Published online Dec 14, 2015. doi: 10.3748/wjg.v21.i46.13042
Peer-review started: March 18, 2015
First decision: June 2, 2015
Revised: June 19, 2015
Accepted: September 28, 2015
Article in press: September 30, 2015
Published online: December 14, 2015
Processing time: 266 Days and 18.7 Hours
AIM: To investigate the anti-obesity and antibacterial effects of Ligustrum robustum (L. robustum) in vivo and in vitro and its possible mechanisms.
METHODS: The effects of L. robustum aqueous extract (LR) on various gut bacteria in vitro were evaluated. The effects of LR on high-fat diet-fed (HFD) rats in vivo were also assessed. Culture methods, quantitative polymerase chain reaction, and terminal-restriction fragment length polymorphism were used to analyze the effects of LR on gut bacteria. Biochemical tests were also performed to detect the changes in obesity-related indicators after LR treatment.
RESULTS: LR treatment lowered adipose weight and decreased Lee’s index, blood glucose, total cholesterol, and lipid in the tested groups relative to control (P < 0.05). To determine the reasons for these changes, we assessed the potential bacteriostatic and bactericidal effects of LR on specific bacterial species in vitro. LR affected the richness, diversity, and evenness of gut bacteria, increased fecal Lactobacillus, and decreased Enterococci in HFD rats (P < 0.05).
CONCLUSION: L. robustum may be a safe and effective food for weight loss and obesity control, and the effects of L. robustum might be mediated by the regulation of gut bacteria.
Core tip: Gut microbes play important roles in fat storage and metabolism. The control of gut microbes is considered promising in the prevention of obesity. In this study, the regulatory effect of Ligustrum robustum aqueous extract (LR) on gut bacteria in vivo and in vitro and body weight was determined. Certain doses of LR prevented obesity without sacrificing daily food or energy intake. LR may affect the richness, diversity, and evenness of gut bacteria by increasing Lactobacillus and decreasing Enterococci in the host gut.
-
Citation: Xie ZM, Zhou T, Liao HY, Ye Q, Liu S, Qi L, Huang J, Zuo HJ, Pei XF. Effects of
Ligustrum robustum on gut microbes and obesity in rats. World J Gastroenterol 2015; 21(46): 13042-13054 - URL: https://www.wjgnet.com/1007-9327/full/v21/i46/13042.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i46.13042
Recently, emerging data have implicated gut microbes in the development of obesity, and the regulation of gut microbes as a potential strategy in the prevention and treatment of obesity has attracted significant attention. Dietary factors, which possess anti-obesity effects, may also play a key role in regulating gut microbes. However, few foods have been investigated for their effects on gut microbes and the development of obesity.
Ligustrum robustum (L. robustum) was classified as food by the Chinese Ministry of Health in 2011[1]. In southwest China, the leaves of L. robustum are processed as Ku-Ding tea. Previous studies have shown that this tea might have anti-oxidative, anti-inflammatory, and anti-obesity effects[2-5]. In addition, it was found that L. robustum may affect a variety of gut microorganisms[6,7]. Thus, we hypothesized that L. robustum might exert anti-obesity effects by regulating gut microbes.
In this study, we examined the effects of L. robustum on different types of gut bacteria. We also assessed the effects of L. robustum on the changes in host body weight and gut flora in high-fat diet-fed rats.
L. robustum was previously identified by Professor Guomin Liu of the Ku-Ding Tea Research Institute, Hainan University, China and was obtained from China’s largest L. robustum provider, Green Hills and Blue Waters Co., Ltd. (Junlian, Sichuan, China) (http://jlqingshanlvshui.1688.com/). The extraction was guided by Professor Jing Huang of the West China School of Pharmacy, Sichuan University and prepared by Chengdu Push Bio-Technology Co., Ltd. (Chengdu, China). In brief, dried L. robustum was ground and extracted with 10 times its volume of water for 60 min at 80 °C. The residue was re-extracted twice, the extracted solution was filtered, and the combined supernatants were concentrated to 1 g/mL in a vacuum evaporator at 60 °C. L. robustum aqueous extract (LR) was sterilized at 115 °C for 15 min and stored in bottles at -20 °C in the dark.
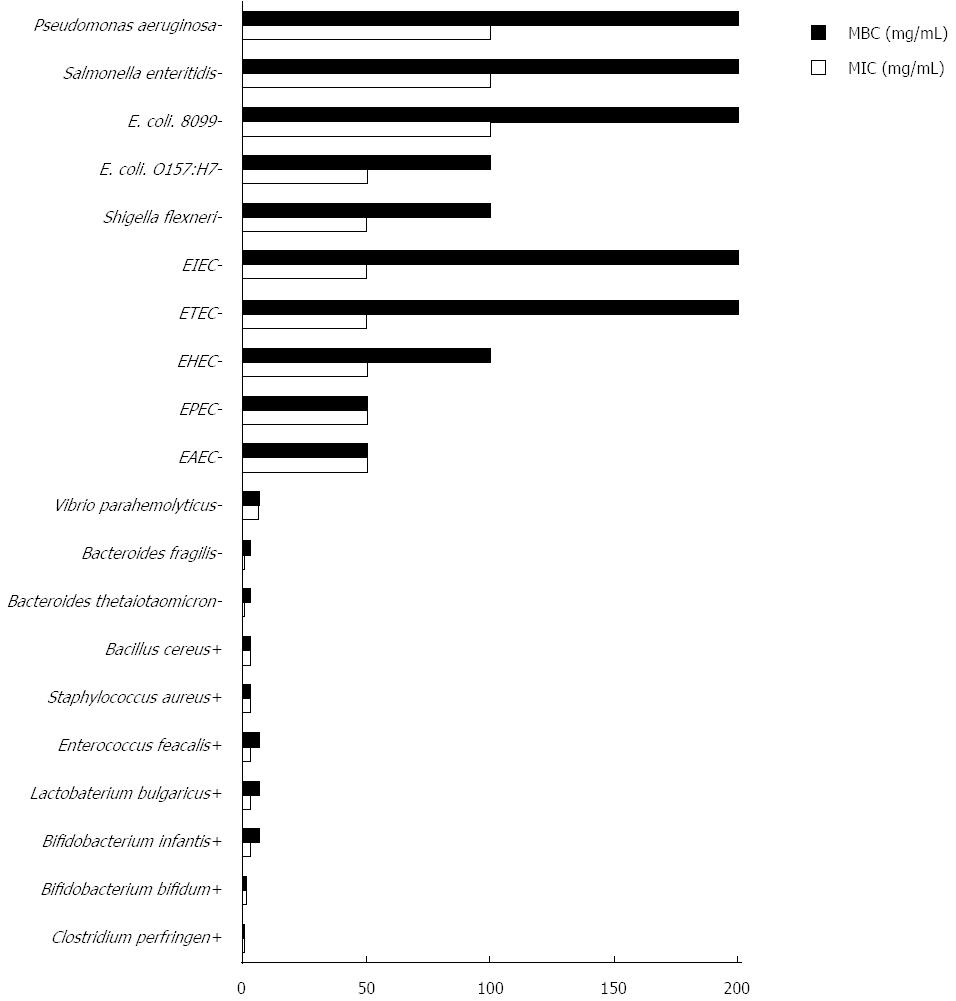
The inhibitory effects of LR on intestinal symbiotic bacteria, conditional pathogenic bacteria, and enteropathogenic bacteria were determined. The symbiotic bacteria included: Escherichia coli (E. coli) ATCC 8099, Bifidobacterium bifidum CICC 6071, Bifidobacterium infantis CICC 6069, Lactobacterium bulagricum (separated from yogurt), Enterococcus faecalis ATCC 29212, and Bacteroides thetaiotaomicron ATCC 29741. The conditional pathogenic bacteria included: Bacteroides fragilis ATCC 25285 and Clostridium perfringens ATCC 13124. The enteropathogenic bacteria included: Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 15442, Salmonella enteritidis CMCC 50335, Shigella flexneri CMCC 51061, E. coli O157:H7 ATCC 882364, Entero-Aggregative E. coli (clinical isolates), Entero-Hemorrhagic E. coli (clinical isolates), Entero-Toxigenic E. coli (clinical isolates), Entero-Pathogenic E. coli (clinical isolates), Entero-Invasive E. coli (clinical isolates), Bacillus cereus (Military Medical Science Academy of the PLA reference strain 4001), and Vibrio parahemolyticus ATCC 17802. All clinical isolates in our laboratory were identified by conventional biochemical and serological tests.
The micro-dilution susceptibility assay was performed to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). Dilutions were prepared in 96-well plates to obtain final concentrations ranging from 200 mg/mL to 0.1 mg/mL of LR in the medium. Fresh target bacteria were prepared (Table 1), and an inoculum of 10 μL (approximately 106 CFU/mL-107 CFU/mL) was inoculated into the microplates, and the tests were performed in a volume of 100 μL. The homogenated 96-well plates were incubated aerobically or anaerobically at 37 °C in duplicate for 24-48 h (Table 1), and then the culture solution containing bacteria was re-suspended. The lowest concentration of the tested samples, which did not show any visual growth after macroscopic valuation, was determined as the MIC. Using the results of the MIC assay, the concentrations showing complete absence of visual bacterial growth were identified, and 10 μL of each culture broth was transferred onto agar plates and incubated for the specified time and temperature, as mentioned above. The complete absence of growth on the agar surface at the lowest concentration of sample was defined as the MBC.
| Bacterial group | Bacteria preparation | MIC | ||
| Medium | Incubation conditions | Medium | Incubation conditions | |
| Bacteroides | GAM1 agar | 37 °C anaerobic, 48 h | BHI2 (10% HS4) | 37 °C Anaerobic, 48 h |
| Bifidobacteria | BBL2 agar | 37 °C anaerobic, 48 h | RC2 | 37 °C Anaerobic, 48 h |
| Lactobacilli | LBS2 agar | 37 °C aerobic with 5% CO2 48 h | RC2 | 37 °C Anaerobic, 48 h |
| C. perfringens | SPS2 agar | 37 °C anaerobic, 48 h | RC2 | 37 °C Anaerobic, 48 h |
| Aerobic E. coli | EMB2 agar | 37 °C aerobic, 18 h | MH2 | 37 °C Aerobic, 18 h |
| Enterococci | BEA2 agar | 37 °C aerobic, 18 h | MH2 | 37 °C Aerobic, 18 h |
| Candida albicans | Salouraud2 agar | 37 °C aerobic, 18 h | Salouraud2 | 37 °C Aerobic, 18 h |
| Staphylococcus aureus | blood plate3 | 37 °C aerobic, 18 h | MH2 | 37 °C Aerobic, 18 h |
| Other bacteria | LB1 agar | 37 °C aerobic, 18 h | MH2 | 37 °C Aerobic, 18 h |
Animals and treatments: Male Sprague Dawley rats [220 ± 20 g, specific pathogen free (SPF) grade, Certified No. SCXK (Jing) 2009-0004], normal chow diets (No. D12450B), and high-fat diets (HFDs, No. D12492) were obtained from the Beijing HFK Bioscience Co., Ltd. (Beijing, China). The animals were kept in an environmentally controlled breeding room (temperature: 23 °C ± 2 °C, humidity: 50%-60%, 12 h dark/light cycle). The protocol was approved by the Ethics Committee of the State Key Laboratory of Oral Diseases, Sichuan University, China. After 3 d of adaptive feeding, the rats were randomly assigned to one of five groups according to their body weight, with each group comprising 10 rats. The treatment was as follows: the control group (Normal) was fed normal chow diet and infused with distilled water; the obese model group (HFD) was fed HFD and infused with distilled water; and low-, medium- and high-dose groups (LR-L, LR-M, LR-H) were fed HFD and infused with a solution of LR at 2.5 mL/kg body weight, 5 mL/kg body weight, and 10 mL/kg body weight per day, respectively.
Body weight and food intake were monitored once a week. Total calorie intake was calculated according to dietary calorie intake and expressed as kcal/rat per day. Freshly collected feces were used for bacteria number assays. After 6 wk of normal chow diet or HFD feeding, the animals were weighed and fasted for 24 h before the experiments. Fasted blood was collected from the femoral artery. Serum was isolated by centrifugation at 3000 g and 4 °C for 10 min and stored at -70 °C until it was used for blood biochemical assays. Following blood collection, the rats were sacrificed by cervical dislocation. Visceral adipose tissue, liver, and spleen were immediately weighed after removal.
Blood glucose (GLU), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using an automatic biochemical analyzer (Olympus AU400; Olympus, Tokyo, Japan).
Fecal samples were obtained before the animals were killed and stored at -70 °C for culture independent analysis. For bacterial cultivation and counting, fresh fecal samples were cultured quantitatively for aerobic, facultative, and anaerobic bacteria using methods defined by the Chinese Ministry of Health[8]. In brief, freshly voided feces (less than 10 min after defecation) were collected in a sterile box. Fecal samples (approximately 1000 mg) were homogenized and serially diluted in sterile anaerobic solution. Appropriate dilutions were incubated aerobically or anaerobically at 37 °C in duplicate using selective media within 2 h of collection. The culture conditions are shown in Table 1. The target bacterial colonies in each medium at the corresponding dilution were counted, and the bacteria were subsequently characterized by Gram staining and polymerase chain reaction (PCR) identification. Colony counts are expressed as the log of colony forming units per gram of wet feces (log colony forming units/g, log CFU/g).
Genomic DNA was extracted from 200 mg (wet weight) of frozen rat feces using a TIANamp Stool DNA Kit (Tiangen, Beijing, China).
In our laboratory, 16S rRNA analysis of the main gut bacteria (Table 2) in feces was performed in accordance with a previously published method[9-13] with slight modifications, which demonstrated good repeatability and specificity[14].
| Target bacteria (amplicon size, bp) | Sequence (5’ to 3’) |
| Bifidobacteria sp. (243) | F: TCGCGTC(C/T)GGTGTGAAAG |
| R: CCACATCCAGC(A/G)TCCAC | |
| Lactobacilli sp. (341) | F: AGCAGTAGGGAATCTTCCA |
| R: CACCGCTACACATGGAG | |
| Clostridium perfringens groups (120) | F: ATGCAAGTCGAGCGA(G/T)G |
| R: TATGCGGTATTAATCT(C/T)CCTTT | |
| Enterobacteriaceae (195) | F: CATTGACGTTACCCGCAGAAGAAGC |
| R: CTCTACGAGACTCAAGCTTGC | |
| Enterococci sp. (144) | F: CCCTTATTGTTAGTTGCCATCATT |
| R: ACTCGTTGTACTTCCCATTGT | |
| Bacteroidetes (126) | F: GGARCATGTGGTTTAATTCGATGAT |
| R: AGCTGACGACAACCATGCAG | |
| Firmicutes (126) | F: GGAGYATGTGGTTTAATTCGAAGCA |
| R: AGCTGACGACAACCATGCAC |
A double fluorescence labeled terminal-restriction fragment length polymorphism (T-RFLP) analysis was performed based on the technique described by Kato et al[15]. Bacterial 16S rRNA genes were amplified with the universal bacterial primers Bac8F (5’-FAM-AGAGTTTGATCCTGGCTCAG) and Univ1492R (5’-HEX-GGTTACCTTGTTACGACTT) (Sangon, Shanghai, China). For T-RFLP, the forward primer was fluorescently 5’-labeled with FAM and the reverse primer was 5’-labeled with HEX, respectively, to enable subsequent detection of terminal restriction fragments (T-RFs).
The PCR mixture contained approximately 50 ng DNA template, 12.5 μL Hot Start Taq Master Mix (Dongsheng Biotech, Guangdong, China), 0.2 μmol/L labeled primer, 0.5 μL Taq Enzyme, and water in a final volume of 25 μL. The PCR reaction was performed in a PCR Thermo cycler (S1000, Bio-Rad, Hercules, CA, United States) using the following program: an initial activation of Taq polymerase at 95 °C for 3 min, 30 cycles of 95 °C for 30 s, 53 °C (56 °C and 60 °C were also tested) for 30 s, and 72 °C for 90 s, with a final extension at 72 °C for 7 min. Negative controls containing all reagents, but without DNA template, were included throughout the analysis to exclude DNA contamination.
Approximately 100 ng purified PCR products (TIANgel Midi Purification Kit DP209, Tiangen, Beijing, China) were digested with 1 μL of the restriction enzyme Hae III or Hha I at 37 °C for 1 h in a final volume of 20 μL following the manufacturer’s instructions (Takara, Dalian, China). DNA fragments were separated by capillary electrophoresis on an ABI 3730xl DNA analyzer (Invitrogen, Shanghai, China). The lengths of fluorescently labeled T-RFs were determined by comparison with an internal standard (GeneScan 1200 LIZ®; Applied Biosystems, Foster City, CA, United States), and data were analyzed using GeneMapper 4.1 software (Applied Biosystems, Foster City, CA, United States). T-RFs > 15 bp with more than 1% abundance of bacterial 16S rRNA gene fragments were identified using the MiCA online software[16,17]. In order to quantify the differences in community structures of the studied groups, the fragment richness, Shannon-Weiner diversity index, and Evenness value were calculated by double fluorescence labeled T-RFLP[16]. The Jaccard similarity index[18] of T-RFs between the HFD group and the other groups were then investigated using the equation, J = C/(A + B - C), in which A is the total size of T-RFs present in group A, B is the total size of T-RFs present in group B, and C is the total size of T-RFs present in both groups.
Continuous variables were expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) or nonparametric tests, where appropriate. Spearman’s correlation was used to determine the relationship between body weight and Jaccard similarity index derived from T-RFLP. P values less than 0.05 were considered statistically significant. SPSS 13.0 software (SPSS Inc., Chicago, IL, United States) was used for all statistical analyses.
The statistical methods of this study were reviewed by assistant professor Xing Zhao from the Department of Biostatistics, West China School of Public Health, Sichuan University, China.
Following culture in vitro, the MIC and MBC of 20 types of bacteria were obtained. The tested strains had different MIC and MBC to LR: 0.20 mg/mL and 0.20 mg/mL for Clostridium perfringens; 0.39 mg/mL and 0.39 mg/mL for Bacteroides thetaiotaomicron and Bacteroides fragilis; 1.56 mg/mL and 1.56 mg/mL for Bifidobacterium bifidum; 3.13 mg/mL and 6.25 mg/mL for Bifidobacterium infantis; 3.13 mg/mL and 6.25 mg/mL for Lactobacterium bulagricum and Enterococcus faecalis; 3.13 mg/mL and 3.13 mg/mL for Staphylococcus aureus and Bacillus cereus; 6.25 mg/mL and 6.25 mg/mL for Vibrio parahemolyticus; 1.56 mg/mL and no bactericidal effect for Candida albicans. The MICs for other strains were all greater than 25 mg/mL. Taken together, nonparametric test results indicated that LR exhibited higher inhibitory effects on gram positive bacteria than gram negative bacteria in the in vitro experiment (P < 0.05, Figure 1).
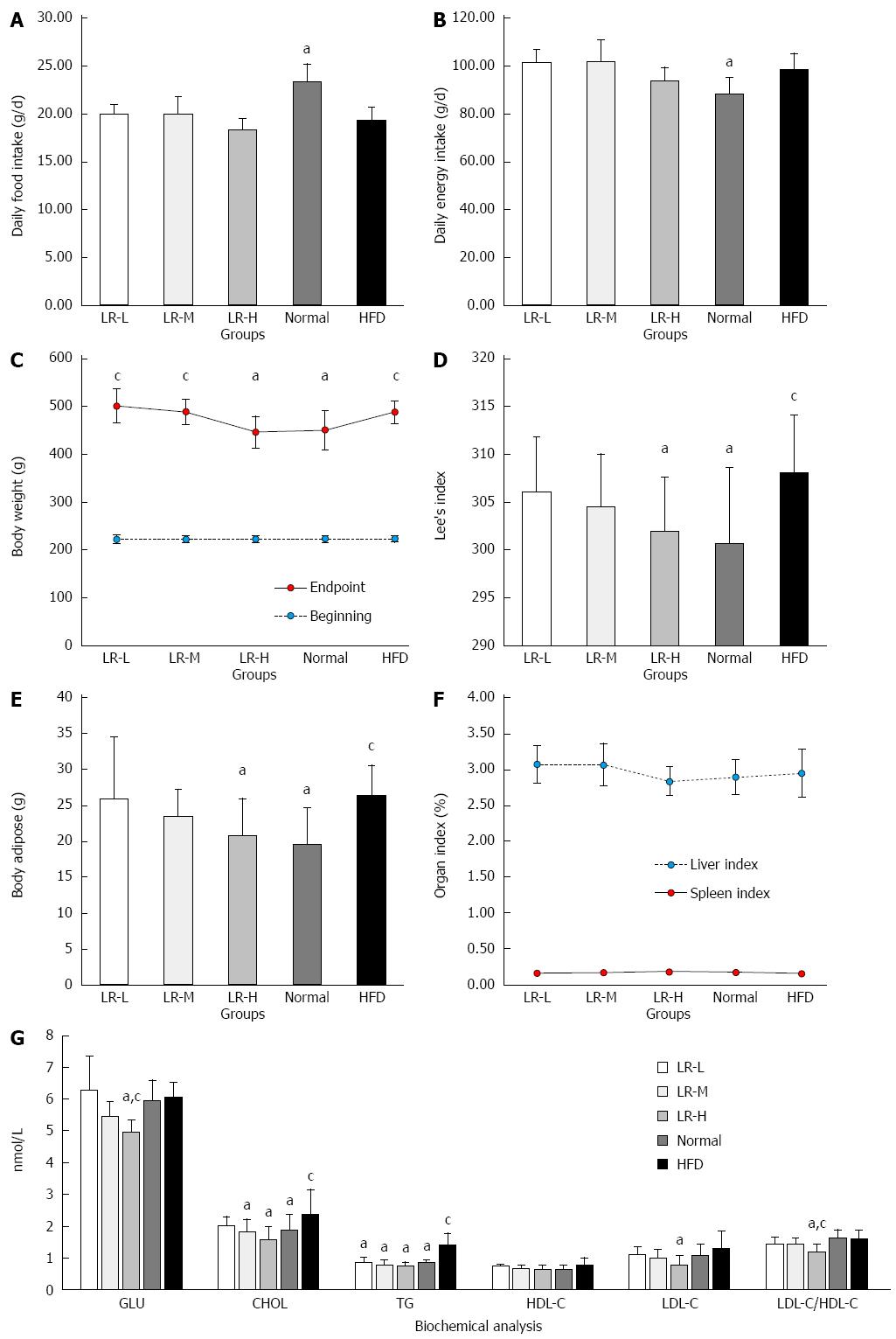
Food and energy intake: As indicated in Figure 2A, the average daily food intake in the Normal group was higher than that in the HFD model group (23.47 ± 1.69 g vs 19.16 ± 1.33 g, P < 0.05). In contrast, the average daily energy intake in the Normal group was markedly lower than that in the HFD model group (98.52 ± 6.67 kcal vs 88.49 ± 6.72 kcal, P < 0.05, Figure 2B), which could lead to greater body weight gain in the HFD group. In addition, LR did not significantly affect average daily food intake (19.95 ± 0.98 g, 19.99 ± 1.78 g, 18.37 ± 1.13 g, respectively) and energy intake (101.74 ± 5.02 g, 101.94 ± 9.07 g, 93.67 ± 5.75 g, respectively) compared with the HFD model group, suggesting that L. robustum did not affect food intake and energy intake.
Body and organ weight: Compared with the control group, the HFD group showed increases in body weight, Lee’s index, and body adipose tissue (P < 0.05, Figure 2C-E). However, no significant increase in Lee’s index was found in LR-L, LR-M, and LR-H compared with the Normal group, and high dose LR suppressed the increases in body weight and fat in HFD rats (P < 0.05, Figure 2F).
Blood glucose and lipid parameters: The high-fat diet increased plasma CHOL and TG relative to the Normal group (P < 0.05, Figure 2G). LR-H reduced the increases in GLU, TG, CHOL, LDL-C, and the ratio of LDL-C to HDL-C relative to the HFD group (P < 0.05, Figure 2G).
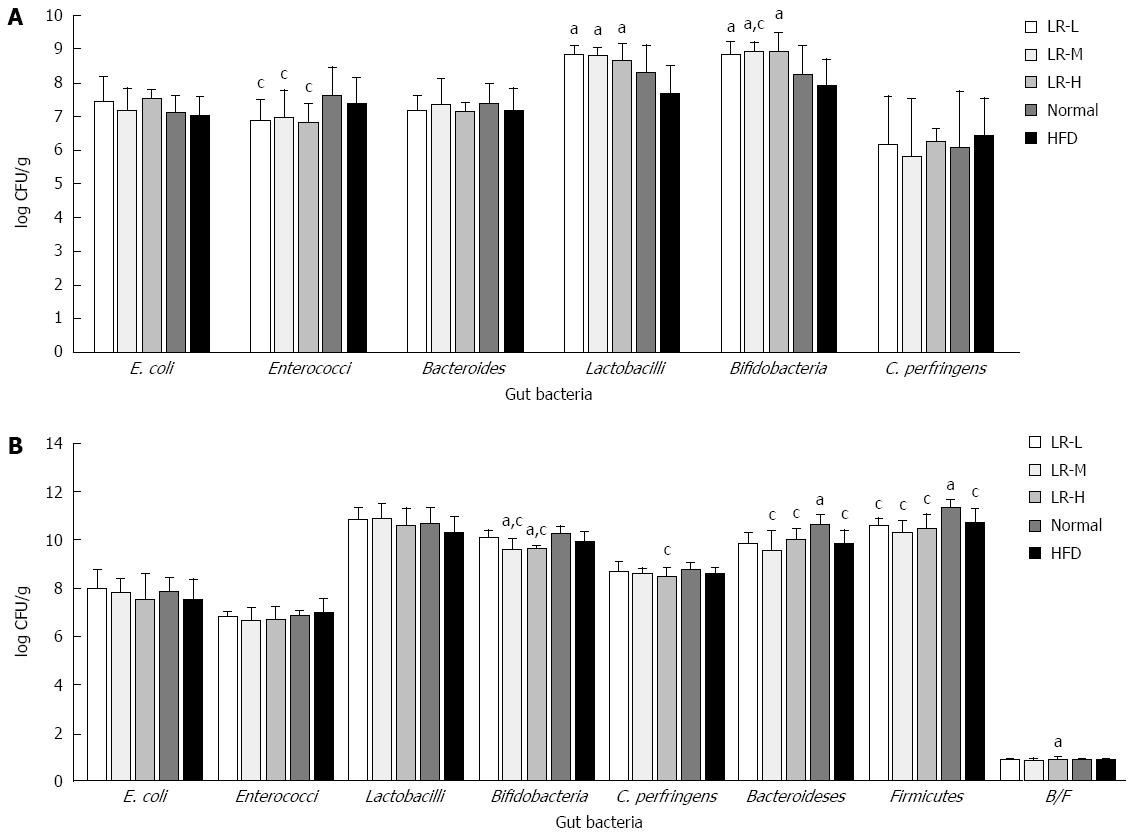
Viable counts of six gut bacteria: After 24-48 h of culture ex vivo, no significant differences in E. coli, Bacteroides, and Clostridium perfringens growth were observed between the experimental groups (Figure 3A). However, compared with the HFD group, the LR groups had a greater increase in the growth of Lactobacilli and Bifidobacteria (P < 0.05), and compared with the Normal group, the LR groups had a lower number of Enterococci (P < 0.05).
Quantitative PCR: As indicated in Figure 3B, there were no significant differences in E. coli, Enterococci, and Lactobacilli, although LR showed a tendency to decrease Enterococci and increase Lactobacilli in the feces of HFD rats, which is in accordance with the results of the culture method. In addition, the HFD group had the lowest ratio of Bacteroidetes to Firmicutes in all the groups, and had a decreased number of Firmicutes compared with the Normal group (P < 0.05). In contrast, certain doses of LR increased the ratio of Bacteroidetes to Firmicutes (P < 0.05) and decreased Clostridium perfringens and Bifidobacteria in the feces of HFD rats (P < 0.05). These data indicated that the effects of LR on certain types of gut bacteria, using quantitative PCR, were partly in accordance with those derived from the culture method (Enterococci and Lactobacilli), although Bifidobacteria was different.
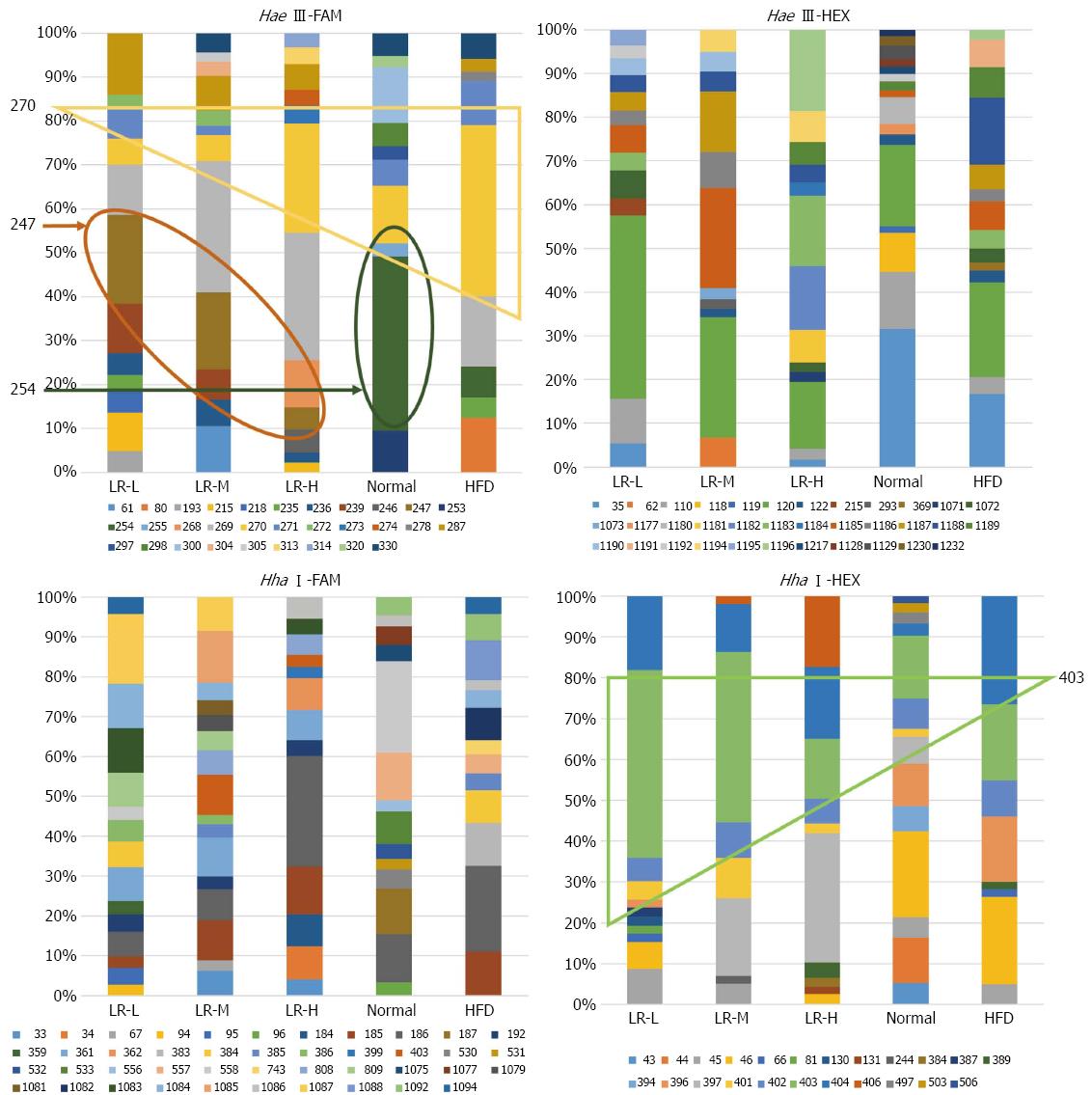
T-RFLP community analysis: In this study, as previously mentioned, the forward primer was labeled with FAM fluorescein and the reverse primer was labeled with HEX fluorescein. Two incision enzymes, Hha I and Hae III, were used separately to obtain T-RFs. Finally, four types of treatment, namely Hha I-HEX, Hha I-FAM, Hae III-HEX, and Hae III-FAM, were analyzed separately. The relative abundance of T-RFs in each sample was determined by calculating the ratio between the size of each peak and the total peak size of all peaks detected in one sample. Distinct T-RF was defined as an abundance of more than 1% (Figure 4). According to the Mica III database, in the Hae III-FAM group, the probable bacteria in T-RF 247 were Lactobacillus gallinarum, uncultured Lactobacillus sp., uncultured Chloroflexi bacteria, and uncultured bacteria. T-RF 247 was found in LR-L, LR-M, and LR-H but not in the Normal or HFD groups, indicating that LR treatment might increase Lactobacillus. T-RF 254 was identified as Clostridium subterminale, uncultured Clostridiales bacteria, uncultured rumen bacteria, and uncultured bacteria. T-RF 254 was detected in the Normal group (19.9%) and HFD group (3.4%). However, T-RF 254 was not detected in the treatment groups, indicating that LR may decrease bacteria such as Clostridium. T-RF 270 was identified as Streptococcus equi subsp., uncultured rumen bacteria, Helicobacter sp., uncultured Acidobacteria bacteria, and uncultured bacteria. T-RF 271, using Hae III-FAM, was identified as Helicobacter pylori, uncultured rumen bacteria, uncultured Fusobacterium sp., Fusobacterium nucleatum subsp. and uncultured bacteria. The HFD group had a higher percentage of T-RF 270 and T-RF 271 than the other groups (T-RF 270: 2.8%, 2.9%, 12.1%, 6.6%, 19.1%; T-RF 271: 3.4%, 1.0%, 0%, 3.0%, 5.0% in the LR-L, LR-M, LR-H, Normal, and HFD groups, respectively), which indicated that the HFD group may contain more Streptococcus equi subsp. and Helicobacter sp. compared with the other groups. In the Hae III-HEX group, the bacteria of T-RF 35 were suggested to be uncultured bacteria. The abundance of T-RF 35 in the LR-L, LR-M, LR-H, Normal and HFD groups was 2.2%, 0%, 1.03%, 20.69%, and 10.25% respectively. In the Hha I-FAM group, bacteria of T-RF 361 were mainly uncultured rumen bacteria. The abundance of T-RF 361 in the LR-L, LR-M, LR-H, Normal, and HFD groups was 3.4%, 4.3%, 3.1%, 0%, and 0%, respectively, indicating that LR treatment may increase the abundance of uncultured rumen bacteria. In the Hha I-HEX group, bacteria of T-RF 403 were mainly Lactobacillus sp. and uncultured bacteria when compared with the database. This also indicated that LR treatment may increase the abundance of Lactobacillus sp. The findings from T-RF 403 (Hha I-HEX) and T-RF 247 (Hae III-FAM) indicated that LR effectively increased some types of Lactobacillus sp. (Figure 4). This finding was partly in accordance with the results obtained from the culture method and quantitative PCR.
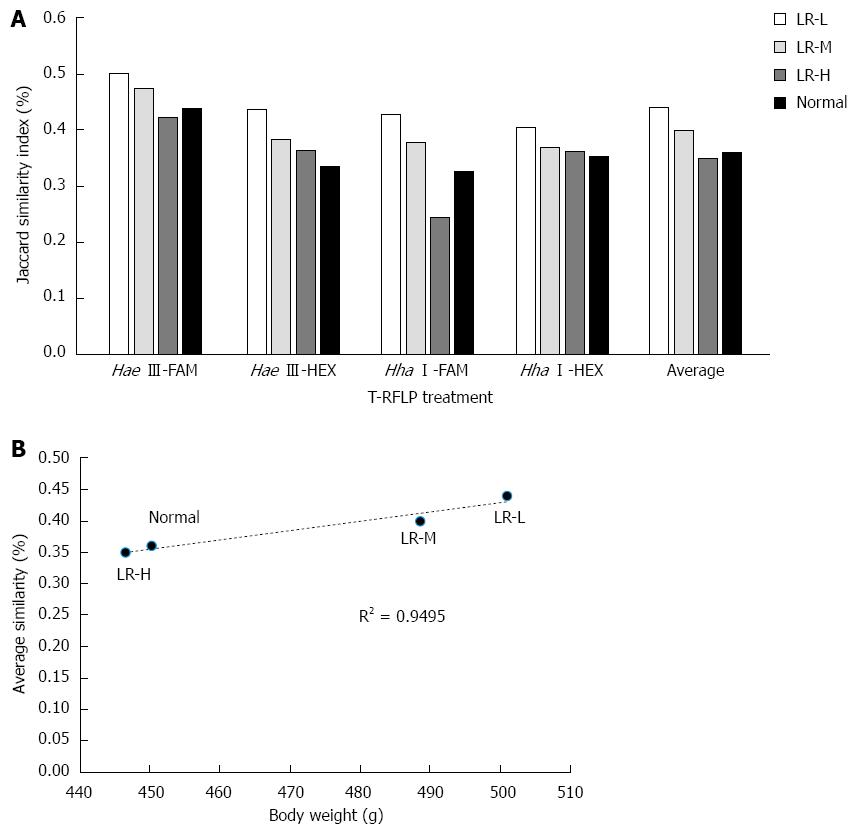
The Jaccard similarity index in the different treatments (i.e., Hae III-FAM, Hae III-HEX, Hha I-FAM, and Hha I-HEX) between the HFD group and the other groups is shown in Figure 5A. The average similarity compared with the HFD group was 0.44%, 0.40%, 0.35%, and 0.36%, respectively. There was a correlation between body weight and average similarity compared with the obese group (P < 0.05, Figure 5B), which indicated that gut microbiota in the heavier body weight group had higher similarity with the HFD group. These results demonstrated that the anti-obesity effect of LR might be derived from its effect on gut bacteria.
In this study, we found that bacterial richness was higher in the LR-L group than in the HFD group when Hae III-FAM and Hae III-HEX were applied (P < 0.05, Table 3). When Hae III-FAM, Hae III-HEX, and Hha I-FAM were used, a higher diversity index was found in the LR-L group compared with the HFD group (P < 0.05). When Hha I-FAM was used, a higher diversity index was also found in the LR-M group compared with the HFD group (P < 0.05). In addition, both the LR-L group and the LR-M group had higher E values compared with the HFD group when using Hha I-FAM (P < 0.05), while no difference between the LH-H and obese group was observed.
| Group | n | Richness | Shannon-Weiner | Evenness | |||||||||
| Hae III-FAM | Hae III-HEX | Hha I-FAM | Hha I-HEX | Hae III-FAM | Hae III-HEX | Hha I-FAM | Hha I-HEX | Hae III-FAM | Hae III-HEX | Hha I-FAM | Hha I-HEX | ||
| LR-L | 10 | 44.80 ± 8.64ac | 32.50 ± 5.20ac | 44.90 ± 14.90 | 24.00 ± 7.77 | 3.16 ± 0.31ac | 2.76 ± 0.22ac | 3.23 ± 0.20a | 2.36 ± 0.16 | 0.84 ± 0.07 | 0.80 ± 0.05 | 0.86 ± 0.03a | 0.76 ± 0.05 |
| LR-M | 10 | 31.70 ± 8.08 | 26.00 ± 5.68 | 39.40 ± 5.32 | 25.00 ± 4.99 | 2.93 ± 0.25 | 2.54 ± 0.18c | 3.19 ± 0.11a | 2.34 ± 0.15 | 0.85 ± 0.04 | 0.79 ± 0.05 | 0.87 ± 0.03a | 0.73 ± 0.05 |
| LR-H | 10 | 31.80 ± 11.79 | 24.50 ± 4.58 | 37.00 ± 7.56 | 26.40 ± 6.11 | 2.88 ± 0.38 | 2.50 ± 0.25c | 3.04 ± 0.18 | 2.51 ± 0.22 | 0.85 ± 0.04 | 0.79 ± 0.06 | 0.85 ± 0.04 | 0.77 ± 0.07 |
| Normal | 9 | 29.11 ± 10.40 | 21.56 ± 8.17 | 35.89 ± 7.03 | 21.44 ± 4.50 | 2.66 ± 0.25 | 2.28 ± 0.23a | 3.09 ± 0.22 | 2.35 ± 0.16 | 0.81 ± 0.08 | 0.77 ± 0.09 | 0.87 ± 0.04a | 0.77 ± 0.06 |
| HFD | 9 | 33.56 ± 11.20 | 27.11 ± 5.80 | 38.67 ± 12.75 | 24.78 ± 6.36 | 2.80 ± 0.47 | 2.50 ± 0.20c | 2.95 ± 0.39 | 2.38 ± 0.23 | 0.81 ± 0.06 | 0.76 ± 0.06 | 0.82 ± 0.04c | 0.75 ± 0.08 |
Various studies have demonstrated that gut bacteria play an important role in obesity development. Here, we analyzed the regulatory effect of LR on gut bacteria in vivo and in vitro and body weight control. We found that a high dose of LR resulted in a significant decrease in body weight and Lee’s index compared with rats fed the HFD not treated with LR, and certain doses of LR decreased CHOL, TG, LDL-C, and LDL-C/HDL-C in rats fed the HFD. These results suggest that LR may have anti-obesity and lipid-reducing effects. To determine whether the effect of weight loss was related to food and energy intake, we evaluated food and energy intake in all LR treated rats and in rats fed the HFD and found no significant differences, indicating that LR may prevent obesity without sacrificing daily food intake or daily energy intake. This led us to further investigate the mechanism of LR on body weight control. It is widely accepted that gut microbes play an important role in host energy metabolism and obesity development. Combined with the already existing evidence that LR has a regulatory effect on microorganisms[6,7], we speculated that the anti-obesity effects of LR may be mediated by the regulation of host gut microbes. To verify our hypothesis, we tested the regulatory effects of LR on gut bacteria in vivo and in vitro using different methods.
We first tested the effect of LR on the changes in rat gut bacteria. The culture results demonstrated that all three groups treated with LR had a lower level of Enterococci (P < 0.05). The quantitative PCR results also indicated a decreasing tendency in the Enterococci population compared with the HFD model group. In our previous study, we found that the number of Enterococci in the obese group was higher than that in the normal weight group[19]. The studies by Mozes et al[20] and Zhou et al[21] showed that an increase in Enterococci might help promote the development of obesity. These findings demonstrated that the anti-obesity effect of LR may be partly due to its ability to decrease the number of Enterococci.
In addition to Enterococci, we also found that the numbers of Bifidobacteria in the LR-L, LR-M, and LR-H groups were higher than that in the HFD group (P < 0.05). However, the results from quantitative PCR demonstrated that the numbers of Bifidobacteria in the LR-M and LR-H groups were lower than those in the HFD model group and control group (P < 0.05). What are the probable reasons contributing to the controversial results obtained from the different detection methods? In Matsuki et al[22], it was reported that not all Bifidobacteria could be identified by the culture method, and various types of Bifidobacteria required different culture conditions to grow on the plates. Thus, BBL, the only medium used, may not satisfy the requirements of all types of Bifidobacteria, especially those requiring strict culture conditions. Moreover, it is worth noting that a previous study indicated that many cultivable Bifidobacteria strains were important for host health and energy intake[22]. Therefore, in our study, the decreased amount of Bifidobacteria detected by quantitative PCR might be vulnerable to LR treatment. According to our other results, this kind of Bifidobacteria might contribute less to the control of host’s body weight than those that could be grown on BBL plates. Besides, there are only 2000 types of bacteria that can be used for 16S rRNA sequencing, and a large proportion of the 16S rRNA sequence is still unknown. Therefore, we speculate that the reason we obtained controversial results for Bifidobacteria may be partly due to the different principles of the detection methods. However, further studies are needed to confirm our speculation.
Lactobacillus consists of more than 90 types of bacteria, and approximately 30% can be cultured from stool samples[23]. The beneficial effect of Lactobacillus has been widely known for a long time. In this study, the culture results showed that the populations of Lactobacillus at different doses of LR were higher than those in the HFD model group (P < 0.05). The quantitative PCR results also demonstrated a similar tendency without significant variance. Furthermore, T-RF 247 (Hae III-FAM) and T-RF 403 (Hha I-HEX) analyses showed that LR treatment increased the abundance of Lactobacillus sp. Several previous studies reported that Lactobacillus regulates host metabolism and energy intake[24,25]. These results may indicate a potential role for cultivable Lactobacillus in LR-mediated weight loss.
Clostridium perfringens is a harmful gut bacterium, which can cause many diseases[26,27]. In this study, C. perfringens in LR-H treated group was lower than that in the normal group (P < 0.05), therefore, LR might inhibit the growth of C. perfringens and result in a healthier gut environment. However, this is a preliminary conclusion, and more studies are needed to confirm these findings.
Bacteroides, the most predominant bacterial group in the gut, is involved in the regulation of host metabolism and energy intake by regulating the metabolism of polysaccharide in the gut[28]. Previous studies have shown that normal rats contained more Bacteroides compared with the obese group[19,20]. In the present study, no significant difference was found using the culture method. However, the number of Bacteroidetes in the control group was higher than that in the group fed a HFD (P < 0.05). This was partly consistent with previous findings.
The relative proportions of gut bacteria can also affect body weight. Bacteroidetes and Firmicutes are the main gut bacteria that affect energy homeostasis[29]. It was reported that obese rats had less Bacteroidetes and more Firmicutes than the lean group[30,31]. In the present study, the HFD model group had the highest Lee’s index and the lowest ratio of Bacteroidetes to Firmicutes compared with the other groups. On the other hand, the RL-H group had the lowest Lee’s index and the highest ratio of Bacteroidetes to Firmicutes (P < 0.05). In addition, our results showed that the numbers of Bacteroidetes and Firmicutes in the normal control group were higher than those in the other four HFD groups, indicating that a HFD may lower the total number of gut bacteria.
In addition to the animal experiment, the different bacteriostatic and bactericidal effects of LR on gut bacteria were studied. Strongly selective bacteriostasis of LR was found by the MIC and MBC tests, and LR showed a higher inhibitory effect on the growth of gram positive bacteria than on gram negative bacteria. Interestingly, Enterococci, Bifidobacteria, Lactobacillus, and C. perfringens are all gram positive bacteria, and it was speculated that the growth of these gram positive bacteria would be inhibited and not enhanced according to the in vitro experiment. However, in the in vivo experiment, the number of some gram positive bacteria, such as Enterococci and C. perfringens decreased, while the number of Lactobacillus increased. This may be attributed to the fact that LR did not reach the effective concentrations required ex vivo when in the intestinal tract. The in vitro experiment targeted specific strains that partly represented the whole gut flora, whereas the results from the animal experiment focused on the genus level of different gut bacteria. Furthermore, LR is a mixture of different components, and the digestive tract is a complicated channel with various enzymes and microorganisms. LR would gradually be degraded in the gastrointestinal tract, thus the effective components in LR would probably be metabolized. As a result, in the ex vivo and in vivo experiments, the active ingredients of LR on gut bacteria would be different. However, we still observed the same trend in some bacteria after LR treatment both in vivo and in vitro.
In the present study, double fluorescence labeled T-RFLP was used to analyze the richness, diversity, and evenness of gut microbiota. Analysis of dominant flora showed that LR changed the advantageous bacterial species and their percentage in the total flora. By analyzing the percentage of advantageous flora in the total flora, we found that a certain amount of LR increased the percentage of Lactobacillus sp. in the total flora. We also found that LR treatment, especially LR-L, increased the richness and species diversity of the gut. Similar analysis of gut microbiota in the LR treated groups and the normal group compared with the HFD group indicated that gut microbiota in the heavier body weight group had higher similarity with the HFD group, which revealed a possible role for LR in obesity prevention by affecting the gut microbiota.
In conclusion, our study demonstrated significant bacteriostatic and bactericidal effects of LR on specific gut bacteria in vitro, and certain doses of LR altered the richness and diversity of gut bacteria in HFD rats. This study also found increased number of fecal Lactobacilli and decreased number of Enterococci, together with a decrease in Lee’s index and blood concentration of fat storage-related indicators in HFD rats. These findings indicated that LR may have a potential anti-obesity effect by regulating gut bacteria.
Gut microbes play important roles in fat storage and metabolism. The control of gut microbes is considered to be a promising method for preventing and treating obesity. The identification of safe and efficient modulatory agents to regulate body weight is a promising strategy for obese individuals and is of great interest to researchers.
To date, few foods have been investigated for their effects on gut microbes and the prevention of obesity. Recent studies indicated that Ligustrum robustum (L. robustum), a safe food-grade drink, can influence body weight and may have different bacteriostatic activities on different bacteria. However, the exact effect and mechanism of L. robustum require further investigation. The relationship between gut bacteria and obesity following treatment with L. robustum remains poorly defined.
In this study, the authors analyzed the regulatory effect of L. robustum aqueous extract (LR) on gut bacteria in vivo and in vitro and its effect on body weight control. The data showed that LR had significant bacteriostatic and bactericidal effects on specific gut bacteria in vitro and in vivo, and certain doses of LR altered the richness and diversity of gut bacteria. This study also found increased numbers of fecal Lactobacilli and decreased numbers of Enterococci together with a decrease in Lee’s index and in the blood concentration of fat storage-related indicators in high-fat diet-fed rats. These findings indicated that LR may have a potential anti-obesity effect by regulating gut bacteria.
The data suggested that L. robustum may be regarded as a safe and effective food for weight loss and obesity control, and these functions might be mediated by the regulation of gut bacteria. These findings may help to boost people’s recognition of L. robustum, promote the consumption of L. robustum, and facilitate the expansion and development of L. robustum.
The authors are provided interesting and original data about the effects of LR on host body weight and gut flora in high-fat diet-fed rats and its possible mechanisms. This study is well carried out, and the applied methods are sound. Overall, the study showed that L. robustum may have a potential anti-obesity effect by regulating gut bacteria.
P- Reviewer: Mallard BL S- Editor: Kong JX L- Editor: Filipodia E- Editor: Ma S
| 1. | Ministry of Health PR, China. The official approval for Ligustrum robustum as a food. Zhonguo Shipin Weisheng Zazhi. 2012;2:165. |
| 2. | Yu ZL, Zeng WC. Antioxidant, antibrowning, and cytoprotective activities of Ligustrum robustum (Rxob.) Blume extract. J Food Sci. 2013;78:C1354-C1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Lau KM, He ZD, Dong H, Fung KP, But PP. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J Ethnopharmacol. 2002;83:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | He ZD, Lau KM, But PP, Jiang RW, Dong H, Ma SC, Fung KP, Ye WC, Sun HD. Antioxidative glycosides from the leaves of Ligustrum robustum. J Nat Prod. 2003;66:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Huang LF, Wan DG. Study on the weight loss effect of Ligustrum robustum derived from Sichuan. Chengdu Zhongyiyao Daxue Xuebao. 2004;9:49. |
| 6. | Ye Q, Zuo HJ, Liao HY, Cao Y, Cao X, Pei XF. Study on in vitro antimicrobial activity of L purpurascents Y.C.Yang. Xiandai Yufang Yixue. 2013;40:1320-1322. |
| 7. | Du XX, Ye Q, Chen F, Zuo HJ, Huang MJ, Dong ZF, Pei XF. Study on the antibacterial activity of Ligustrum robustum combined with antibiotics to antibiotic resistant Staphylococcus aureus isolates. Xiandai Yufang Yixue. 2014;41:894-897. |
| 8. | Method for the Assessment of Regulating Gastrointestinal Tract Flora Function. Ministry of Health PR, China. Technical Standards for Testing & Assessment of Health Food. Beijing: People’s Medical Publishing House 2003; 148-153. |
| 9. | Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 825] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 10. | Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br J Nutr. 2008;100:1269-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Metzler-Zebeli BU, Hooda S, Pieper R, Zijlstra RT, van Kessel AG, Mosenthin R, Gänzle MG. Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl Environ Microbiol. 2010;76:3692-3701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Guo X, Xia X, Tang R, Wang K. Real-time PCR quantification of the predominant bacterial divisions in the distal gut of Meishan and Landrace pigs. Anaerobe. 2008;14:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Bahl MI, Bergström A, Licht TR. Freezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysis. FEMS Microbiol Lett. 2012;329:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Cheng Y, Zuo HJ, Liao HY, Chen JY, Zheng TL, Pei XF, Xu X. Establishment of real-time PCR method for detection of intestinal bacteria. Xiandai Yufang Yixue. 2014;41:4338-4341. |
| 15. | Kato M, Ishige A, Anjiki N, Yamamoto M, Irie Y, Taniyama M, Kibe R, Oka J, Benno Y, Watanabe K. Effect of herbal medicine Juzentaihoto on hepatic and intestinal heat shock gene expression requires intestinal microflora in mouse. World J Gastroenterol. 2007;13:2289-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Shyu C, Soule T, Bent SJ, Foster JA, Forney LJ. MiCA: a web-based tool for the analysis of microbial communities based on terminal-restriction fragment length polymorphisms of 16S and 18S rRNA genes. Microb Ecol. 2007;53:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Blasco L, Kahala M, Tampio E, Ervasti S, Paavola T, Rintala J, Joutsjoki V. Dynamics of microbial communities in untreated and autoclaved food waste anaerobic digesters. Anaerobe. 2014;29:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Bellou N, Papathanassiou E, Dobretsov S, Lykousis V, Colijn F. The effect of substratum type, orientation and depth on the development of bacterial deep-sea biofilm communities grown on artificial substrata deployed in the Eastern Mediterranean. Biofouling. 2012;28:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zuo HJ, Xie ZM, Zhang WW, Li YR, Wang W, Ding XB, Pei XF. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J Gastroenterol. 2011;17:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 20. | Mozes S, Bujnáková D, Sefcíková Z, Kmet V. Intestinal microflora and obesity in rats. Folia Microbiol (Praha). 2008;53:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Zhou LP, Hu LX, Deng XM, Li WZ, Yang HY, Ding WJ. Study on the gut bacteria alternation of patient undergo acupuncture weight control therapy. Sichuan Zhongyiyao Daxue Xuebao. 2011;29:124-126. |
| 22. | Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 376] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 23. | Hase K, Ohno H. [Epithelial cells as sentinels in mucosal immune barrier]. Nihon Rinsho Meneki Gakkai Kaishi. 2006;29:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One. 2013;8:e54617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Miyoshi M, Ogawa A, Higurashi S, Kadooka Y. Anti-obesity effect of Lactobacillus gasseri SBT2055 accompanied by inhibition of pro-inflammatory gene expression in the visceral adipose tissue in diet-induced obese mice. Eur J Nutr. 2014;53:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 27. | Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 421] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 28. | Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1495] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 29. | Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri JM, Moreno LA, Martin-Matillas M, Campoy C, Martí A, Moleres A. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond). 2009;33:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 242] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 30. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4639] [Cited by in RCA: 4466] [Article Influence: 223.3] [Reference Citation Analysis (1)] |
| 31. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6408] [Article Influence: 337.3] [Reference Citation Analysis (0)] |