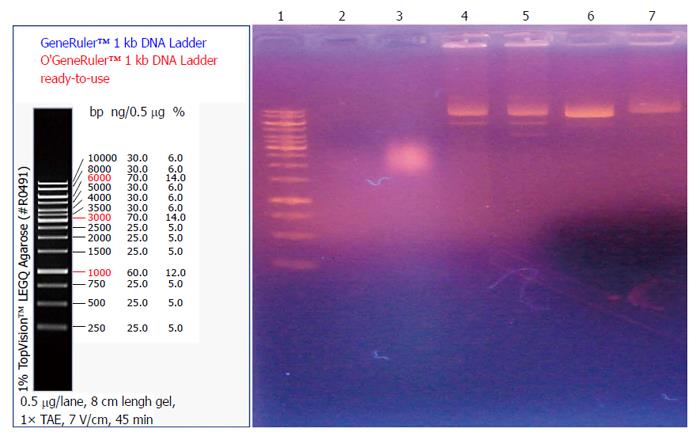
For gel preparation, 0.2 g of agarose was mixed with 20 mL of 1 × TAE and heated for 1 min. While the agarose was cooling, 1 μL of EB was added. The cooled agarose was then poured into a casting tray. Ten microliters of the samples (crude extract 2 mg/mL + plasmid, crude extract 80 mg/mL + plasmid, TNE + plasmid, plasmid alone, gentamicin 50 mg/mL + plasmid, and gentamicin 100 mg/mL + plasmid) were mixed with 2 μL of 6 × loading buffer and loaded into slots. Three microliters of 1 kDa DNA marker was also loaded. After loading, electrophoresis was conducted at a voltage of 80 V for about 20 min.
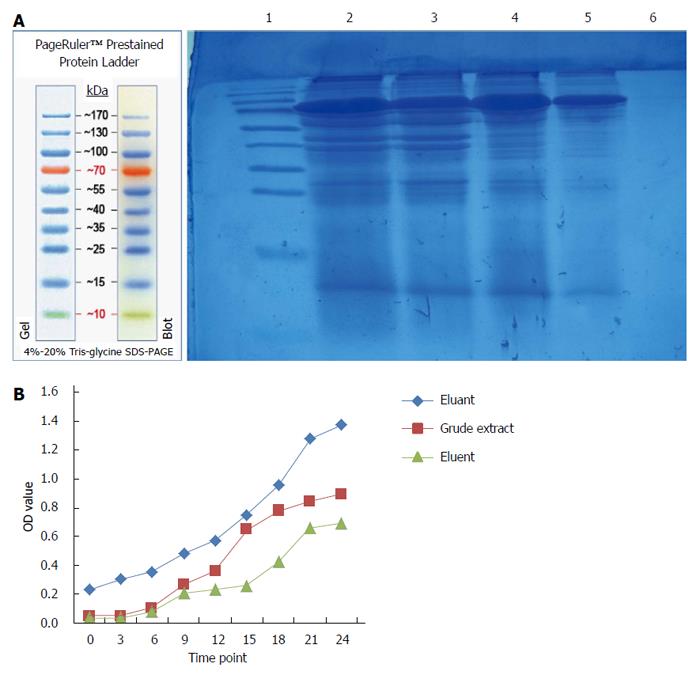
DEAE-Sepharose Fast Flow ion exchange chromatography and Sephacryls-200HR gel filtration chromatography were used to separate and purify the crude extract of maggots[18-20]. The eluent and eluant were obtained.
Two hundred microliters of the crude extract of maggots, eluent or eluant were mixed with 22 μL of 10% sterile tryptone, respectively. The mixture (150 μL) was added into a sterile 96 well plate, followed by the addition of 30 μL of diluted bacterial suspension. 1% sterile tryptone was used as a negative control. The plate was incubated at 37 °C, and the OD at 589 nm was measured with an automated microplate reader at 0, 3, 6, 9, 12, 15, 18, 21 and 24 h after zeroing the absorbance with a blank solution. The OD-T curve was plotted with time on X-axis and OD value on Y-axis.
As shown in Figure 1, the antibacterial effects of the crude extract of maggots at concentrations > 0.5 mg/mL were significant. The antibacterial effects of the crude extract of maggots at concentrations of 1.0, 1.5 and 2.0 mg/mL did not differ significantly. However, the antibacterial effects of the crude extract of maggots at a concentration of 0.5 mg/mL were significantly poorer than those at a concentration of 1.0 mg/mL. The antibacterial effect of the crude extract of maggots at a concentration of 0.1 mg/mL was the poorest.
As shown in Figure 2, the results of fluorescence probe analysis showed that the rate of membrane permeability change was 35.4% in the 20 mg/mL gentamicin group, 40.0% in the 50 mg/mL gentamicin group, 42.3% in the 100 mg/mL gentamicin group, 1223.1% in the 2 mg/mL crude extract group, and 1300.0% in the 80 mg/mL crude extract group. The rates of membrane permeability change were significantly higher in the crude extract groups than in the gentamicin groups.
As shown in Figure 3, no plasmid DNA band was detected in the two lanes containing 2 mg/mL and 80 mg/mL of the crude extract.
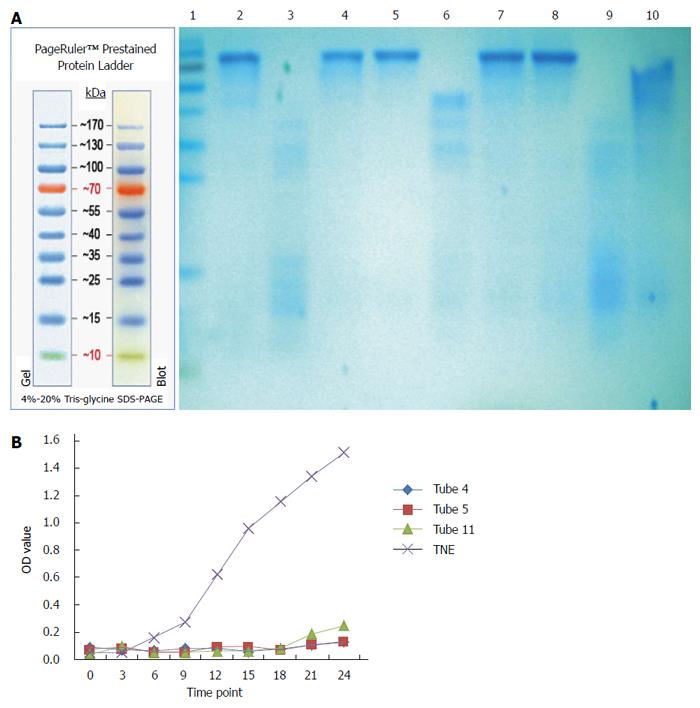
As shown in Figure 4, a lower molecular weight protein band (about 15 kDa) was detected in the crude extract of maggots and eluent, but not in eluant from DEAE-Sepharose ion exchange chromatography. The antibacterial effects of the crude extract of maggots and eluent were superior to those of eluant, with the antibacterial effects of eluents being better than those of the crude extract of maggots.
As shown in Figure 5, of 24 tubes of filtrates obtained, the antibacterial effects of filtrates in tubes 4, 5 and 11 were significantly higher than those of the control. The molecular weight of proteins in filtrates in tubes 4, 5 and 11 was about 15 kDa.
The discovery of antibiotics is a milestone in the battle of humans against infectious diseases. However, the abuse of antibiotics in recent years has led to the emergence of multidrug resistant strains, and even super-resistant strains, bringing an enormous obstacle to clinical management of infections[21]. This makes the development of new antibacterial drugs become an urgent task in the field of medical research. With the extraction of small molecule proteins with antimicrobial activity (antimicrobial peptides) from various insects by both domestic and foreign scientists, the direction of antibiotic research has gradually shifted from conventional antibacterial drugs to biological antibacterial drugs. The antibacterial effects of maggots have become a research hotspot. E. coli colonizes the intestine of humans and is a major member of the human intestinal flora, forming a symbiotic relationship with the body. However, when the body’s defense declines, for example in cases of hepatitis, cirrhosis, cancer and immune system disorders, patients are prone to opportunistic infections with E. coli, such as spontaneous bacterial peritonitis and E. coli bacteremia. In recent years, the overuse of antibiotics has led to the generation of multidrug resistant E. coli. For this reason, the present study investigated the antibacterial effects of a crude extract of maggots against E. coli and explored the underlying mechanisms by co-culturing the crude extract and E. coli.
This study firstly co-cultured different concentrations of the crude extract of maggots and E. coli to observe the antibacterial effects of the crude extract, and then detected the cell membrane permeability and DNA synthesis in E. coli. We experimentally found that as the concentration of the crude extract increased, the antibacterial effects became increasingly significant. When the concentration of the crude extract reached 1.0 mg/mL, the strongest antibacterial effects were achieved and no obvious bacterial growth was further observed. This finding suggests that the crude extract of maggots can exert antibacterial effects against E. coli in a concentration-dependent manner. The finding, however, raises the following questions: How do maggots inhibit the growth of E. coli? Which ingredients of maggots inhibit the growth E. coli?
In order to investigate the mechanism of antibacterial effects of maggots, we conducted the following two experiments. First, we examined the effects of different concentrations of the crude extract and gentamicin on the rate of membrane permeability change of E. coli by fluorescence probe technique. The experimental results showed that the rate of membrane permeability change was 35.4% in the 20 mg/mL gentamicin group, 40.0% in the 50 mg/mL gentamicin group, 42.3% in the 100 mg/mL gentamicin group, 1223.1% in the 2 mg/mL crude extract group, and 1300.0% in the 80 mg/mL crude extract group. This finding suggests that compared with gentamicin, the crude extract of maggots can significantly change the membrane permeability of E. coli. By changing the membrane permeability of E. coli, the crude extract of maggots can affect the exchange of substances inside and outside the bacteria, thereby inhibiting bacterial growth. Since gentamicin exerts antibacterial effects by inhibiting bacterial protein synthesis, its impact on bacterial growth was less significant. Second, we performed agarose gel electrophoresis to detect the effects of different concentrations of the crude extract of maggot and gentamicin on plasmid DNA replication of E. coli. Plasmids are a class of covalently closed double-stranded DNA molecules that are physically separate from chromosomal DNA and can replicate autonomously in bacterial or fungal cells. They often contain a variety of genes that confer bacterial resistance or are related with bacterial metabolism. Therefore, inhibition of plasmid replication can inhibit bacterial metabolism to achieve bacteriostatic and bactericidal effects. Our experimental results showed that no plasmid DNA bands were observed in the two lanes containing 2 mg/mL and 80 mg/mL of the crude extract of maggots. In other words, the levels of plasmid DNA were low in E. coli incubated with 2 mg/mL and 80 mg/mL of the crude extract, suggesting that the above two concentrations of the crude extract can inhibit plasmid DNA synthesis and thereby inhibit bacterial growth.
In addition to exploring the antibacterial mechanism of the crude extract of maggots, this study also preliminarily separated and purified the crude extract of maggots. First, we separated the crude extract by DEAE-Sepharose ion chromatography. DEAE-Sepharose Fast Flow is an anion exchanger, and the technical principle of DEAE-Sepharose ion-exchange chromatography is that the isolated protein (as the crude extract of maggots in this study) carries a net negative charge in Tris-HCl buffer (pH = 8.0) and may be bound by the anion exchanger in the column. When gradient elution was conducted, exchange of ions occurred between the proteins bound by the ion exchanger and the elution solution, and the proteins were finally eluted into the solution. Due to variations of the charges of different proteins, their affinities to the ion exchanger are not the same[19]. Therefore, they are eluted in different orders and thereby separated[20]. In this study, we selected 1 mg/mL of the crude extract, 0.5 mg/mL of the crude extract, eluent and eluant obtained from ion exchange chromatography to conduct SDS-PAGE and antibacterial experiments. The experimental results showed that a approximately 15 kDa protein band was detected in the crude extract of maggots and eluent, but not in eluant. Antibacterial experiments with 1 mg/mL of the crude extract, eluent and eluant showed that the antibacterial effects of the crude extract of maggots and eluent were superior to those of eluant, with the antibacterial effects of eluent being better than those of the crude extract of maggots. This finding suggests that both the crude extract and eluent contained the active antibacterial ingredient and that the content of the antibacterial ingredient in the eluate was higher. Second, we used Sephacryls-200HR gel filtration chromatography for the separation and purification of the crude extract. Gel filtration chromatography, also known as molecular sieve[22,23], separates proteins based on the molecular weight of proteins, does not require binding between proteins and columns, and can significantly reduce the loss and inactivation of proteins caused by irreversible binding. When different substances in the sample pass through the gel column with the eluent, molecules with a size larger than the pore size of the gel can not enter the pores of the gel and have to enter the gap between the gel particles, therefore having the same flow rate as the eluent. If the molecule is smaller than the gel pore size, it can enter the pores of the gel and therefore does not have the same flow rate as the eluent. Based on this principle, molecules of different sizes can be separated[19]. In this study, we collected the eluents at different time points, detected their molecular weights by SDS-PAGE electrophoresis, and assessed their antibacterial effects. The experimental results showed that of 24 tubes of eluents, a suspicious 15 kDa band was detected in tubes 4, 5 and 11, and that the filtrates in tubes 4, 5 and 11 had significant antibacterial effects, indicating that the molecular size of the active ingredient of the crude extract of maggots may be approximately 15 kDa.
In short, this study found that the crude extract of maggots can inhibit E. coli growth, exhibiting obvious, dose-dependent antibacterial effects. The crude extract exerts bacteriostatic effects by changing the bacterial membrane permeability and inhibiting plasmid DNA replication, but their specific targets and mechanisms of action need to be further studied. In addition, whether maggots exert antibacterial effects through other mechanisms also needs further study. The present study also separated and purified the crude extract of maggots by ion exchange chromatography and gel filtration chromatography and found that a protein with a molecular weight of about 15 kDa had significant antibacterial effects. The identity of this protein as well as its structure and composition remains to be studied. The research on maggots as a novel biological antimicrobial drug is still in the initial stage, and there are many problems awaiting us to solve. Our efforts in research of maggots may create a new research area for biological antimicrobial drugs.
Currently abuse of antibiotics results in resistance of bacteria, which brings along great difficulties in treatment of clinical infectious diseases. The maggots are proven to have antibacterial effects, but the antibacterial substances and mechanisms are still not clear.
In this study, DEAE-Sepharose ion exchange chromatography and Sephacryls-200HR gel filtration chromatography were used to isolate and purify the crude extract of the maggots.
The authors obtained small molecular proteins of the maggots with antibacterial effects and explored the antibacterial mechanisms of the maggots.
The purified antimicrobial peptides from the maggots can be used to treat infectious diseases in future.
Maggots are the larvae of Lucilia sericata, Chrysomya megacephala and their relatives, belonging to the Calliphoridae family. Maggots are nontoxic and cold-natured. After being dried and ground to a powder, they can be externally applied to treat ecthyma and lip pustules. Antimicrobial peptide is a general name of short peptides and constitutes of the main part of natural immunity. It has characteristics of broad-spectrum, high efficiency, selective toxicity, stability and not being easy to cause drug resistance.
This study obtained small molecular peptides from maggots using advanced molecular biological techniques and elucidated the antibacterial mechanisms of the peptides, which provides a theoretical basis for clinical treatment of infectious diseases.
P- Reviewer: Bello BL, Salman N S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Xu BH, Chen ZY, Ai YC. A preliminary study on rearing Lucilia sericata. Xinxiang Yixueyuan Xuebao. 1999;17:30-31. |
| 2. | Xie G, editor . Grand Dictionary of Chinese Medicine. Shenyang: Liaoning Science and Technology Publishing House 1994; 1366. |
| 3. | Jiangsu New Medical College. Chinese Medical Dictionary (volume 1). Shanghai: Science and Technology Publishing House 1977; 384. |
| 4. | Li YC, editor . Grand Dictionary of Chinese Medicine. Beijing: People’s Medical Publishing House 1982; 63. |
| 5. | Li SZ. Compendium of Materia Medica (revision). Beijing: People’s Medical Publishing House 1982; 2291-2296. |
| 6. | Baer WS. The classic: The treatment of chronic osteomyelitis with the maggot (larva of the blow fly). 1931. Clin Orthop Relat Res. 2011;469:920-944. [PubMed] |
| 7. | Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R. Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care. 2007;16:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Steenvoorde P, Jukema GN. The antimicrobial activity of maggots: in-vivo results. J Tissue Viability. 2004;14:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Tantawi TI, Gohar YM, Kotb MM, Beshara FM, El-Naggar MM. Clinical and microbiological efficacy of MDT in the treatment of diabetic foot ulcers. J Wound Care. 2007;16:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Nigam Y, Bexfield A, Thomas S, Ratcliffe NA. Maggot therapy: the science and implication for CAM part II-maggots combat infection. Evid Based Complement Alternat Med. 2006;3:303-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Wang G, Watson KM, Buckheit RW. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob Agents Chemother. 2008;52:3438-3440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Luque-Ortega JR, van’t Hof W, Veerman EC, Saugar JM, Rivas L. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 2008;22:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Wong JH, Ng TB. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides. 2005;26:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Hou L, Shi Y, Zhai P, Le G. Inhibition of foodborne pathogens by Hf1, a novel antibacterial peptide from the larvae of the housefly (Musca domestica) in medium and orange juice. Food Control. 2007;18:1350-1357. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Wang SY, Lv DC, Wang JN. A preliminary study on antibacterial effects of maggot secretion extracts on infected wound. J Tissue Engineer Reconstruct Surg. 2005;6:344-360. |
| 16. | Wang S. The healing and antibacterial function research of maggot secretion on the ulcer area. Seoul, South Korea: Special Speech. The Seventh International Conference on Biotherapy 2007; 109. |
| 17. | Wang SY, Lv DC, Wang YY, Cao PA. Technical study of medical maggot of Chinese medicine preparation. Dalian Yikedaxue Xuebao. 2008;30:90-92. |
| 18. | Harris ELV, Angal S. Protein Purification Methods a practical approach. New York: Oxford University Press 1999; 202-215. |
| 19. | Zou GL, Zhu RF. Enzymology (1st ed). Wuhan: Wuhan University Press 1997; 44-71. |
| 20. | Yan Z, Zhang YQ. Techniques of Protein Research. Xi’an: Fourth Military Medical University Press 2007; 45-55. |
| 21. | Verhelst X, De Vos M, Van Vlierberghe H. Optimal use of corticosteroids in gastroenterology and hepatology. J Translat Intern Med. 2014;2:53-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Harris ELV, Angal S, eds . Separation on the basis of size: Gel permeation chromagraphy protein purification methods: A Practical Approach. New York: Springer-Verlag 1989; . |
| 23. | Stellwagen E. Gel filtration. Methods Enzymol. 1990;182:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |