Published online Sep 28, 2015. doi: 10.3748/wjg.v21.i36.10299
Peer-review started: April 21, 2015
First decision: May 18, 2015
Revised: July 11, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 28, 2015
Processing time: 160 Days and 18.9 Hours
Hepatitis C virus (HCV) is a hepatotrophic virus and a major cause of chronic liver disease, including hepatocellular carcinoma, worldwide. The life cycle of HCV is closely associated with the metabolism of lipids and lipoproteins. The main function of lipoproteins is transporting lipids throughout the body. Triglycerides, free cholesterol, cholesteryl esters, and phospholipids are the major components of the transported lipids. The pathway of HCV assembly and secretion is closely linked to lipoprotein production and secretion, and the infectivity of HCV particles largely depends on the interaction of lipoproteins. Moreover, HCV entry into hepatocytes is strongly influenced by lipoproteins. The key lipoprotein molecules mediating these interactions are apolipoproteins. Apolipoproteins are amphipathic proteins on the surface of a lipoprotein particle, which help stabilize lipoprotein structure. They perform a key role in lipoprotein metabolism by serving as receptor ligands, enzyme co-factors, and lipid transport carriers. Understanding the association between the life cycle of HCV and lipoprotein metabolism is important because each step of the life cycle of HCV that is associated with lipoprotein metabolism is a potential target for anti-HCV therapy. In this article, we first concisely review the nature of lipoprotein and its metabolism to better understand the complicated interaction of HCV with lipoprotein. Then, we review the outline of the processes of HCV assembly, secretion, and entry into hepatocytes, focusing on the association with lipoproteins. Finally, we discuss the clinical aspects of disturbed lipid/lipoprotein metabolism and the significance of dyslipoproteinemia in chronic HCV infection with regard to abnormal apolipoproteins.
Core tip: Hepatitis C virus (HCV) and lipids interact closely at multiple stages in the HCV life cycle. HCV infection may have a profound influence on lipid metabolism, while lipids can regulate HCV replication. Infectious HCV forms lipo-viral particles that possess the features of lipoproteins. Examination of lipoprotein sub-fractions and apolipoproteins is inevitable for evaluating the nature of disturbed lipid metabolism. Among apolipoproteins, apolipoprotein E is a key molecule required for HCV entry, and is one of the possible therapeutic targets for interrupting HCV infection. Understanding the disturbed lipid metabolism may shed light on the pathophysiology of HCV infection and help develop novel therapeutics.
- Citation: Aizawa Y, Seki N, Nagano T, Abe H. Chronic hepatitis C virus infection and lipoprotein metabolism. World J Gastroenterol 2015; 21(36): 10299-10313
- URL: https://www.wjgnet.com/1007-9327/full/v21/i36/10299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i36.10299
Hepatitis C virus (HCV) is one of the most important viruses affecting human health. It is estimated to infect about 2% of the world’s population[1]. Liver cirrhosis and hepatocellular carcinoma (HCC) develop after long-term HCV infection[2]. As natural eradication of chronic HCV infection is extremely rare, interferon (IFN)-based antiviral therapy had been carried out, but its efficacy was limited[3]. The application of direct-acting antivirals (DAAs) has been progressing in recent years[4]. IFN free regimens with combinations of DAA preparations having different action mechanisms are recommended as a standard treatment in various countries. It is expected that combinations of DAAs without IFN might help abolish most chronic HCV infections within the end of the next decade in Japan. However, treatment with DAAs is time consuming and very expensive.
HCV is a unique virus that uses the host lipid metabolism at multiple key steps of the HCV life cycle[5]. HCV is secreted from the liver as highly infective lipo-viral particles (LVPs), which express hallmarks of lipoprotein particles such as apolipoprotein C (apo C) and apolipoprotein E (apo E) on the surface[6]. This strongly indicates that there is an extremely close connection between the infectivity of HCV and lipoprotein metabolism.
Understanding the tight association between HCV life cycle and lipoprotein metabolism is very important because HCV infection is a unique model wherein the virus causes chronic infection while coexisting with the host and simultaneously taking over the host’s metabolism[6]. Moreover, each step of the HCV life cycle that is connected to lipoprotein metabolism is a potential target for anti-HCV therapy. The need to identify such targets arises despite the approval of DAA-based treatment, because the high cost and comparative long duration of DAA treatment warrant other types of concomitant drugs or substitutive drugs to overcome the limitations. In addition, the anomalous lipid metabolism caused by HCV infection may lead to liver injury and hepatocarcinogenesis.
Hepatologists may not necessarily be familiar with the details of lipoprotein metabolism even though the liver is the central organ for the function of this protein. Therefore, in this review, we provide a concise overview of human lipoprotein, with attention to recent acquired knowledge. Next, we provide an outline of the life cycle of HCV, focusing on the interaction between HCV and lipoprotein metabolism in view of anti-HCV therapeutic targets. Finally, the clinical aspects of disturbed lipid/lipoprotein metabolism and recent data on dyslipoproteinemia in chronic HCV infection, including the abnormality of circulating apolipoprotein, have been summarized.
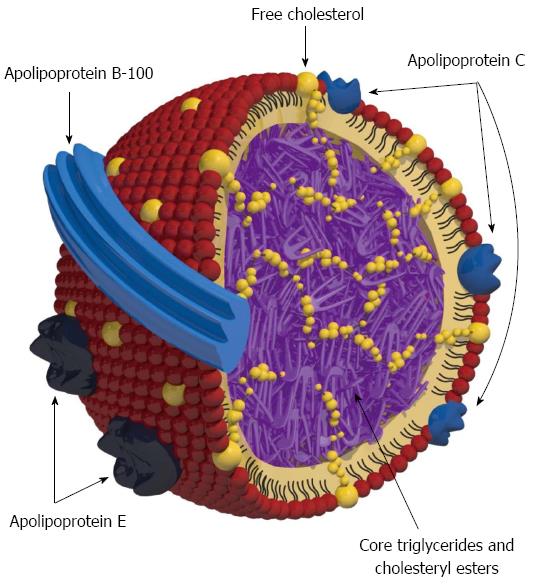
A lipoprotein is a particle comprising a single outer layer of amphipathic phospholipid covering a non-polar central core. The main function of lipoproteins is transporting lipids throughout the body. Triglycerides (TGs), cholesteryl esters (CEs), free cholesterol, CEs, and phospholipids are the major components of the lipids. The structure of lipoprotein (very-low-density lipoprotein; VLDL) is illustrated in Figure 1.
Lipoproteins are usually classified based on buoyant density by ultracentrifugation into five classes, namely, chylomicrons (CMs), VLDLs, intermediate-density lipoproteins (IDLs), low-density lipoproteins (LDLs), and high-density lipoproteins (HDLs). Alternatively, lipoprotein can be classified by the mobility of electrophoresis as β lipoprotein, pre-β lipoprotein, or α lipoprotein. CMs and VLDLs are TG-rich lipoproteins, while LDLs and HDLs contain abundant cholesterol. CM particles are the biggest, while HDL particles are the smallest. Buoyant density of HLD is the highest, while it is the lowest in CMs. The physicochemical natures of the five classes of lipoprotein by buoyant density are presented in Table 1.
| Class name | Density (g/mL) | Diameter (nm) | % Protein | % Cholesterol (free cholesterol + cholesteryl ester) | % Phospholipids | % Triglycerides | % Free fatty acids |
| CM | < 0.95 | 100-1000 | < 2 | 4-8 | 7-8 | 84-88 | 0 |
| VLDL | 0.950-1.006 | 30-80 | 7-10 | 20-25 | 18-20 | 50-55 | 1 |
| IDL | 1.006-1.019 | 25-50 | 10-18 | 29-45 | 22-27 | 25-31 | 1 |
| LDL | 1.019-1.063 | 18-28 | 20-25 | 45-58 | 20-28 | 10-15 | 1 |
| HDL | 1.063-1.210 | 5-15 | 33-57 | 17-40 | 26-46 | 3-15 | 0-6 |
Apolipoproteins are amphipathic proteins on the surface of a lipoprotein particle that help stabilize the lipoprotein structure. They perform a key role in lipoprotein metabolism by serving as receptor ligands, enzyme co-factors, and lipid transport carriers. Apolipoproteins are classified into apolipoprotein A (apo A), apolipoprotein B (apo B), apo C, or apo E. Apolipoproteins can also be divided into two groups on the basis of biological and structural features, namely exchangeable and non-exchangeable apolipoproteins. Apo B-100 is a non-exchangeable protein irreversibly associated with the LDL and VLDL particle, whereas others are transportable proteins. Apo A-I is the major protein component of HDL. Apo C-II is a co-factor of lipoprotein lipase (LPL), which mediates the hydrolysis of TGs in the core of CM and VLDL particles, while apo C-III inhibits the function of LPL. These exchangeable apolipoproteins exist mostly on the surface of CM, VLDL, and HDL particles[7]. The nature and function of the major apolipoproteins are summarized in Table 2.
| Name | Molecular weight (Da) | Origin | Lipoprotein association | Principal function |
| apo A-I | 28016 | Hepatocyte | HDL>>CM | Cofactor of LCAT |
| Intestinal epithelial cell | Prostacyclin stabilizer | |||
| Ligand of SR-B1 | ||||
| apo A-II | 17414 | Hepatocyte | HDL | Inhibits LCAT |
| Intestinal epithelial cell | Inhibits HL | |||
| apo A-IV | 31570 | Intestinal epithelial cell | CM | (Activates LCAT?) |
| (hepatocyte?) | (Activates CETP?) | |||
| apo B-48 | 241000 | Intestinal epithelial cell (splicing variant of apo B-100) | CM and CM remnant | Formation of CM particle |
| apo B-100 | 545000 | Hepatocyte | VLDL, IDL, and LDL | Formation of VLDL/LDL particle |
| Ligand of LDL receptor | ||||
| apo C-I | 6600 | Hepatocyte | CM, VLDL, IDL, and HDL | Inhibits CETP by altering the electric charge of HDL |
| apo C-II | 8800 | Hepatocyte | CM, VLDL, IDL, and HDL | Cofactor of LPL |
| apo C-III | 8750 | Hepatocyte | CM, VLDL, IDL, and HDL | Inhibits LPL and HL |
| Promotes assembly and secretion of VLDL | ||||
| apo C-IV | Hepatocyte | CM, VLDL, IDL, and HDL | Not specified | |
| apo E | 34100 | Hepatocyte | CM, CM remnant, VLDL, IDL and HDL | Ligand of LDL receptor/LDL receptor-related protein |
| Macrophage | Binds to HSPGs |
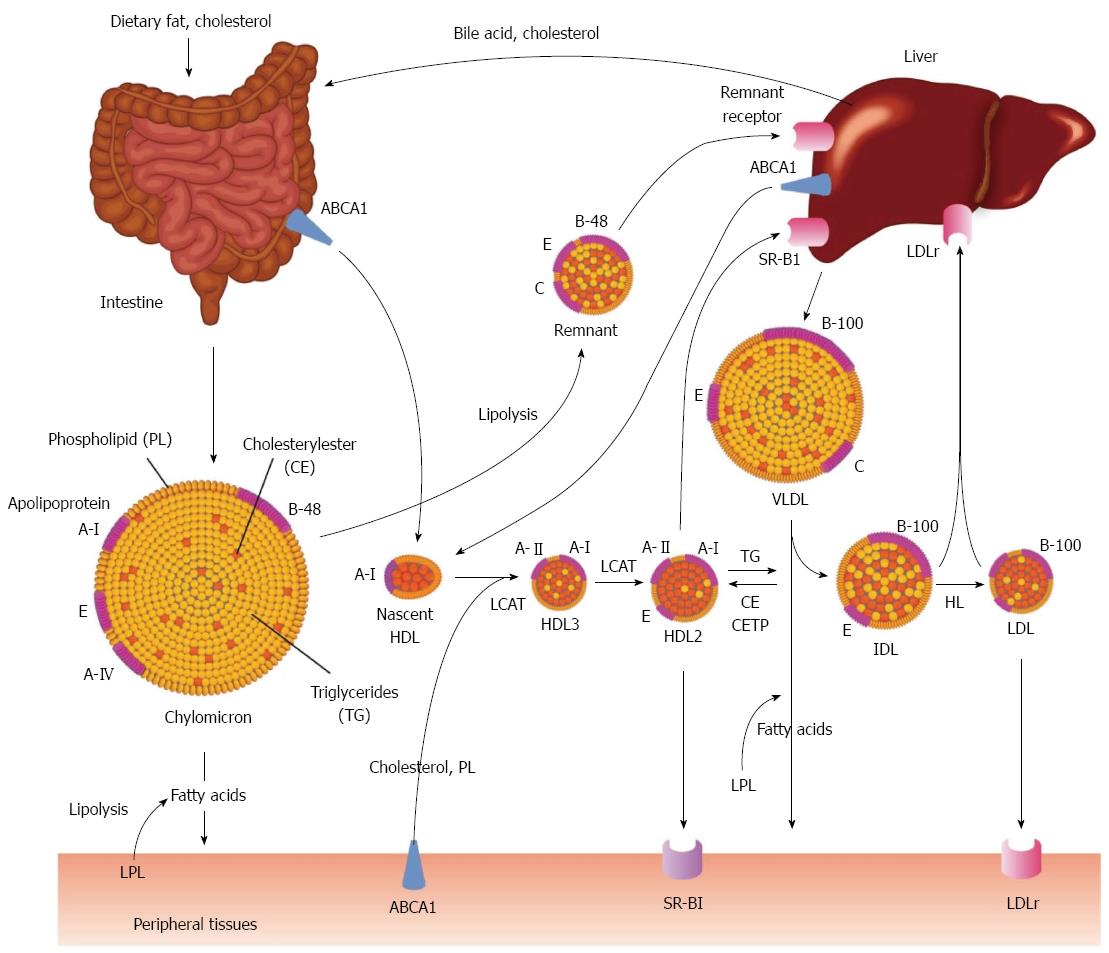
Lipoprotein metabolic pathways consist of exogenous and endogenous pathways[8]. In the exogenous pathway, lipids are absorbed through intestinal epithelial cells, while in the endogenous pathway, lipoproteins are synthesized mainly in the hepatocytes or intestinal epithelial cells.
Exogenous pathway: About 90% of dietary lipid consists of TGs. In contrast, the daily diet contains only 400 mg/d of cholesterol. The major source of cholesterol is bile, in which about 1500 mg of cholesterol is secreted every day. The average absorption rate of cholesterol is about 50%, and non-absorbed cholesterol is lost in the feces. Absorbed lipids are assembled with apo B-48 in the intestinal epithelial cells, which form nascent CMs. Then, nascent CM particles are secreted into lymphatic vessels and flow into systemic circulation via the thoracic duct. Nascent CMs then receive apo C-II and apo E from HDL particles and become mature CMs. CMs are very large and less dense particles. TGs in CMs are hydrolyzed by LPL, which is located on vascular endothelial cells and releases one molecule of monoacylglycerol and two molecules of free fatty acids. They are taken into the tissues, while CMs are degraded into remnants[9]. The CM remnant is attached to the hepatocyte by interaction of apo E with the remnant receptor and is absorbed into hepatocytes.
Endogenous pathway: The liver is the main organ involved in the endogenous pathway. Hepatocytes secrete VLDL particles. Assembling VLDLs begins in the endoplasmic reticulum. In the beginning of VLDL formation, TGs are incorporated by the action of microsomal TG transfer protein (MTP) into a growing particle in which apoB-100 is the major component of an outer surface of the particles. Then, CEs and apo E are incorporated into the particle as well, followed by exocytosis of the nascent VLDL particles into the blood. Secreted nascent VLDL particles acquire more apo E and apo Cs from HDL particles. Mature VLDL particles are catalyzed by the action of LPL. Apo C-II activates LPL, while apo C-III impairs LPL activity and the hepatic uptake of VLDL remnants[10]. Fatty acids released by the degradation of VLDL are mainly incorporated into the muscle or adipose tissue for energy sources or stored as fats.
VLDL particles are consistently produced and secreted from the liver, and 1018 particles are released into the circulation every 24 h. Large and TG super-rich VLDL (VLDL1) is secreted after a meal, while small VLDL2 is secreted during starvation[11]. The catalyzed VLDL named VLDL remnant or intermediate-density lipoprotein (IDL) is incorporated into the liver through the interaction of apo E and remnant receptor, or further hydrolyzed by hepatic lipase (HL).
After hydrolysis by HL, IDLs transform to LDLs, which have high cholesterol content. LDL particles provide cholesterol to peripheral tissues or liver cells via interaction of apoB-100 with LDL receptors (LDLr). LDL particles are attached and internalized by endocytosis and hydrolyzed in lysosomes.
Apo A-I, the major apolipoprotein of HDL, is synthesized and secreted from hepatocytes or intestinal epithelial cells. Apo A-I is attached to ATP-binding cassette transporter A1 (ABCA1), a cellular cholesterol efflux pump, and lipidated by free cholesterol and phospholipids[12]. Apo A-I carries lecithin acyl cholesterol acyltransferase (LCAT), which esterifies cholesterol to CE and makes discoidal nascent HDL. Then, nascent HDL particles change to spherical particles by receiving more CE, and increases in size. Smaller HDL is called HDL3, while larger HDL is named HDL2. Apo A-II, the second most common apolipoprotein in HDL, may present predominantly on the HDL3. Mature HDL2 delivers cholesterol to the peripheral tissues[13]. In this process, HDL2 is de-lipidated by transferring cholesterol to the tissues through the scavenger receptor class B type I (SR-BI). Then, HDL particle returns to the hepatocyte or intestinal epithelial cell, and attaches to ABCA1 to receive surplus cholesterol in these cells (re-lipidation). Alternatively, HDL2 attaches to SR-BI on the hepatocyte for providing cholesterol to the liver (reverse cholesterol transport; RCT). Cholesterol excess in any cell can be removed and transported to the hepatocytes through RCT.
HDL and apo B-related lipoprotein exchange CE for TG by the action of CE transport protein (CETP). If TG content increases in apo B-related lipoprotein, one molecule of CE in HDL is exchanged with one molecule of TG in apo B-related lipoprotein. Then, CE is returned to the hepatocytes through endocytosis via LDLr (indirect RCT). Cholesterol in hepatocytes is excreted into bile directly or after being metabolized to bile acid.
A summary of the lipoprotein metabolism is illustrated in Figure 2.
HCV replication is reported to begin in a membranous web on the endoplasmic reticulum (ER) membrane[14,15]. The membranous web contains non-structural HCV proteins (NS3/4A, 4B, NS5A/5B) and newly synthesized HCV-RNA. Phosphatidylinositol-4-kinase IIIa (PI4KIIIa) affects the generation of the membranous web by altering the phosphorylation status of the HCV NS5A protein[16,17]. An initiation phase of assembly occurs on the cytosolic side of the ER membrane, which interacts with cytosolic lipid droplets (LDs)[18]. HCV core protein is associated with LDs[19] and is then recruited to the HCV assembly site by interacting with NS2 and NS3-4A[20].
In the late assembly steps, a lipid envelope is acquired, and the E1 and E2 envelope glycoproteins are incorporated into virions[21]. The transmembrane protein NS2 plays a critical role in the assembly of virions by mediating the interaction of immature particles with E1/E2 membranous glycoprotein[22,23]. Nascent virus particles combine with pre-VLDLs during maturation[24]. In this manner, lipids in the luminal LDs, apo B-100, apo E, and apo C-I participate in the generation of LVPs, which form true hybrid particles of HCV and VLDL[25]. However, apo B-100 is not an absolute requirement for HCV-LVP morphogenesis. Instead, apo C-I and E are indispensable for the intracellular morphogenesis of HCV-LVP[26-28]. In any case, engagement with the VLDL assembly pathway facilitates virion maturation. In this way, the process of maturation and secretion of HCV particles is linked with VLDL assembly and secretion[22]. More details on the precise process of HCV replication, assembly, and secretion have been summarized in several reviews[5,29-32]. In chronic HCV infection, about 1012 HCV virions are secreted into circulation every 24 h.
Replication of HCV is suppressed by inhibition of MTP, which is a key molecule for the generation of VLDL[33]. Apo B-100 production is interfered by HCV, and over storage of TG leads to hepatic steatosis[34]. Inhibition of apo E, a key molecule of both HCV-LVP[35] and VLDL, impairs the secretion of infectious HCV-LVP[36]. These findings indicate close association between HCV replication and VLDL production, and imply the significance of VLDL as a possible target of anti-HCV therapy.
The metabolism of fatty acids and phospholipids is crucial in HCV replication. Fatty acid synthase is upregulated during HCV replication[37,38]. HCV replication is inhibited by polyunsaturated fatty acids (PUFAs)[39,40] but stimulated by monounsaturated fatty acids[41]. The inhibition of HCV replication by PUFAs may be mediated by the peroxidation of PUFAs, which can be blocked by vitamin E[42] or a reduction in cellular cholesterol level[40]. Meanwhile, HCV replication induces sphingosine kinase 2-mediated peroxidation of PUFAs, which suppress HCV replication[43]. This feedback mechanism may regulate the HCV-replication runaway and participate in long-term perpetuation of HCV infection. These findings strongly suggested that manipulation of lipid metabolism in hepatocytes may be an important therapeutic strategy for impairing HCV replication.
HMG-CoA inhibitors (statins), which inhibit cholesterol synthesis, have been used as adjuvants for anti-HCV therapy[44-46]. Addition of statins to the standard pegylated (peg) IFN and ribavirin therapy for HCV genotype 1 may improve the rate of sustained virological response. As mentioned earlier, increased synthesis of geranylgeranyl pyrophosphate is involved in HCV replication[41]. Statins decrease geranylgeranylation and thus function against HCV infection[47]. In addition, eicosapentaenoic acid (an omega-3 PUFA) and fibrate may also improve the outcome of peg IFN and ribavirin therapy[48,49].
A micro RNA (miRNA) is a small non-coding RNA molecule, which functions on silencing of RNA and regulates post-transcriptional gene expression. miR-122 binds near the 5’ end of the HCV genome and stabilizes HCV RNA, which may participate in positive regulation of HCV replication[50]. miR-122 also upregulates genes participating in the synthesis of cholesterol[51]. Therefore, miR-122 can be an important target of anti-HCV therapy. Treatment of chronic HCV infection by Miravirsen (locked nucleic acid-modified oligonucleotide complementary to miR-122) leads to long-lasting suppression of serum HCV[52,53] accompanied by a decrease of serum cholesterol. miR-27a is preferentially expressed in the HCV-infected liver and regulates lipid metabolism by targeting the lipid synthetic transcription factor retinoid X receptor α (RXRα) and ABCA1. Moreover, miR-27a represses the gene expression of many lipid metabolism-related genes. Suppression of miR-27a increases the cellular lipid content, and increases HCV replication and infectivity[54]. Manipulation of miR-27a may also be a possible target against HCV infection.
In summary, replication of HCV is largely affected by lipid metabolism. Suppression of VLDL production, manipulation of lipid metabolism and miRNA may be a possible target against HCV infection.
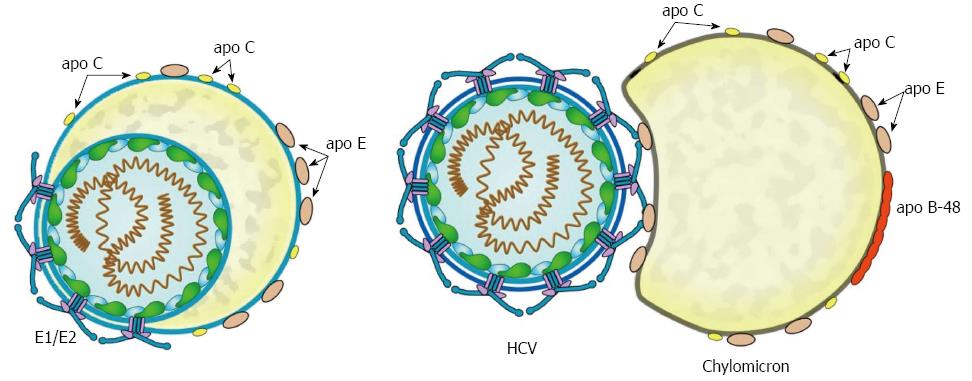
HCV particles have unusually low and heterogeneous buoyant density compared with other enveloped RNA viruses[30,32]. Infectious HCV particles have densities of 1.03-1.10 g/mL. There may be two different type of infectious HCV particles: first, hybrid particles of lipoproteins and HCV virions that share a common envelope (true HCV-LVP), and second, transient association of HCV virions with lipoprotein particles (transient HCV-LVP) (Figure 3). True HCV-LVP may further transiently unite to host lipoprotein particles. The existence of transient HCV-LVP is supported by a report that serum-derived HCV particles are associated with apo B-48-containing CMs[55].
Highly infectious HCV particles have a buoyant density of less than 1.07 g/mL, which corresponds to the density of LDL or VLDL[56], and their average particle size is 73 nm, which corresponds to the size of VLDL1[57]. Thus, physical properties of infectious HCV particles closely resemble those of VLDL particles. Infectious HCV particles have apo E and apo C-I to C-III on the surface, just as VLDLs have[28,58-60]. Due to the physicochemical similarities with lipoproteins, infectious HCV particles have been named HCV-LVPs.
In a study of chronic HCV G1 infection, the quantity of plasma HCV-LVPs correlated with plasma TG/HDL-cholesterol ratio, HOMA-IR, and non-response to IFN-based antiviral therapy, suggesting the importance of lipid/glucose metabolism in the generation and persistence of circulating HCV-LVPs[57]. An HCV-LVP may contain more than 300 molecules of apo E on the surface, while VLDL has only 5-7 molecules[59]. Therefore, apo E may be one of the key molecules for determining the direction of circulating HCV-LVPs.
The density of circulating HCV is dramatically altered after oral intake of TGs[61]. HCV-LVPs with a density less than 1.025 g/mL increase by 26-fold after the intake of a fatty meal, together with a rise in TG-super-rich VLDL1 and CM. This very low-density HCV-LVP bears apo B-48 or apo B-100 and very rapidly increases after meal; thereafter, it is rapidly cleared from circulation. Marked increase of postprandial HCV-LVPs indicates that non-infectious HCV particles can be transferred onto TG-super-rich VLDL1 or CM by a similar manner that transfers exchangeable apolipoproteins from HDL to VLDL1 or CM postprandially. This study clearly indicates that HCV could be transiently associated with TG-rich lipoprotein particles after meal, as shown in Figure 3.
HCV-LVP can be catalyzed by LPL akin to the catalysis of CM and VLDL. This catalyzed HCV-LVP may lose infectivity. Infectivity of HCV is significantly inhibited by exogenous LPL in vitro[62]. In addition, not only LPL but also HL reduces the infectivity of HCV by catalyzing HCV-LVP[63]. Abundant apo C-III on HCV-LVP or VLDL reduces LPL-mediated inhibition of HCV infection[64].
The activity of HL is affected by HCV infection, and the transcription level of HL is strongly downregulated in HCV G1b-infected livers compared to that in hepatitis B virus (HBV)-infected or non-alcoholic steatohepatitis (NASH) livers[65]. In addition, posttranscriptional regulation mechanism of HL activity is important for activation of HL[66]. HL is anchored in cell-surface heparan sulfate proteoglycans (HSPGs) on the hepatocytes, and is inactivated. After a meal, HL is dissociated from HSPGs and binds to HDL. Apo A-II may enhance the displacement of HL to HDL, and inhibit the activation of HL together with apo A-I[67,68]. Apo E interferes with the association of HL with HDL, while stimulates the activity of HL on HDL. Therefore, the balance of apo E, apo A-I, and A-II might be responsible for HL activity.
Recent evidence indicates that there are many “empty” HCV particles that express E1/E2 glycoprotein on the surface, but do not contain the core protein and HCV-RNA in the blood of HCV patients. “Empty” HCV particles may be 106 times more numerous than infectious HCV-LVPs[56], and may have a role on host lipoprotein metabolism. In addition, “empty” HCV particles could be bound to anti-E1 and E2-specific antibodies[56]. Therefore, infectious HCV-LVP may be hidden among the numerous “empty” HCV particles. This might be one of the mechanisms enabling HCV to escape from host immunological surveillance system.
In summary, HCV-LVP demonstrates dynamic changes in circulation and loses infectivity by LPL or HL. In view of anti-HCV therapy, the manipulation of HCV-LVP may not be an excellent therapeutic strategy because there are dynamic transformations between non-infectious HCV and HCV-LVP. In addition, E1/E2 envelope proteins on the surface of infectious HCV are hidden among the numerous “empty” HCV particles. This finding renders E1/E2 envelope proteins less amenable as promising target molecules.
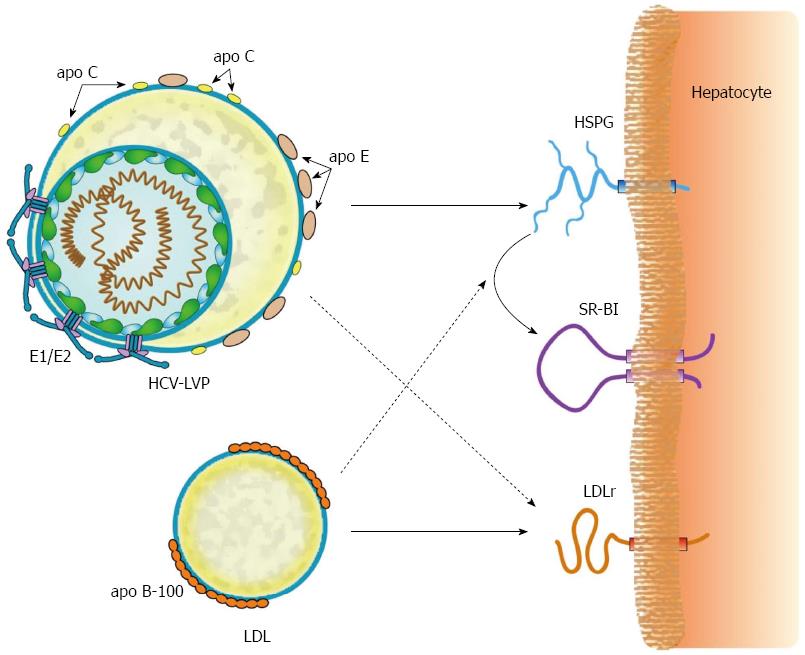
HCV entry into human hepatocytes is a complex, multistep process involving a couple of receptors. The full description of the mechanisms of this process is beyond the scope of this review. Details are available in numerous related reviews[5,29,32,69-71]. As a first step of HCV entry, HCV-LVP attaches to the liver cell surface by adhering to HSPGs, which are abundant on the liver cell surface. HCV-LVP uses syndecan-1 or syndecan-4 HSPGs for the initiation of entry into hepatocytes[72]. Apo E on the surface of HCV-LVP and HCV envelope proteins adheres to HSPGs[73-76]. The minimal unit required for infection is a decasaccharide[77].
The binding of apo B-100 on the surface of HCV-LVP to LDLr was proposed to involve the penetration of HCV into hepatocytes[78,79]. However, a productive entry process does not seem to operate following the binding of HCV-LVP to LDLr, but rather a non-productive pathway that degrades HCV particles is elicited[80]. Though apo E on the surface of HCV-LVP can participate in the binding to LDLr, HCV-LVP competes with the excess of LDL or VLDL for occupation of LDLr.
Alternatively, productive HCV entry arises by interaction with SR-BI[81,82], a receptor for the uptake of cholesterol from HDL particles (Figure 4). The precise process of HCV adhesion to SR-BI is a complex[83], multistep one[84]. In brief, the initial step might be an interaction between apo E on the HCV-LVP and SR-BI. In the second step, the lipid transfer activity of SR-BI (entry of cholesterol from mature HDL) facilitates HCV entry by binding of specific residues in hypervariable region 1 of E2 and SR-BI[85]. Thereafter, exposed determinants in E2 bind to CD81[86] and move to tight junction protein claudin 1. Claudin 1 interacts with CD81 and contributes to the next step of HCV internalization[87]. Finally, internalization of HCV is completed by clathrin-mediated endocytosis[88].
Transferrin receptor 1 and other receptors may act as factors promoting HCV entry[89,90]. Niemann-Pick C1-like 1 (NPC1L1) was reported as a receptor for HCV entry[91]. However, the significance of NPC1L1 is controversial because this molecule is expressed only on the apical side of the hepatocyte[92].
As an anti-HCV therapy target, apo E is one of the most promising target molecules for disrupting HCV infection. Apo E is preferentially distributed on the surface of HCV-LVP and plays a critical role in the entry of HCV. Under short-term suppression of apo E, serious adverse effects may not arise. Furthermore, SR-BI is a potential target molecule for anti-HCV therapy[84], because SR-BI is essential for productive entry of HCV.
ABCA1 is also a potential target molecule. Recently, the critical role of ABCA1 on HCV-cell fusion and HCV entry was reported[93]. ABCA1 mediates cholesterol efflux from hepatocytes to extracellular apo A-I, which initiates the formation of HDL. Upregulation of ABCA1 expression and its cholesterol efflux function[94] impairs HCV infection and decreases virus production. Stimulation of the ABCA1-dependent cholesterol efflux pathway disrupts membrane cholesterol homeostasis, thereby inhibiting HCV-cell fusion and entry.
In summary, apo E on the surface of HCV-LVP is the key molecule for harboring HCV-LVPs on the surface of hepatocytes and for the initial attachment of HCV-LVPs to SR-BI. HCV entry is inhibited by cholesterol efflux via ABCA1 and is facilitated by the activity of SDR-BI. These findings suggest a close connection between HDL-related cholesterol metabolism and the persistence of HCV infection. One of the chief mediators determining the influx and efflux of cholesterol might be the intracellular concentration of cholesterol.
Low serum cholesterol (or low LDL-cholesterol) level is a well-known characteristic feature of chronic HCV infection[95,96]. The magnitude of altered lipid metabolism depends on host IFNL3 polymorphism and HCV genotype. The CC rs12979860 polymorphism (major IFNL3 genotype) is significantly associated with higher serum cholesterol and LDL-cholesterol levels in HCV genotype 1 (G1) patients but not in patients with genotype 3 (G3), genotype 4, or non-infected controls[97]. Disturbed lipids are corrected after the eradication of HCV[98]. Therefore, HCV infection by itself, or inflammation and fibrosis accompanied by chronic HCV infection might be affected by the disturbance of lipoprotein metabolism. In this regard, a study from Japan clearly indicated that HCV itself directly caused hypolipidemia[99].
Development of hepatic steatosis in HCV core transgenic mice indicates a tight association between HCV infection and the generation of steatosis[100]. Furthermore, HCC development after a long-term observation of HCV transgenic indicated carcinogenic potential of the HCV core protein, owing to persistent disturbance of lipid metabolism[101].
Steatosis in chronic hepatitis C (CHC) may associate with inflammation and progression of liver fibrosis. Steatosis is more tightly associated with inflammation in CHC than in chronic hepatitis B (CHB)[102]. In addition, PNPLA3 genetic variants and body mass index are involved in hepatic steatosis in non-obese CHC[103]. Advanced fibrosis in HCV patients is associated with concurrent diabetes mellitus, liver steatosis, and obesity[104]. Furthermore, the PNPLA3 variant may contribute to the severity of CHC[105] and HCC development[106].
These findings suggest that liver steatosis and fibrosis might be strongly influenced by disturbed host metabolic factors that can induce NASH. Therefore, correction of disturbed metabolic factors is important for preventing the progression of HCV-related liver disease.
Disturbed serum lipoprotein and apolipoprotein levels in chronic HCV infection may be affected by deteriorated liver function induced by HCV. However, a decrease in the serum VLDL-TG level and the reciprocal increase in non-VLDL-TG were found in very early stages of chronic HCV-G1b infection[107]. In addition, Moriya et al[108] reported that apo B, apo C-II, and apo C-III levels were significantly reduced in HCV G1b infection, while apo A-I, A-II, and E levels were similar in patients infected with HCV G1b, G2a, or HBV. These findings suggest that HCV infection by itself may be involved in the disturbance of serum lipoproteins/apolipoproteins. Therefore, examining serum lipoproteins/apolipoproteins is beneficial for monitoring disturbed lipid metabolism induced by HCV.
As mentioned above, low LDL-cholesterol level is one of the features of chronic HCV infection. Nevertheless, current evidence indicates that HCV by itself or factors associated with HCV infection can promote the occurrence and progression of atherosclerosis[109-111]. A recent meta-analysis indicates that HCV infection is significantly associated with carotid atherosclerosis independent of classical risk factors such as type 2 diabetes (or insulin resistance) and hepatic steatosis[109]. There are various factors possibly associated with atherosclerosis in CHC, especially elevation of proinflammatory cytokines in CHC may be participated in the progression of atherosclerosis.
In a view of anomalous lipoproteins, we found that an increase in TG in the small-VLDL fraction (equivalent to VLDL remnant) is a characteristic feature of chronic HCV G1b infection, independent of other metabolic factors [manuscript in preparation]. This elevation is not found in the subjects cured of HCV infection. As TG-rich lipoprotein remnants are unequivocally mentioned as atherogenic[112,113], our finding of the increasing atherogenic VLDL remnant may be involved in the promotion and progression of atherosclerosis in chronic HCV infection.
In contrast to VLDL and LDL, the interaction of HDL with chronic HCV infection is poorly understood. However, SR-BI is a receptor for both HCV and matured HDL, and is a possible target for anti-HCV therapy[83]. In HCV G3 infection, the HCV-LVP load correlates inversely with HDL cholesterol[114], but such a correlation is not found in HCV G1 infection. This finding may indicate that there is a difference in the role of lipoprotein among infections by different HCV genotypes. Although apo B-associated cholesterol level has received attention as a determinant of treatment outcome in patients receiving IFN plus ribavirin[115], dyslipoproteinemia in HCV infection is not limited to apo B-related lipoprotein, because the metabolic pathways of HDL and apo B-related lipoprotein are closely related to each other. In the near future, the total picture of dyslipoproteinemia associated with the different HCV genotypes is expected to be elucidated more clearly.
As described earlier, apolipoprotein plays a key role in lipoprotein metabolism. Therefore, monitoring apolipoproteins may be beneficial for understanding the anomalous lipoprotein metabolism in chronic HCV infection. The amphipathic α-helices of transportable apolipoproteins are crucial for maintaining the shape of HCV-LVP[60]. These exchangeable apolipoproteins can easily be transported between lipoprotein and HCV-LVP. Therefore, the dynamics of exchangeable apolipoprotein in HCV patients on stabilizing the structure of circulating HCV-LVP cannot be ignored.
Serum levels of apo B (apo B-100) have been examined in connection with the persistence of HCV infection and treatment outcome of IFN-based therapy[116,117]. Serum apo B level is strongly correlated with LDL-cholesterol, and is partially determined by the amino acid changes of core and NS5A protein[116,118], suggesting a close interaction between HCV replication and lipoprotein production in the liver.
Apo E is one of the key molecules of HCV assembly and entry into hepatocytes. Among the three isotypes of apo E (E2, E3, and E4)[119], E3 is the most frequent. E2 variant binds poorly to cell surface receptors[120], and is associated with a 3-5-fold reduction in the risk of chronic HCV infection[121], suggesting that this allele protects against viral persistence via defective binding of HCV-LVPs to the receptors involved in the entry of HCV-LVPs[122]. Association of E4 with atherosclerosis, Alzheimer disease, and degenerative diseases has been implicated. The E4 allele is positively associated with higher levels of vitamin D. High vitamin D levels are also linked to a favorable outcome of antiviral therapy for chronic HCV infection[123]. The apo E4 allele might have a protective effect against severe liver damage caused by HCV[124], while being associated with poor treatment response in HCV G1b patients[125]. Unfortunately, the connection between apo E4 and serum vitamin D in these patients has not been studied. Moreover, in an apo E3/E3 homozygote, fibrosis progression might be accelerated in chronic HCV with persistently normal transaminases[126]. However, the concept that apo E isoforms may play critical roles in the morbidity of chronic HCV infection has not been widely accepted.
Low levels of circulating apo E are reported to associate with favorable response to IFN-based therapy and with low HCV-LVP level in HCV G1 infection[127]. However, the serum apo E level is negatively correlated with the quantity of HCV-LVP in HCV G3[114]. Therefore, the manner of interaction with host lipid metabolism may vary according to the HCV genotype. Our recent study suggested that serum apo E level is elevated in HCV G1b patients and not in patients with HCV G2[128].
With regard to apo As, apo A-I has been shown to be involved in HCV RNA replication and virion production[129]. However, we did not find any changes in serum apo A-I levels in HCV G1b and G2 patients in a previous study[128]. Apo A-II binds directly to the C-terminal domain of the HCV core protein[130]. Apo A-II was significantly associated with HOMA-IR and leptin concentrations in HCV patients[131]. Therefore, apo A-II may contribute to hepatic steatosis progression in HCV infection. In our study, however, the serum level of apo A-II seemed to increase in HCV G1b patients without a concomitant increase in BMI[128]. Further studies are needed to determine the significance of serum apo A-II in chronic HCV infection.
Apo C-I associates with s morphogenesis of HCV virions, HCV replication[28] and membrane fusion of HCV[132]. Human apo C-I accounts for the ability of HDLs to inhibit the CETP activity[133]. However, the information on serum level of apo C-I in HCV infection is not available. Serum apo C-III was reported to be higher in donors with resolved HCV infection than in donors with chronic infection[134]. Apo C-III might be a candidate biomarker associated with HCV-related progression of hepatic fibrosis. Relative to other lipoproteins, low serum apo C-III levels are reported to have the strongest association with chronic versus cleared infection and a decline with increasing severity of hepatic fibrosis[134]. LPL is an anti-HCV factor that hydrolyzes HCV-LVPs, and apo C-III on HCV-LVPs reverses the LPL-mediated inhibition of HCV infection[64]. In contrast, apo C-II gives rise to the catalytic activity of LPL and may promote LPL-mediated inhibition of HCV infection. Therefore, the balance of apo C-II and apo C-III may determine the infectivity of HCV-LVPs.
In our study, we found that both serum apo C-II and apo C-III decreased in chronic HCV infection and advanced liver fibrosis. Although serum apo C-II and apo C-III levels were strongly correlated with each other, multiple regression analysis revealed that a decline in serum apo C-II alone significantly contributes to advanced fibrosis in HCV G1b infection[128]. Therefore, relative depletion of apo C-II in chronic HCV infection with advanced fibrosis might help protect HCV from LPL-mediated loss of infectivity and contribute to persistent infection of HCV.
In a previous study, apo C-II and apo C-III levels were significantly reduced in HCV G1b infection compared with G2a infection with similar liver disease progression[108]. However, in our multivariate analysis, apo A-II and E significantly increased in HCV G1b infection compared with HCV G2 infection, whereas apo C-II and apo C-III decreased in HCV infection regardless of HCV genotype[128]. This discrepancy remains as one of the issues that should be solved in the future.
In summary, the significance and the precise mechanisms of anomalous circulating apolipoprotein levels in HCV patients, especially the differences among infections by different HCV genotypes, have not been fully elucidated yet.
HCV is a unique virus, which ingeniously uses the host’s lipoprotein metabolism for persistence of infection. HCV and lipid interact closely at the critical steps of HCV life cycle (replication, assembly, secretion, and entry into hepatocyte). Disturbed lipid metabolism is tightly associated with inflammatory activity, progression of liver fibrosis, and HCC development. Circulating lipoprotein particles transport lipids throughout the body and carry out important functions for lipid metabolism. Our recent knowledge of the close interaction between HCV and lipoprotein suggests that a specific molecule on the lipoprotein could be a target for anti-HCV therapy. Our study on lipoproteins suggests that the increase in the atherogenic VLDL remnant may contribute to the progression of atherosclerosis in chronic HCV infection.
Monitoring of dyslipoproteinemia and correction of disturbed lipoprotein metabolism in chronic HCV patients might be important aspects in a strategy for predicting and preventing the generation of liver cirrhosis and HCC. Until now, the whole aspect of disturbed lipoprotein metabolism in chronic HCV infection had not been clarified. Further understanding of disturbed lipoprotein metabolism might unravel novel targets for anti-HCV therapy and help prevent complications of HCV infection.
P- Reviewer: Licata A, Mihaila RG, Tanaka N S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 424] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 2. | Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Siebert U, Sroczynski G; German Hepatitis C Model GEHMO Group; HTA Expert Panel on Hepatitis C. Effectiveness and cost-effectiveness of initial combination therapy with interferon/peginterferon plus ribavirin in patients with chronic hepatitis C in Germany: a health technology assessment commissioned by the German Federal Ministry of Health and Social Security. Int J Technol Assess Health Care. 2005;21:55-65. [PubMed] |
| 4. | Gogela NA, Lin MV, Wisocky JL, Chung RT. Enhancing our understanding of current therapies for hepatitis C virus (HCV). Curr HIV/AIDS Rep. 2015;12:68-78. [PubMed] |
| 5. | Popescu CI, Riva L, Vlaicu O, Farhat R, Rouillé Y, Dubuisson J. Hepatitis C virus life cycle and lipid metabolism. Biology (Basel). 2014;3:892-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Bassendine MF, Sheridan DA, Bridge SH, Felmlee DJ, Neely RD. Lipids and HCV. Semin Immunopathol. 2013;35:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Bassendine MF, Sheridan DA, Felmlee DJ, Bridge SH, Toms GL, Neely RD. HCV and the hepatic lipid pathway as a potential treatment target. J Hepatol. 2011;55:1428-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Lusis AJ, Fogelman AM, Fonarow GC. Genetic basis of atherosclerosis: part I: new genes and pathways. Circulation. 2004;110:1868-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Giammanco A, Cefalù AB, Noto D, Averna MR. The pathophysiology of intestinal lipoprotein production. Front Physiol. 2015;6:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kawakami A, Yoshida M. Apolipoprotein CIII links dyslipidemia with atherosclerosis. J Atheroscler Thromb. 2009;16:6-11. [PubMed] |
| 11. | Gill JM, Brown JC, Bedford D, Wright DM, Cooney J, Hughes DA, Packard CJ, Caslake MJ. Hepatic production of VLDL1 but not VLDL2 is related to insulin resistance in normoglycaemic middle-aged subjects. Atherosclerosis. 2004;176:49-56. [PubMed] |
| 12. | Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053-33058. [PubMed] |
| 13. | Grow TE, Fried M. Interchange of apoprotein components between the human plasma high density lipoprotein subclasses HDL2 and HDL3 in vitro. J Biol Chem. 1978;253:8034-8041. [PubMed] |
| 14. | Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974-5984. [PubMed] |
| 15. | Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, Bienz K, Moradpour D. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol. 2003;77:5487-5492. [PubMed] |
| 16. | Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, Kaderali L, Poenisch M, Blankenburg H, Hiet MS. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 406] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Reiss S, Harak C, Romero-Brey I, Radujkovic D, Klein R, Ruggieri A, Rebhan I, Bartenschlager R, Lohmann V. The lipid kinase phosphatidylinositol-4 kinase III alpha regulates the phosphorylation status of hepatitis C virus NS5A. PLoS Pathog. 2013;9:e1003359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. [PubMed] |
| 19. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. [PubMed] |
| 20. | Counihan NA, Rawlinson SM, Lindenbach BD. Trafficking of hepatitis C virus core protein during virus particle assembly. PLoS Pathog. 2011;7:e1002302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Vieyres G, Dubuisson J, Pietschmann T. Incorporation of hepatitis C virus E1 and E2 glycoproteins: the keystones on a peculiar virion. Viruses. 2014;6:1149-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Suzuki R, Matsuda M, Watashi K, Aizaki H, Matsuura Y, Wakita T, Suzuki T. Signal peptidase complex subunit 1 participates in the assembly of hepatitis C virus through an interaction with E2 and NS2. PLoS Pathog. 2013;9:e1003589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Shanmugam S, Saravanabalaji D, Yi M. Detergent-resistant membrane association of NS2 and E2 during hepatitis C virus replication. J Virol. 2015;89:4562-4574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848-5853. [PubMed] |
| 25. | Boyer A, Dumans A, Beaumont E, Etienne L, Roingeard P, Meunier JC. The association of hepatitis C virus glycoproteins with apolipoproteins E and B early in assembly is conserved in lipoviral particles. J Biol Chem. 2014;289:18904-18913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Coller KE, Heaton NS, Berger KL, Cooper JD, Saunders JL, Randall G. Molecular determinants and dynamics of hepatitis C virus secretion. PLoS Pathog. 2012;8:e1002466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Lee JY, Acosta EG, Stoeck IK, Long G, Hiet MS, Mueller B, Fackler OT, Kallis S, Bartenschlager R. Apolipoprotein E likely contributes to a maturation step of infectious hepatitis C virus particles and interacts with viral envelope glycoproteins. J Virol. 2014;88:12422-12437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Meunier JC, Russell RS, Engle RE, Faulk KN, Purcell RH, Emerson SU. Apolipoprotein c1 association with hepatitis C virus. J Virol. 2008;82:9647-9656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle--an update. J Hepatol. 2014;61:S3-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Lindenbach BD. Virion assembly and release. Curr Top Microbiol Immunol. 2013;369:199-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Bartenschlager R, Penin F, Lohmann V, André P. Assembly of infectious hepatitis C virus particles. Trends Microbiol. 2011;19:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 32. | Lindenbach BD, Rice CM. The ins and outs of hepatitis C virus entry and assembly. Nat Rev Microbiol. 2013;11:688-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 33. | Mirandola S, Bowman D, Hussain MM, Alberti A. Hepatic steatosis in hepatitis C is a storage disease due to HCV interaction with microsomal triglyceride transfer protein (MTP). Nutr Metab (Lond). 2010;7:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Mancone C, Montaldo C, Santangelo L, Di Giacomo C, Costa V, Amicone L, Ippolito G, Pucillo LP, Alonzi T, Tripodi M. Ferritin heavy chain is the host factor responsible for HCV-induced inhibition of apoB-100 production and is required for efficient viral infection. J Proteome Res. 2012;11:2786-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Jiang J, Luo G. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol. 2009;83:12680-12691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Hueging K, Doepke M, Vieyres G, Bankwitz D, Frentzen A, Doerrbecker J, Gumz F, Haid S, Wölk B, Kaderali L. Apolipoprotein E codetermines tissue tropism of hepatitis C virus and is crucial for viral cell-to-cell transmission by contributing to a postenvelopment step of assembly. J Virol. 2014;88:1433-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Yang W, Hood BL, Chadwick SL, Liu S, Watkins SC, Luo G, Conrads TP, Wang T. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Nasheri N, Joyce M, Rouleau Y, Yang P, Yao S, Tyrrell DL, Pezacki JP. Modulation of fatty acid synthase enzyme activity and expression during hepatitis C virus replication. Chem Biol. 2013;20:570-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Leu GZ, Lin TY, Hsu JT. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem Biophys Res Commun. 2004;318:275-280. [PubMed] |
| 40. | Pollock S, Nichita NB, Böhmer A, Radulescu C, Dwek RA, Zitzmann N. Polyunsaturated liposomes are antiviral against hepatitis B and C viruses and HIV by decreasing cholesterol levels in infected cells. Proc Natl Acad Sci USA. 2010;107:17176-17181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. [PubMed] |
| 42. | Huang H, Chen Y, Ye J. Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc Natl Acad Sci USA. 2007;104:18666-18670. [PubMed] |
| 43. | Yamane D, McGivern DR, Wauthier E, Yi M, Madden VJ, Welsch C, Antes I, Wen Y, Chugh PE, McGee CE. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nat Med. 2014;20:927-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Selic Kurincic T, Lesnicar G, Poljak M, Meglic Volkar J, Rajter M, Prah J, Baklan Z, Kotar T, Maticic M. Impact of added fluvastatin to standard-of-care treatment on sustained virological response in naïve chronic hepatitis C Patients infected with genotypes 1 and 3. Intervirology. 2014;57:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Bader T, Hughes LD, Fazili J, Frost B, Dunnam M, Gonterman A, Madhoun M, Aston CE. A randomized controlled trial adding fluvastatin to peginterferon and ribavirin for naïve genotype 1 hepatitis C patients. J Viral Hepat. 2013;20:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Atsukawa M, Tsubota A, Kondo C, Itokawa N, Narahara Y, Nakatsuka K, Hashimoto S, Fukuda T, Matsushita Y, Kidokoro H. Combination of fluvastatin with pegylated interferon/ribavirin therapy reduces viral relapse in chronic hepatitis C infected with HCV genotype 1b. J Gastroenterol Hepatol. 2013;28:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Sidorkiewicz M, Józwiak B, Durys B, Majda-Stanislawska E, Piekarska A, Kosciuk N, Ciechowicz J, Majewska E, Bartkowiak J. Mevalonate pathway modulation is associated with hepatitis C virus RNA presence in peripheral blood mononuclear cells. Virus Res. 2009;145:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Sheridan DA, Bridge SH, Crossey MM, Felmlee DJ, Fenwick FI, Thomas HC, Neely RD, Taylor-Robinson SD, Bassendine MF. Omega-3 fatty acids and/or fluvastatin in hepatitis C prior non-responders to combination antiviral therapy - a pilot randomised clinical trial. Liver Int. 2014;34:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 49. | Grammatikos G, Farnik H, Bon D, Böhlig A, Bader T, Berg T, Zeuzem S, Herrmann E. The impact of antihyperlipidemic drugs on the viral load of patients with chronic hepatitis C infection: a meta-analysis. J Viral Hepat. 2014;21:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Henke JI, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, Jünemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300-3310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 527] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 51. | Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1638] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 52. | Janssen HL, Kauppinen S, Hodges MR. HCV infection and miravirsen. N Engl J Med. 2013;369:878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res. 2014;111:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 54. | Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, Sakai A, Okada H, Watanabe R, Murakami S. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol. 2013;87:5270-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 55. | Diaz O, Delers F, Maynard M, Demignot S, Zoulim F, Chambaz J, Trépo C, Lotteau V, André P. Preferential association of Hepatitis C virus with apolipoprotein B48-containing lipoproteins. J Gen Virol. 2006;87:2983-2991. [PubMed] |
| 56. | Scholtes C, Ramière C, Rainteau D, Perrin-Cocon L, Wolf C, Humbert L, Carreras M, Guironnet-Paquet A, Zoulim F, Bartenschlager R. High plasma level of nucleocapsid-free envelope glycoprotein-positive lipoproteins in hepatitis C patients. Hepatology. 2012;56:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Bridge SH, Sheridan DA, Felmlee DJ, Nielsen SU, Thomas HC, Taylor-Robinson SD, Neely RD, Toms GL, Bassendine MF. Insulin resistance and low-density apolipoprotein B-associated lipoviral particles in hepatitis C virus genotype 1 infection. Gut. 2011;60:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Merz A, Long G, Hiet MS, Brügger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem. 2011;286:3018-3032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 59. | Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418-2428. [PubMed] |
| 60. | Fukuhara T, Wada M, Nakamura S, Ono C, Shiokawa M, Yamamoto S, Motomura T, Okamoto T, Okuzaki D, Yamamoto M. Amphipathic α-helices in apolipoproteins are crucial to the formation of infectious hepatitis C virus particles. PLoS Pathog. 2014;10:e1004534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Felmlee DJ, Sheridan DA, Bridge SH, Nielsen SU, Milne RW, Packard CJ, Caslake MJ, McLauchlan J, Toms GL, Neely RD. Intravascular transfer contributes to postprandial increase in numbers of very-low-density hepatitis C virus particles. Gastroenterology. 2010;139:1774-1783, 1783.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Andréo U, Maillard P, Kalinina O, Walic M, Meurs E, Martinot M, Marcellin P, Budkowska A. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell Microbiol. 2007;9:2445-2456. [PubMed] |
| 63. | Shimizu Y, Hishiki T, Sugiyama K, Ogawa K, Funami K, Kato A, Ohsaki Y, Fujimoto T, Takaku H, Shimotohno K. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCV-associated lipoproteins. Virology. 2010;407:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Sun HY, Lin CC, Lee JC, Wang SW, Cheng PN, Wu IC, Chang TT, Lai MD, Shieh DB, Young KC. Very low-density lipoprotein/lipo-viro particles reverse lipoprotein lipase-mediated inhibition of hepatitis C virus infection via apolipoprotein C-III. Gut. 2013;62:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Shinohara Y, Imajo K, Yoneda M, Tomeno W, Ogawa Y, Fujita K, Kirikoshi H, Takahashi J, Funakoshi K, Ikeda M. Hepatic triglyceride lipase plays an essential role in changing the lipid metabolism in genotype 1b hepatitis C virus replicon cells and hepatitis C patients. Hepatol Res. 2013;43:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Chatterjee C, Sparks DL. Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am J Pathol. 2011;178:1429-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 67. | Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J Lipid Res. 2004;45:849-858. [PubMed] |
| 68. | Ramsamy TA, Neville TA, Chauhan BM, Aggarwal D, Sparks DL. Apolipoprotein A-I regulates lipid hydrolysis by hepatic lipase. J Biol Chem. 2000;275:33480-33486. [PubMed] |
| 69. | Zhu YZ, Qian XJ, Zhao P, Qi ZT. How hepatitis C virus invades hepatocytes: the mystery of viral entry. World J Gastroenterol. 2014;20:3457-3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Vercauteren K, Mesalam AA, Leroux-Roels G, Meuleman P. Impact of lipids and lipoproteins on hepatitis C virus infection and virus neutralization. World J Gastroenterol. 2014;20:15975-15991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Douam F, Lavillette D, Cosset FL. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci. 2015;129:63-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Lefèvre M, Felmlee DJ, Parnot M, Baumert TF, Schuster C. Syndecan 4 is involved in mediating HCV entry through interaction with lipoviral particle-associated apolipoprotein E. PLoS One. 2014;9:e95550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, Van Kuppevelt TH, Depla E. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003-41012. [PubMed] |
| 74. | Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80:10579-10590. [PubMed] |
| 75. | Jiang J, Cun W, Wu X, Shi Q, Tang H, Luo G. Hepatitis C virus attachment mediated by apolipoprotein E binding to cell surface heparan sulfate. J Virol. 2012;86:7256-7267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Koutsoudakis G, Dragun J, Pérez-Del-Pulgar S, Coto-Llerena M, Mensa L, Crespo G, González P, Navasa M, Forns X. Interplay between basic residues of hepatitis C virus glycoprotein E2 with viral receptors, neutralizing antibodies and lipoproteins. PLoS One. 2012;7:e52651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Xu Y, Martinez P, Séron K, Luo G, Allain F, Dubuisson J, Belouzard S. Characterization of hepatitis C virus interaction with heparan sulfate proteoglycans. J Virol. 2015;89:3846-3858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Monazahian M, Böhme I, Bonk S, Koch A, Scholz C, Grethe S, Thomssen R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J Med Virol. 1999;57:223-229. [PubMed] |
| 79. | Wünschmann S, Medh JD, Klinzmann D, Schmidt WN, Stapleton JT. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J Virol. 2000;74:10055-10062. [PubMed] |
| 80. | Albecka A, Belouzard S, Op de Beeck A, Descamps V, Goueslain L, Bertrand-Michel J, Tercé F, Duverlie G, Rouillé Y, Dubuisson J. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 2012;55:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 81. | Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624-41630. [PubMed] |
| 82. | Catanese MT, Graziani R, von Hahn T, Moreau M, Huby T, Paonessa G, Santini C, Luzzago A, Rice CM, Cortese R. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol. 2007;81:8063-8071. [PubMed] |
| 83. | Vercauteren K, Van Den Eede N, Mesalam AA, Belouzard S, Catanese MT, Bankwitz D, Wong-Staal F, Cortese R, Dubuisson J, Rice CM. Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanized mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology. 2014;60:1508-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Zahid MN, Turek M, Xiao F, Thi VL, Guérin M, Fofana I, Bachellier P, Thompson J, Delang L, Neyts J. The postbinding activity of scavenger receptor class B type I mediates initiation of hepatitis C virus infection and viral dissemination. Hepatology. 2013;57:492-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 85. | Dao Thi VL, Granier C, Zeisel MB, Guérin M, Mancip J, Granio O, Penin F, Lavillette D, Bartenschlager R, Baumert TF. Characterization of hepatitis C virus particle subpopulations reveals multiple usage of the scavenger receptor BI for entry steps. J Biol Chem. 2012;287:31242-31257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Sharma NR, Mateu G, Dreux M, Grakoui A, Cosset FL, Melikyan GB. Hepatitis C virus is primed by CD81 protein for low pH-dependent fusion. J Biol Chem. 2011;286:30361-30376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Harris HJ, Farquhar MJ, Mee CJ, Davis C, Reynolds GM, Jennings A, Hu K, Yuan F, Deng H, Hubscher SG. CD81 and claudin 1 coreceptor association: role in hepatitis C virus entry. J Virol. 2008;82:5007-5020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964-6972. [PubMed] |
| 89. | Martin DN, Uprichard SL. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc Natl Acad Sci USA. 2013;110:10777-10782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 90. | Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 569] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 91. | Sainz B, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18:281-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 92. | Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 93. | Bocchetta S, Maillard P, Yamamoto M, Gondeau C, Douam F, Lebreton S, Lagaye S, Pol S, Helle F, Plengpanich W. Up-regulation of the ATP-binding cassette transporter A1 inhibits hepatitis C virus infection. PLoS One. 2014;9:e92140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R. Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res. 2004;45:1410-1417. [PubMed] |
| 95. | Mihm S, Fayyazi A, Hartmann H, Ramadori G. Analysis of histopathological manifestations of chronic hepatitis C virus infection with respect to virus genotype. Hepatology. 1997;25:735-739. [PubMed] |
| 96. | Hofer H, Bankl HC, Wrba F, Steindl-Munda P, Peck-Radosavljevic M, Osterreicher C, Mueller C, Gangl A, Ferenci P. Hepatocellular fat accumulation and low serum cholesterol in patients infected with HCV-3a. Am J Gastroenterol. 2002;97:2880-2885. [PubMed] |
| 97. | Rojas Á, del Campo JA, Maraver M, Aparcero R, García-Valdecasas M, Diago M, Carmona I, Andrade RJ, Solà R, Romero-Gómez M. Hepatitis C virus infection alters lipid metabolism depending on IL28B polymorphism and viral genotype and modulates gene expression in vivo and in vitro. J Viral Hepat. 2014;21:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Serfaty L, Andreani T, Giral P, Carbonell N, Chazouillères O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428-434. [PubMed] |
| 99. | Miyazaki T, Honda A, Ikegami T, Saitoh Y, Hirayama T, Hara T, Doy M, Matsuzaki Y. Hepatitis C virus infection causes hypolipidemia regardless of hepatic damage or nutritional state: An epidemiological survey of a large Japanese cohort. Hepatol Res. 2011;41:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] |
| 101. | Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T. Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor alpha in transgenic mice: implications for HCV-associated hepatocarcinogenesis. Int J Cancer. 2008;122:124-131. [PubMed] |
| 102. | Moroşan E, Mihailovici MS, Giuşcă SE, Cojocaru E, Avădănei ER, Căruntu ID, Teleman S. Hepatic steatosis background in chronic hepatitis B and C - significance of similarities and differences. Rom J Morphol Embryol. 2014;55:1041-1047. [PubMed] |
| 103. | Petta S, Vanni E, Bugianesi E, Rosso C, Cabibi D, Cammà C, Di Marco V, Eslam M, Grimaudo S, Macaluso FS. PNPLA3 rs738409 I748M is associated with steatohepatitis in 434 non-obese subjects with hepatitis C. Aliment Pharmacol Ther. 2015;41:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Dyal HK, Aguilar M, Bhuket T, Liu B, Holt EW, Torres S, Cheung R, Wong RJ. Concurrent Obesity, Diabetes, and Steatosis Increase Risk of Advanced Fibrosis Among HCV Patients: A Systematic Review. Dig Dis Sci. 2015;60:2813-2824. [PubMed] |
| 105. | Huang CF, Dai CY, Yeh ML, Huang CI, Tai CM, Hsieh MH, Liang PC, Lin YH, Hsieh MY, Yang HL. Association of diabetes and PNPLA3 genetic variants with disease severity of patients with chronic hepatitis C virus infection. J Hepatol. 2015;62:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Sato M, Kato N, Tateishi R, Muroyama R, Kowatari N, Li W, Goto K, Otsuka M, Shiina S, Yoshida H. Impact of PNPLA3 polymorphisms on the development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Hepatol Res. 2014;44:E137-E144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Nishimura M, Yamamoto H, Yoshida T, Seimiya M, Sawabe Y, Matsushita K, Umemura H, Sogawa K, Takizawa H, Yokosuka O. Decreases in the serum VLDL-TG/non-VLDL-TG ratio from early stages of chronic hepatitis C: alterations in TG-rich lipoprotein levels. PLoS One. 2011;6:e17309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 108. | Moriya K, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Yotsuyanagi H, Iino S, Kimura S, Koike K. Serum lipid profile of patients with genotype 1b hepatitis C viral infection in Japan. Hepatol Res. 2003;25:371-376. [PubMed] |
| 109. | Huang H, Kang R, Zhao Z. Is hepatitis C associated with atherosclerotic burden? A systematic review and meta-analysis. PLoS One. 2014;9:e106376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Ishizaka N, Ishizaka Y, Yamkado M. Atherosclerosis as a possible extrahepatic manifestation of chronic hepatitis C virus infection. Clin Med Insights Cardiol. 2014;8:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Olubamwo OO, Onyeka IN, Miettola J, Kauhanen J, Tuomainen TP. Hepatitis C as a risk factor for carotid atherosclerosis - a systematic review. Clin Physiol Funct Imaging. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Nakada Y, Kurosawa H, Tohyama J, Inoue Y, Ikewaki K. Increased remnant lipoprotein in patients with coronary artery disease--evaluation utilizing a newly developed remnant assay, remnant lipoproteins cholesterol homogenous assay (RemL-C). J Atheroscler Thromb. 2007;14:56-64. [PubMed] |
| 113. | Takeichi S, Yukawa N, Nakajima Y, Osawa M, Saito T, Seto Y, Nakano T, Saniabadi AR, Adachi M, Wang T. Association of plasma triglyceride-rich lipoprotein remnants with coronary atherosclerosis in cases of sudden cardiac death. Atherosclerosis. 1999;142:309-315. [PubMed] |
| 114. | Bridge SH, Sheridan DA, Felmlee DJ, Crossey MM, Fenwick FI, Lanyon CV, Dubuc G, Seidah NG, Davignon J, Thomas HC. PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: evidence for genotype-specific regulation of lipoprotein metabolism. J Hepatol. 2015;62:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Sheridan DA, Price DA, Schmid ML, Toms GL, Donaldson P, Neely D, Bassendine MF. Apolipoprotein B-associated cholesterol is a determinant of treatment outcome in patients with chronic hepatitis C virus infection receiving anti-viral agents interferon-alpha and ribavirin. Aliment Pharmacol Ther. 2009;29:1282-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | Aizawa Y, Yohizawa K, Aida Y, Ishiguro H, Abe H, Tsubota A. Genotype rs8099917 near the IL28B gene and amino acid substitution at position 70 in the core region of the hepatitis C virus are determinants of serum apolipoprotein B-100 concentration in chronic hepatitis C. Mol Cell Biochem. 2012;360:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Yoshizawa K, Abe H, Aida Y, Ishiguro H, Ika M, Shimada N, Tsubota A, Aizawa Y. Serum apolipoprotein B-100 concentration predicts the virological response to pegylated interferon plus ribavirin combination therapy in patients infected with chronic hepatitis C virus genotype 1b. J Med Virol. 2013;85:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 118. | Aizawa Y, Abe H, Yoshizawa K, Ishiguro H, Aida Y, Shimada N, Tsubota A. Dyslipoproteinemia in Chronic HCV Infection. Lipoproteins - Role in Health and Diseases. Chapter 30. Croatia: InTech 2012; . [DOI] [Full Text] |
| 119. | Ghebranious N, Ivacic L, Mallum J, Dokken C. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res. 2005;33:e149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 120. | Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518-2521. [PubMed] |
| 121. | Price DA, Bassendine MF, Norris SM, Golding C, Toms GL, Schmid ML, Morris CM, Burt AD, Donaldson PT. Apolipoprotein epsilon3 allele is associated with persistent hepatitis C virus infection. Gut. 2006;55:715-718. [PubMed] |
| 122. | Hishiki T, Shimizu Y, Tobita R, Sugiyama K, Ogawa K, Funami K, Ohsaki Y, Fujimoto T, Takaku H, Wakita T. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol. 2010;84:12048-12057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 123. | Atsukawa M, Tsubota A, Shimada N, Abe H, Kondo C, Itokawa N, Nakagawa A, Iwakiri K, Kawamoto C, Aizawa Y. Serum 25(OH)D3 levels affect treatment outcomes for telaprevir/peg-interferon/ribavirin combination therapy in genotype 1b chronic hepatitis C. Dig Liver Dis. 2014;46:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 124. | Wozniak MA, Itzhaki RF, Faragher EB, James MW, Ryder SD, Irving WL. Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology. 2002;36:456-463. [PubMed] |
| 125. | Mueller T, Gessner R, Sarrazin C, Graf C, Halangk J, Witt H, Köttgen E, Wiedenmann B, Berg T. Apolipoprotein E4 allele is associated with poor treatment response in hepatitis C virus (HCV) genotype 1. Hepatology. 2003;38:1592; author reply 1592-1593. [PubMed] |
| 126. | Fabris C, Vandelli C, Toniutto P, Minisini R, Colletta C, Falleti E, Smirne C, Pirisi M. Apolipoprotein E genotypes modulate fibrosis progression in patients with chronic hepatitis C and persistently normal transaminases. J Gastroenterol Hepatol. 2011;26:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Sheridan DA, Bridge SH, Felmlee DJ, Crossey MM, Thomas HC, Taylor-Robinson SD, Toms GL, Neely RD, Bassendine MF. Apolipoprotein-E and hepatitis C lipoviral particles in genotype 1 infection: evidence for an association with interferon sensitivity. J Hepatol. 2012;57:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Seki N, Sugita T, Aida Y, Itagaki M, Ishiguro H, Sutoh S, Abe H, Tsubota A, Matsushima M, Aizawa Y. Assessment of the features of serum apolipoprotein profiles in chronic HCV infection: difference between HCV genotypes 1b and 2. Hepatol Int. 2014;8:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Mancone C, Steindler C, Santangelo L, Simonte G, Vlassi C, Longo MA, D’Offizi G, Di Giacomo C, Pucillo LP, Amicone L. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut. 2011;60:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 130. | Sabile A, Perlemuter G, Bono F, Kohara K, Demaugre F, Kohara M, Matsuura Y, Miyamura T, Bréchot C, Barba G. Hepatitis C virus core protein binds to apolipoprotein AII and its secretion is modulated by fibrates. Hepatology. 1999;30:1064-1076. [PubMed] |
| 131. | Petit JM, Jooste V, Duvillard L, Minello A, Texier V, Galland F, Gambert P, Verges B, Hillon P. Apolipoprotein-AII concentrations are associated with liver steatosis in patients with chronic hepatitis C. Dig Dis Sci. 2007;52:3431-3434. [PubMed] |
| 132. | Dreux M, Boson B, Ricard-Blum S, Molle J, Lavillette D, Bartosch B, Pécheur EI, Cosset FL. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. J Biol Chem. 2007;282:32357-32369. [PubMed] |
| 133. | Gautier T, Masson D, de Barros JP, Athias A, Gambert P, Aunis D, Metz-Boutigue MH, Lagrost L. Human apolipoprotein C-I accounts for the ability of plasma high density lipoproteins to inhibit the cholesteryl ester transfer protein activity. J Biol Chem. 2000;275:37504-37509. [PubMed] |
| 134. | Rowell J, Thompson AJ, Guyton JR, Lao XQ, McHutchison JG, McCarthy JJ, Patel K. Serum apolipoprotein C-III is independently associated with chronic hepatitis C infection and advanced fibrosis. Hepatol Int. 2012;6:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |