Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1158
Peer-review started: June 17, 2014
First decision: July 21, 2014
Revised: August 4, 2014
Accepted: September 18, 2014
Article in press: September 19, 2014
Published online: January 28, 2015
Processing time: 223 Days and 19.5 Hours
AIM: To investigate the prevalence of significant liver fibrosis assessed using transient elastography (TE) and its predictors in asymptomatic general population.
METHODS: A total of 159 subjects without chronic viral hepatitis who underwent comprehensive medical health check-up between January 2012 and July 2012 were prospectively recruited. Significant liver fibrosis was defined as liver stiffness value > 7.0 kPa.
RESULTS: The mean age and body mass index (BMI) of the study population (men 54.7%) was 56.0 years and 24.3 kg/m2. Among the study subjects, 11 (6.9%) showed significant liver fibrosis. On univariate analysis, BMI, alanine aminotransferase (ALT), homeostasis model assessment of insulin resistance, carotid intimal media thickness (IMT), number of calcified plaques on carotid ultrasound, and visceral fat area on computed tomography were significantly higher in subjects with significant liver fibrosis than in those without (all P < 0.05). However, on multivariate analysis, BMI [odds ratio (OR) =1.487; P = 0.045], ALT (OR = 1.078; P = 0.014), carotid IMT (OR = 3.244; P = 0.027), and the number of calcified carotid plaques (OR = 1.787; P = 0.031) were independent predictors of significant liver fibrosis.
CONCLUSION: The prevalence of significant liver fibrosis assessed using TE was 6.9% in apparently healthy subjects. High BMI, high ALT, thicker carotid IMT, and higher numbers of calcified carotid plaques were independently associated with the presence of significant liver fibrosis.
Core tip: This is the first study which investigated the prevalence of significant liver fibrosis assessed using transient elastography and the correlation between comprehensive clinical metabolic parameters (body weight, visceral adiposity, insulin resistance, hepatic steatosis, and atherosclerosis) and the presence of significant liver fibrosis in asymptomatic general subjects. Finally, we found that the prevalence of significant liver fibrosis was fairly high (6.9%) and several factors including higher body mass index, higher alanine aminotransferase, thicker carotid intimal media thickness, and higher numbers of calcified carotid plaques on carotid sonography were significantly correlated to the risk of significant liver fibrosis.
- Citation: You SC, Kim KJ, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Lee WJ, Han KH. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol 2015; 21(4): 1158-1166
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1158
Along with tremendous progress in antiviral agents and treatment strategies, a vigorous national vaccination program has resulted in a gradually decreasing prevalence of chronic viral hepatitis. Despite this, the socioeconomic cost for managing chronic liver diseases (CLDs) is continuously increasing every year in South Korea[1]. This phenomenon is attributed to other CLDs, particularly non-alcoholic fatty liver disease (NAFLD), which has an estimated prevalence of up to 45% in the Asian population[2].
The prognosis of CLDs, including chronic viral hepatitis and NAFLD, generally depends on the severity of liver fibrosis. However, a non-negligible fraction of patients with CLDs, especially NAFLD, remain asymptomatic and even show normal liver function tests[3,4]. This makes it difficult to prevent the progression of liver fibrosis to liver cirrhosis and hepatocellular carcinoma. Thus, accurate and timely determination of significant liver fibrosis is important to prevent progression of liver fibrosis in patients with CLDs and this effective screening will eventually reduce medical costs and improve prognosis.
Although liver biopsy has been regarded as the gold standard for detecting liver fibrosis, it is innately invasive and can lead to mortality, misdiagnosis due to interpretational variability, and sampling errors due to uneven fibrosis distribution within the liver parenchyma[5]. Recently, liver stiffness (LS) measurement using transient elastography (TE) has emerged as a promising noninvasive tool for assessing the degree of liver fibrosis. This technique is non-invasive, accurate, reproducible, convenient, and useful for serial measurement in various CLDs[6-9]. However, few studies have investigated TE for use identifying asymptomatic subjects with significant liver fibrosis by screening the population without overt liver diseases[10,11].
Hence, this study investigated whether TE can screen asymptomatic general population without history of chronic viral hepatitis who received comprehensive medical health check-ups and identify subjects with significant liver fibrosis. In addition, we examined which factors other than chronic viral hepatitis are associated with the presence of significant liver fibrosis in this study population.
This study prospectively and consecutively included 190 apparently healthy subjects who underwent a comprehensive medical health check-up including TE examination in Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, between January 2012 and July 2012.
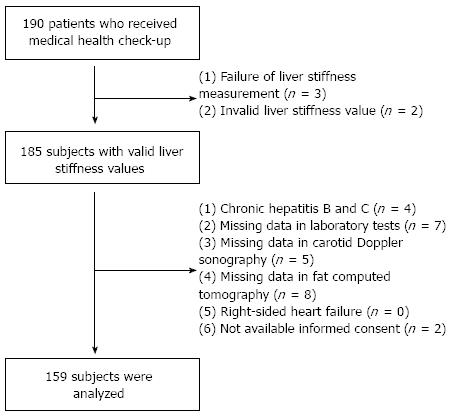
According to the exclusion criteria (Figure 1), 31 patients were excluded; the remaining 159 patients were selected for statistical analysis. Five patients were excluded due to LS measurement failure (no valid shot) or unreliable LS value. An additional 26 patients were excluded due to (1) chronic hepatitis B or C (n = 4); (2) missing laboratory test data (n = 7); (3) missing carotid sonography data (n = 5); (4) missing fat computed tomography data (CT) (n = 8); (6) right-sided heart failure (n = 0); or (7) lack of informed consent (n = 2). Baseline characteristics of the 31 excluded subjects were not statistically different from the 159 subjects included in this study (all P > 0.05, data not shown).
The study was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from each participant or a responsible family member. This study was approved by the Institutional Review Board of Severance Hospital.
In addition to demographic data, a comprehensive medical health check-up with laboratory and imaging studies was performed on the same day of LS measurement using TE. Aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, platelet count, fasting plasma glucose (FPG), Homeostatsis Model Assessment of Insulin Resistance (HOMA-IR), and HbA1c were measured. HOMA-IR was calculated using the following formula: [(Fasting plasma insulin (μU/mL)*FPG (mg/dL))/405][12]. Imaging studies including fat CT and carotid sonography. Liver ultrasonography measured degree of hepatic steatosis as described previously[13].
The principles of LS and controlled attenuation parameter (CAP) measurement using TE have been described previously[14,15]. TE was performed by one experienced technician (> 10000 examinations) blind to clinical subject data. TE results are expressed as kilopascals (kPa) for LS and dB/m for CAP. The interquartile range (IQR) was defined as an index of the intrinsic variability of LS and CAP values corresponding to the interval of LS and CAP results containing 50% of the valid measurements between the 25th and 75th percentiles. The median value of successful measurements was selected as representative of the LS and CAP values of a given patient. As an indicator of variability, the ratio of the IQR of LS and CAP values to the median values (IQR/M and IQR/MCAP, respectively) was calculated. CAP was only calculated when the LS measurement was valid for the same signals, ensuring that liver ultrasonic attenuation was achieved simultaneously and in the same volume of liver parenchyma as the LS measurement.
In this study, only procedures with at least ten valid measurements, a success rate of at least 60%, and an IQR/M of LS value < 0.3 were considered reliable and used for statistical analysis. Because the influence of IQR/MCAP on the accuracy of CAP has not been fully validated, IQR/MCAP was not adopted as a determinant for invalid CAP values.
In this study, a cut-off LS value of 7.0 kPa was defined to identify significant liver fibrosis. This value is based on a previous study from South Korea that proposed a normal LS range of 3.7-7.0 kPa in men and 3.3-6.8 kPa in women[16] and a study from Hong Kong that proposed the optimal cut-off LS value as 7.0 kPa to diagnose significant liver fibrosis (≥ F2) in patients with NAFLD[17].
Results were expressed as mean ± SD, median (range), or n (%), as appropriate. The means or percentages of baseline characteristics between patients with and without significant fibrosis were compared using independent Student t-tests or Mann-Whitney U tests for continuous variables and χ2 tests or Fisher’s exact tests for categorical variables. Univariate and multivariate logistic regression analyses were performed for variables that were significantly different between groups with and without significant fibrosis. All statistical analyses were performed with standard procedures (SAS, version 18). Statistical significance was considered at P < 0.05.
Baseline characteristics of the 159 study subjects are shown in Table 1. The mean age and body mass index (BMI) of the study population (87 men and 72 women) was 56.0 years and 24.3 kg/m2, respectively. The prevalence of obesity, defined as BMI > 25 kg/m2, was 41.5% (n = 66). Mean AST and ALT levels were 23.3 and 23.1 IU/L, respectively. On carotid sonography, mean intimal media thickness (IMT) was 0.75 mm. A carotid plaque was noted in 34 (21.4%) subjects, and the mean number of calcified carotid plaques was 0.48. Mean CAP and LS values were 248.3 dB/m and 4.7 kPa, respectively.
| Variables | Values |
| Demographic variables | |
| Age, yr | 56.0 ± 10.6 |
| Male gender | 87 (54.7) |
| Body mass index, kg/m2 | 24.3 ± 3.1 |
| Diabetes mellitus | 19 (11.9) |
| Hypertension | 40 (25.2) |
| Daily alcohol intake, mg | 15.4 ± 30.5 |
| Laboratory variables | |
| Aspartate aminotransferase, IU/L | 23.3 ± 8.5 |
| Alanine aminotransferase, IU/L | 23.1 ± 12.2 |
| Platelet count, 109/L | 235.5 ± 57.9 |
| Fasting plasma glucose, mg/dL | 100.5 ± 24.5 |
| HOMA-IR | 1.84 ± 1.35 |
| HbA1c, % | 6.0 ± 0.7 |
| Imaging variables | |
| Severe fatty liver on sonography | 30 (18.9) |
| Carotid sonography | |
| Intimal media thickness, mm | 0.75 ± 0.57 |
| Presence of carotid plaque | 34 (21.4) |
| Calcified carotid plaque, n | 0.48 ± 1.13 |
| Computed tomography | |
| Visceral fat area, cm2 | 112.4 ± 54.8 |
| Transient elastography | |
| Controlled attenuation parameter, dB/m | 248.3 ± 44.4 |
| Interquartile rangeCAP, dB/m | 35.91 ± 13.56 |
| Interquartile range/medianCAP | 0.16 ± 0.06 |
| Liver stiffness value, kPa | 4.7 ± 2.2 |
| Interquartile rangeLS, kPa | 0.52 ± 0.21 |
| Interquartile range/medianLS | 0.13 ± 0.06 |
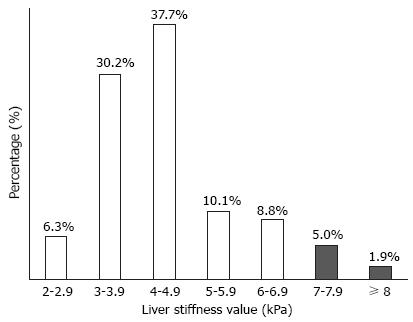
LS values were statistically similar between genders (4.5 kPa in men and 4.8 kPa in women, P = 0.360). Among the study subjects, most subjects (n = 60, 37.7%) had LS values of 4.0-4.9 kPa, whereas 11 (6.9%) subjects had LS values higher than 7 kPa, indicating the presence of significant liver fibrosis (Figure 2). Characteristics of patients with significant fibrosis are shown in Table 2, published online.
| No. | Age (yr) | Gender | BMI (kg/m2) | Alcohol intake (g/d) | ALT (IU/L) | CAP (dB/m) | Degree of fatty liver on US | LS value (kPa) |
| 1 | 47 | F | 24.7 | 0 | 11.0 | 259 | Normal | 7.1 |
| 2 | 69 | F | 28.1 | 0 | 46.0 | 348 | Moderate | 7.1 |
| 3 | 52 | F | 25.9 | 23 | 43.0 | 212 | Mild | 7.2 |
| 4 | 77 | M | 27.9 | 0 | 13.0 | 185 | Normal | 7.6 |
| 5 | 53 | M | 30.2 | 29 | 70.0 | 324 | Moderate | 7.6 |
| 6 | 68 | M | 26.5 | 38 | 42.0 | 274 | Mild | 7.8 |
| 7 | 77 | M | 33.9 | 0 | 35.0 | 281 | Moderate | 7.8 |
| 8 | 56 | M | 23.1 | 11 | 22.0 | 258 | Mild | 7.9 |
| 9 | 52 | M | 30.3 | 5 | 41.0 | 287 | Mild | 8.0 |
| 10 | 67 | M | 25.7 | 0 | 24.0 | 259 | Mild | 14.3 |
| 11 | 77 | F | 26.6 | 0 | 44.0 | 247 | Mild | 25.7 |
Various characteristics were compared between subjects with and without significant liver fibrosis (Table 3). BMI, ALT, HOMA-IR, carotid IMT, number of calcified carotid plaques on carotid sonography, and visceral fat area on CT were significantly higher in subjects with significant liver fibrosis than in those without (27.5 vs 24.1 kg/m2; 32.3 vs 22.2 IU/L; 2.9 vs 1.7; 1.4 vs 0.7 mm; 1.5 vs 0.4, and 171.5 vs 107.2 cm2, respectively; all P < 0.05). CAP value was elevated in patients with significant liver fibrosis but failed to show significant differences between the groups.
| Variables | Patients without significant fibrosis [n = 148 (93.1%), LS value ≤7 kPa] | Patients with significant fibrosis [n = 11 (6.9%), LS value > 7 kPa] | P value |
| Age, yr | 55.5 ± 10.4 | 63.2 ± 11.5 | NS |
| Male gender | 80 (54.1) | 7 (63.6) | NS |
| Body mass index, kg/m2 | 24.1 ± 3.0 | 27.5 ± 3.0 | 0.001 |
| Diabetes mellitus | 17 (11.5) | 2 (18.2) | NS |
| Hypertension | 38 (25.7) | 2 (18.2) | NS |
| Daily alcohol intake, mg | 16.2 ± 31.7 | 11.0 ± 16.4 | NS |
| Alanine aminotransferase, IU/L | 22.2 ± 11.4 | 32.3 ± 17.1 | 0.024 |
| Platelet count, 109/L | 235.6 ± 55.5 | 234.2 ± 92.0 | NS |
| Fasting plasma glucose, mg/dL | 99.7 ± 24.3 | 106.1 ± 24.9 | NS |
| HOMA-IR | 1.7 ± 1.2 | 2.9 ± 2.0 | 0.034 |
| HbA1c, % | 6.0 ± 0.7 | 6.2 ± 0.6 | NS |
| Presence of fatty liver on Sonography, n | 53 (35.8%) | 9 (81.8%) | 0.004 |
| Intimal media thickness, mm | 0.7 ± 0.2 | 1.4 ± 2.1 | 0.011 |
| Presence of carotid plaque | 31 (20.9) | 3 (27.3) | NS |
| Calcified carotid plaque, n | 0.4 ± 1.0 | 1.5 ± 1.8 | 0.019 |
| Visceral fat area, cm2 | 107.2 ± 47.7 | 171.5 ± 93.0 | 0.002 |
| Controlled attenuation parameter, dB/m | 246.4 ± 43.8 | 266.7 ± 45.6 | NS |
On multivariate logistic regression analysis using variables that were significant in univariate analysis (age, BMI, ALT, HOMA-IR, visceral fat area on CT, number of calcified carotid plaques, and carotid IMT), BMI [odds ratio (OR) = 1.487; 95% confidence interval (CI): 1.009-2.193; P = 0.045], ALT (OR = 1.078, 95%CI: 1.015-1.145; P = 0.014), carotid IMT (OR = 3.244, 95%CI: 1.140-9.234; P = 0.027), and number of calcified carotid plaques (OR = 1.787, 95%CI: 1.055-3.026; P = 0.031) were selected as independent predictors of significant liver fibrosis based on positive correlations (Table 4).
| Variable | Univariate | Multivariate | ||
| P value | Odds ratio | 95%CI | P value | |
| Age, yr | 0.021 | 1.065 | 0.981-1.156 | NS |
| Body mass index, kg/m2 | 0.001 | 1.487 | 1.009-2.193 | 0.045 |
| Alanine aminotransferase, IU/L | 0.010 | 1.078 | 1.015-1.145 | 0.014 |
| HOMA-IR | 0.013 | 0.950 | 0.555-1.626 | NS |
| Visceral fat area, cm2 | 0.004 | 0.995 | 0.979-1.011 | NS |
| Intimal media thickness, mm | 0.195 | 3.244 | 1.140-9.234 | 0.027 |
| Calcified carotid plaque, n | 0.008 | 1.787 | 1.055-3.026 | 0.031 |
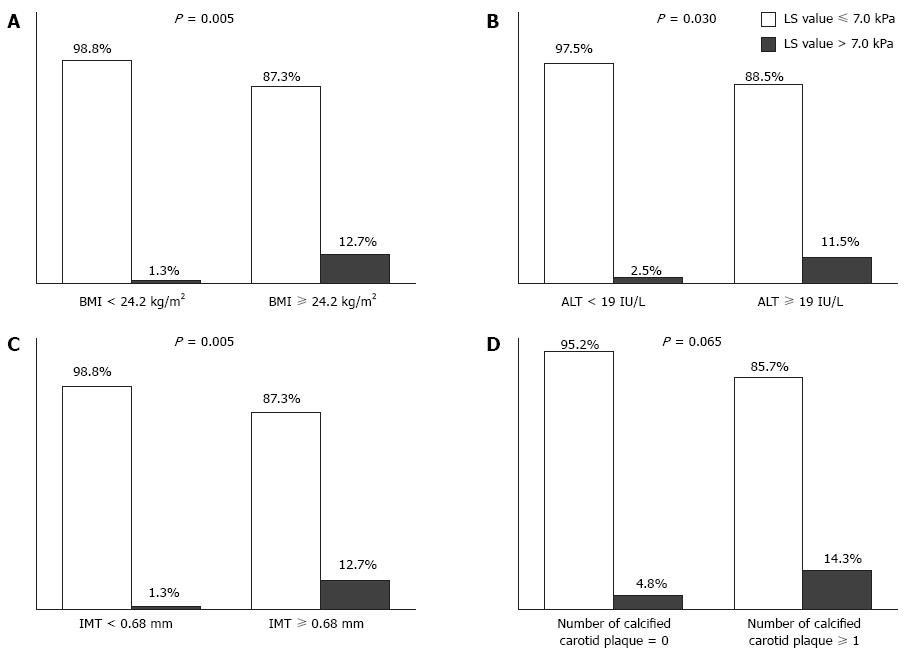
The study population was divided according to the medians of four independent factors (24.2 kg/m2 for BMI, 19 IU/L for ALT, 0.68 mm for carotid IMT, and one or more calcified carotid plaques) to calculate the relative risks between groups (Figure 3). Significant liver fibrosis was observed in ten of 79 (12.7%) subjects with BMI ≥ 24.2 kg/m2, nine of 78 (11.5%) subjects with ALT ≥ 19 IU/L, and ten of 79 (12.7%) subjects with carotid IMT ≥ 0.68 mm. In contrast, significant liver fibrosis was noted in one of 80 (1.3%) subjects with BMI < 24.2 kg/m2 (P = 0.005; OR = 11.4, 95%CI: 1.2-91.7), two of 81 (2.5%) subjects with ALT < 19 IU/L (P = 0.030; OR = 5.2, 95%CI: 1.1-24.7), and one of 80 (1.3%) subjects with carotid IMT < 0.68 mm (P = 0.005; OR = 11.4, 95%CI: 1.4-91.7). In addition, the prevalence of significant liver fibrosis tended to be higher in subjects with calcified carotid plaques than in those without [5/35 (14.3%) vs 6/124 (4.8%); P = 0.065].
Despite the increasing popularity and reliability of LS measurement using TE to assess the degree of liver fibrosis in subjects with CLDs, only a few Western studies have shown the applicability of screening the general population[10,11]. Thus, we tried to demonstrate the applicability of TE as a screening tool to identify subjects with potential significant liver fibrosis. For this aim, we included a cohort of apparently healthy native Korean subjects without history of chronic viral hepatitis who underwent a comprehensive medical health check-up. The prevalence of significant liver fibrosis was fairly high (6.9%) in this cohort. In addition, the associations between significant liver fibrosis and other clinical factors were investigated. BMI, ALT, carotid IMT, and number of calcified carotid plaques were identified as independent predictors of significant liver fibrosis. Although an exact comparison is not feasible due to differences in ethnicity and BMI, lack of histological information, and potential bias caused by reasons for the medical health check-up, the prevalence of significant liver fibrosis in this study was similar to that of a previous French study (6.9% and 7.5%, respectively) that adopted 8 kPa as the cutoff value for screening the general population[11].
Regardless of etiology, significant liver fibrosis is associated with a risk of fibrosis progression and poor prognosis[18,19]. Thus, it is of paramount importance to establish a screening strategy using non-invasive tools such as TE to detect potential underlying significant liver fibrosis in the asymptomatic general population. This will eventually reduce the social burden of liver fibrosis while improving patients’ quality of life and survival by preventing disease progression. BMI, ALT, carotid IMT, and number of calcified carotid plaques, four independent predictors of significant liver fibrosis in this study, are generally known to have significant correlation to NAFLD[20-22], which is the leading cause of CLDs in the general population, with no evidence of chronic viral hepatitis worldwide[23-25]. This indicates that most subjects with significant liver fibrosis in the current study cohort may have NAFLD. Indeed, most patients with significant fibrosis (n = 9, 81.8%) showed more than mild fatty liver on ultrasonography. Of these, 6 patients showed elevated ALT level.
To evaluate the relative risks of significant liver fibrosis, the study population was divided into two groups by BMI, ALT, carotid IMT, and number of calcified carotid plaques. Subjects with ALT higher than a cutoff value of 19 IU/L had significantly increased risk of significant liver fibrosis. This finding that subjects with normal ALT level are not completely free from the risk of significant liver fibrosis is consistent with the results of previous studies. Elevated ALT level, even within the current normal range (≤ 35-40 IU/L), has been associated with a risk of metabolic syndrome[26], CLDs[27], NASH[28], and mortality in the Korean population[29,30].
Subjects with BMI > 24.2 kg/m2 showed a higher prevalence of significant liver fibrosis. Weight gain has been shown to be closely related to liver fibrosis progression[19,31]. Although the exact mechanism for fibrotic progression in patients with NAFLD has not been fully recognized, obesity with a combination of insulin resistance and visceral adiposity causes hepatic lipid accumulation with various free fatty acid influx into the liver, up-regulation of hepatic lipogenic transcription factors, and inhibition of free fatty acid oxidation[32].
Interestingly, liver fibrosis had a significant relationship with carotid IMT and the number of calcified carotid plaques in this study. In a previous study, the close association between the degree of NAFLD-related liver fibrosis and increased carotid IMT independent of metabolic syndrome and insulin resistance was reported[33]. This suggests that atherosclerosis can independently influence risk of liver fibrosis progression. Further studies are required to reveal how cardio- or cerebrovascular diseases due to atherosclerosis can be affected by liver fibrosis in subjects with NAFLD[19,24]. In addition, studies are needed to determine whether TE can be incorporated into screening protocols to identify high-risk subjects with cardio- or cerebrovascular diseases.
Age and prevalence of diabetes mellitus, which may have significant correlation with NAFLD or liver fibrosis[28], were higher in subjects with significant liver fibrosis, but the results were not statistically significant. These negative findings should be interpreted with caution due to a relatively small sample size. Unexpectedly, CAP values were not significantly different between groups, although we assumed that most subjects with significant liver fibrosis suffered from NAFLD and would have increased CAP values. This can be explained by that CAP, similar to other imaging modalities[15], cannot generally differentiate NASH which is related to fibrosis progression from simple steatosis, although CAP does accurately reflect the amount of steatosis[34].
Our study still involves several unresolved issues. First, although previous studies[10,11] and our current study have demonstrated the applicability of TE as a screening tool for diagnosing significant liver fibrosis in the general population, insufficient histological examinations for subjects assumed to have significant liver fibrosis can be a major limitation. Since significant hepatic dysfunction is rarely observed in the asymptomatic general population, liver biopsies are not usually justified to confirm the histological diagnosis of liver disease. Thus, well-designed prospective studies that include a sufficient number of subjects with high LS value and histological evaluation are required. Second, we cannot be sure that the study participants are representative of the general population due to potential selection bias caused by recruiting subjects from health check-ups. However, because most baseline characteristics of the study subjects were similar to those in the general population, including daily alcohol intake[35], prevalence of hypertension and DM[36], carotid IMT thickness[37], and LS value[16], the results were not significantly influenced. Third, because this study was cross-sectional, further studies are needed to trace dynamic changes in LS values according to treatment interventions and to investigate the influence of LS changes on long-term clinical outcomes.
In conclusion, the prevalence of significant liver fibrosis assessed using TE was high in asymptomatic Korean general population, despite no evidence of underlying CLDs. High BMI, high ALT, thicker carotid IMT, and higher numbers of calcified carotid plaques were independently associated with the presence of significant liver fibrosis. Our results are helpful for identifying subjects who are at risk of significant asymptomatic liver fibrosis and for developing guidelines regarding the optimal utilization of TE in the general population.
The prevention from progression of mild liver fibrosis to liver cirrhosis and hepatocellular carcinoma is essential in management of chronic liver diseases (CLD). However, a considerable number of patients with CLD remains asymptomatic and even show normal liver function tests.
Recently, liver stiffness measurement assessed using transient elastography (TE) has emerged as a promising noninvasive tool for serial measurement of liver fibrosis in various chronic liver diseases. However, few studies have investigated TE for identifying asymptomatic subjects with significant liver fibrosis by screening apparently healthy population.
The prevalence of significant liver fibrosis was 6.9% in apparently healthy Korean population. Body mass index, alanine aminotransferase, carotid intima media thickness, and number of calcified carotid plaques were identified as independent predictors of significant liver fibrosis in this study.
Significant liver fibrosis in apparently healthy subjects raises necessity of rigorous screening strategy for CLDs in general Korean population. Predictors of significant fibrosis demonstrated in this study can help to identify subjects who are at risk.
The TE is the machine using a non-invasive probe, similar to an ultrasound probe. Ultrasound transducer of TE generates elastic shear wave propagating through the liver and measures its velocity. Since elastic shear wave runs faster in stiffer tissue, the velocity of shear wave is proportional to liver stiffness. Many previous studies reported that liver stiffness measurement assessed using TE accurately reflects degree of fibrosis in liver.
This is a good cross-sectional study. The authors investigated the prevalence and predictors of liver fibrosis assessed by non-invasive TE in Korean general population. This study will give us useful information regarding optimal selection of patients who require monitoring for chronic liver disease.
P- Reviewer: Ikuta S, Koch TR S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Keum DK, Kim BI. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 2. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 355] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 3. | Sorrentino P, Tarantino G, Conca P, Perrella A, Terracciano ML, Vecchione R, Gargiulo G, Gennarelli N, Lobello R. Silent non-alcoholic fatty liver disease-a clinical-histological study. J Hepatol. 2004;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1549] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 6. | Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. 2012;18:163-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Abenavoli L, Beaugrand M. Transient elastography in non-alcoholic fatty liver disease. Ann Hepatol. 2012;11:172-178. [PubMed] |
| 8. | Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Lédinghen V, Douvin C, Marcellin P, Ganne-Carrié N, Trinchet JC, Beaugrand M. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008;49:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Chon YE, Choi EH, Song KJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Casey SP, Kemp WW, McLean CA, Topliss DJ, Adams LA, Roberts SK. A prospective evaluation of the role of transient elastography for the detection of hepatic fibrosis in type 2 diabetes without overt liver disease. Scand J Gastroenterol. 2012;47:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, Le Clesiau H, Beaugrand M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24518] [Article Influence: 613.0] [Reference Citation Analysis (1)] |
| 13. | Hong JW, Kim JY, Kim YE, Lee EJ. Metabolic parameters and nonalcoholic fatty liver disease in hypopituitary men. Horm Metab Res. 2011;43:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Kim SU, Ahn SH, Park JY, Kang W, Kim do Y, Park YN, Chon CY, Han KH. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol. 2009;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Kim BK, Kim SU, Choi GH, Han WK, Park MS, Kim EH, Park JY, Kim do Y, Choi JS, Yang SC. “Normal” liver stiffness values differ between men and women: a prospective study for healthy living liver and kidney donors in a native Korean population. J Gastroenterol Hepatol. 2012;27:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 970] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 18. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2345] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 19. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1708] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 20. | Lee S, Jin Kim Y, Yong Jeon T, Hoi Kim H, Woo Oh S, Park Y, Soo Kim S. Obesity is the only independent factor associated with ultrasound-diagnosed non-alcoholic fatty liver disease: a cross-sectional case-control study. Scand J Gastroenterol. 2006;41:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 307] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Verma S, Jensen D, Hart J, Mohanty SR. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013;33:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 23. | Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 562] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 25. | Lee SS, Byoun YS, Jeong SH, Kim YM, Gil H, Min BY, Seong MH, Jang ES, Kim JW. Type and cause of liver disease in Korea: single-center experience, 2005-2010. Clin Mol Hepatol. 2012;18:309-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Wu WC, Wu CY, Wang YJ, Hung HH, Yang HI, Kao WY, Su CW, Wu JC, Chan WL, Lin HC. Updated thresholds for serum alanine aminotransferase level in a large-scale population study composed of 34 346 subjects. Aliment Pharmacol Ther. 2012;36:560-568. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Park HN, Sinn DH, Gwak GY, Kim JE, Rhee SY, Eo SJ, Kim YJ, Choi MS, Lee JH, Koh KC. Upper normal threshold of serum alanine aminotransferase in identifying individuals at risk for chronic liver disease. Liver Int. 2012;32:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, Goodman Z, Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 431] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 30. | Kim BK, Han KH, Ahn SH. “Normal” range of alanine aminotransferase levels for Asian population. J Gastroenterol Hepatol. 2011;26:219-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1469] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 33. | Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Chon YE, Jung KS, Kim SU, Park JY, Park YN, Kim do Y, Ahn SH, Chon CY, Lee HW, Park Y. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: a prospective study of a native Korean population. Liver Int. 2014;34:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Organization for Economic Co-operation and Development. Health at a Glance 2011 [Internet]. Paris: Organisation for Economic Co-operation and Development; 2011; Available from: http://www.oecd-ilibrary:content/book/health_glance-2011-en. |
| 36. | Available from: http://knhanes.cdc.go.kr/. |
| 37. | Lee YH, Shin MH, Kweon SS, Rhee JA, Ryu SY, Ahn HR, Choi JS. Metabolic syndrome and carotid artery parameter in Koreans aged 50 years and older. Circ J. 2010;74:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |