Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.905
Peer-review started: March 27, 2014
First decision: August 6, 2014
Revised: August 23, 2014
Accepted: November 8, 2014
Article in press: November 11, 2014
Published online: January 21, 2015
Processing time: 299 Days and 12.7 Hours
AIM: To investigate the expression of Ras homolog (Rho)C, vascular endothelial growth factor (VEGF) and CD105 in esophageal squamous cell carcinoma.
METHODS: Semi-quantitative reverse transcriptase polymerase chain reaction, in situ hybridization and immunohistochemical streptavidin-biotin- peroxidase methods were used to detect expression of RhoC mRNA and protein, and VEGF protein in 62 cases with esophageal squamous cell carcinoma, 31 cases with adjacent atypical hyperplastic tissues, and 62 cases with normal esophageal mucosa. CD105 antibody labeling was used to measure microvascular density. Expression levels were compared according to clinicopathologic and patient parameters.
RESULTS: Expression of RhoC mRNA showed a positive correlation with the protein level in esophageal squamous cell carcinoma, as well as with VEGF protein levels. RhoC mRNA expression was mainly located within the cytoplasm of the tumor cells, appearing as blue to purple particles by in situ hybridization. The differences in RhoC mRNA expression in esophageal squamous cell carcinoma, adjacent atypical hyperplasia and normal esophageal mucosa were significant (P < 0.05). The relative expression of RhoC mRNA in cancer tissues with lymph node metastasis was significantly higher than in the tissues without lymph node metastasis (P < 0.05). VEGF protein expression was consistent with microvascular density (t = 25.52, P < 0.05). Positive expression of VEGF protein in esophageal squamous cell carcinoma of different histologic gradings did not differ significantly. Positive expression of VEGF protein in carcinoma tissues with deep infiltration was significantly higher than in tissues with only superficial infiltration (P < 0.05). The positive expression of VEGF protein in cancer tissues with lymph node metastasis was significantly higher than in the tissues without lymph node metastasis (P < 0.05).
CONCLUSION: RhoC protein may upregulate VEGF expression, thereby promoting tumor angiogenesis. RhoC mRNA and protein expression was correlated with metastasis.
Core tip: Recent studies have found that Ras homolog (Rho)C plays an important role in promoting angiogenesis in breast cancer. RhoC overexpression in breast cancer epithelial cells can improve expression of vascular endothelial growth factor (VEGF) and other angiogenic factors. Detection of the expression of RhoC, VEGF and microvascular density in esophageal squamous cell carcinoma and the relationship with metastasis and invasion have not been reported.
- Citation: Zhao ZH, Tian Y, Yang JP, Zhou J, Chen KS. RhoC, vascular endothelial growth factor and microvascular density in esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21(3): 905-912
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.905
Ras homolog (Rho) family members (RhoA, RhoB and RhoC) are a class of small GTP binding proteins, which play an important role in cell signaling pathways, cell transformation, and tumor cell infiltration and metastasis[1,2]. The relationship between RhoC and malignancy has become one of the research hotspots in oncology. Recent studies have found that RhoC plays an important role in promoting angiogenesis in breast cancer[3,4]. RhoC overexpression in breast cancer epithelial cells can improve the expression of vascular endothelial growth factor (VEGF) and other angiogenic factors. Detection of the expression of RhoC, VEGF and microvascular density (MVD) in esophageal squamous cell carcinoma and the relationship with metastasis and invasion have not been reported.
In the present study, we detected expression of RhoC mRNA and protein and VEGF protein in esophageal squamous cell carcinoma, adjacent atypical hyperplasia, and normal esophageal mucosa. CD105 antibody labeling was also used to detect MVD in esophageal squamous cell carcinoma to explore the relationship with RhoC and VEGF expression, and to clarify the clinicopathologic significance of the RhoC, VEGF, and MVD in the occurrence and development of esophageal squamous cell carcinoma.
We collected surgical specimens from 62 cases of esophageal squamous cell carcinoma at the Tumor Hospital of Henan Anyang City between February and March 2006. There were 36 male and 26 female cases, aged 38-75 years (mean: 60.6 ± 9.5 years). None of the patients had a preoperative history of chemotherapy, radiotherapy or immunotherapy. Esophageal squamous cell carcinoma was divided into four types[5]: ulcer type (n = 28), medullary type (n = 29), mushroom umbrella type (n = 4), and narrow type (n = 1). Fifteen cases were Grade I, 25 were Grade II, and 22 were Grade III; 20 were accompanied by lymph node metastasis, and 42 were not. Seven cases (superficial group) had tumor infiltration of the mucosa, submucosa or superficial muscles, and 55 cases (deep group) had tumor infiltration of the deep muscular or outer layer. All cases were confirmed to be esophageal squamous cell carcinoma by conventional pathology. Specimens were taken from outside the necrosis area, ≤ 3 cm mucosa tissue adjacent to carcinoma, and remote normal mucosa.
Each section was fixed with 40 g/L formaldehyde, conventionally dehydrated, paraffin-embedded, and serially sectioned at a thickness of 4-6 μm for hematoxylin and eosin staining, in situ hybridization, and immunohistochemical staining.
The total RNA of the tissue was extracted using the RNAiso Plus kit (Takara, Otsu, Shiga, Japan) and RT-PCR was performed using PrimeScript One Step RT-PCR Kit Ver. 2 (Takara). The results were analyzed with the GeneGenius Gel Imaging System (Syngene, Cambridge, United Kingdom). The ratio of the gray value of the RhoC amplified bands to the gray value of the β-actin bands was taken as the relative expression level of target gene mRNA.
In situ hybridization was carried out as described previously[6]. The probe sequence of RhoC was 5’-CGT TGG GGC AGA AGT GCT TCA-3’, which was synthesized by Beijing Bioko Biotechnology Co. Ltd.
Immunohistochemical staining was conducted using a streptavidin-biotin-peroxidase assay kit and in strict accordance with manufacturer’s instructions (Beijing Zhongshan Golden Bridge Biotechnology Development Company, China). Breast biopsy specimens with known positive expression of RhoC were taken as positive controls. Antibody was replaced by normal goat serum as a negative control and PBS as a blank control. The results were observed in a double-blind manner as described previously[7]. Yellow or brown particles in the cytoplasm or cell membrane were considered to be positive. Unstained cells were negative. Stained cells, regardless of the area and intensity, were all taken as positive.
SPSS version 13.0 software (SPSS Inc., Chicago, IL, United States) was used to conduct χ2 or rank sum tests. A P < 0.05 was considered to be statistically significant.
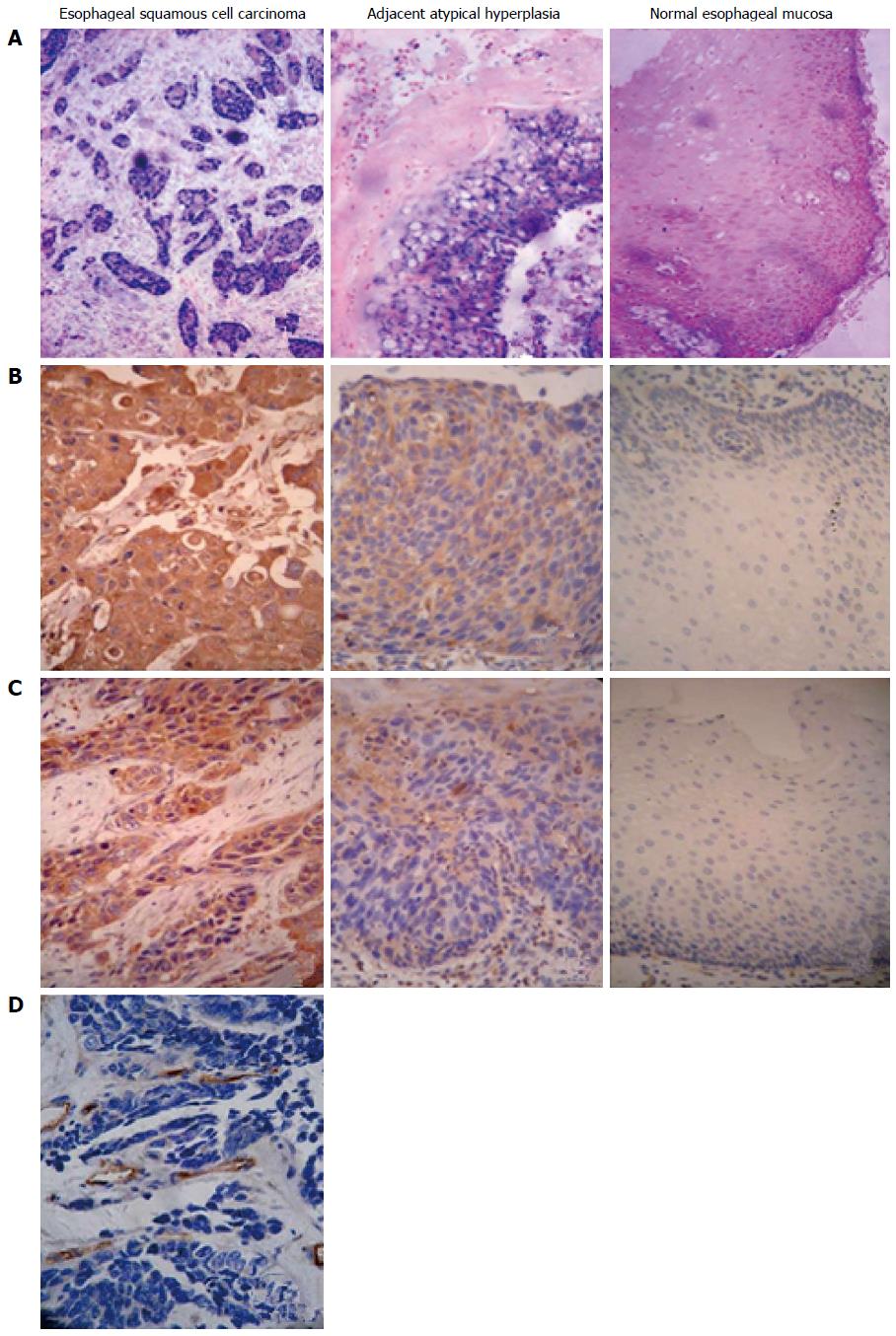
RhoC mRNA expression was mainly located within the cytoplasm of the tumor cells, showing as blue to purple particles (Figure 1A). RhoC mRNA levels significantly differed among normal esophageal mucosa, adjacent atypical hyperplasia, and carcinoma sample (P < 0.05) (Table 1). The lower the degree of differentiation, the higher the expression of RhoC mRNA, and the difference was significant by rank sum test (P < 0.05) (Table 2). The difference in relative RhoC mRNA expression levels in the carcinoma tissues of the shallow and deep infiltration groups was significant (P < 0.05). The relative expression of RhoC mRNA in cancer tissues with lymph node metastasis was significantly higher than in the group without lymph node metastasis (P < 0.05). Sex, age and other factors of patients with esophageal squamous cell carcinoma were independent of the relative content of RhoC mRNA (P > 0.05).
| Site | Cases (n) | RhoC mRNA relative content | χ2 | P value | RhoC mRNA expression | χ2 | P value | RhoC protein expression | χ2 | P value | VEGF protein expression | χ2 | P value | ||||||
| - | + | Positive rate | - | + | Positive rate | - | + | Positive rate | |||||||||||
| Normal esophageal mucosa tissues | 62 | 0.653 ± 0.069 | 49 | 13 | 21% | 50 | 12 | 19.4% | 44 | 18 | 29% | ||||||||
| Adjacent atypical hyperplasia tissues | 31 | 0.731 ± 0.065 | 99.629 | 0.000 | 21 | 10 | 32.3% | 47.735 | 0.000 | 20 | 11 | 35.5% | 54.25 | 0.000 | 14 | 17 | 54.8% | 18.994 | 0.000 |
| Cancer tissues | 62 | 0.902 ± 0.119 | 12 | 50 | 80.6% | 10 | 52 | 83.9% | 20 | 42 | 67.7% | ||||||||
| Clinicopathologic parameters | Cases(n) | RhoC mRNA relative content | F(t, χ2) | P value | RhoC mRNA expression | χ2 | P value | RhoC protein expression | χ2 | P value | VEGF protein expression | χ2 | P value | ||||||
| - | + | Positive rate | - | + | Positive rate | - | + | Positive rate | |||||||||||
| Gender | |||||||||||||||||||
| Male | 36 | 0.922 ± 0.123 | 0.586 | 0.118 | 7 | 29 | 80.6 | 0.000 | 0.983 | 6 | 30 | 83.3 | 0.018 | 0.892 | 11 | 25 | 69.4 | 0.004 | 0.950 |
| Female | 26 | 0.874 ± 0.109 | 5 | 21 | 80.8 | 4 | 22 | 84.6 | 9 | 17 | 65.4 | ||||||||
| Age (yr) | |||||||||||||||||||
| ≥ 60 | 33 | 0.911 ± 0.108 | 0.619 | 0.538 | 6 | 27 | 81.8 | 0.062 | 0.803 | 7 | 26 | 78.8 | 1.348 | 0.246 | 12 | 21 | 63.6 | 0.217 | 0.642 |
| < 60 | 29 | 0.892 ± 0.132 | 6 | 23 | 79.3 | 3 | 26 | 89.7 | 8 | 21 | 72.4 | ||||||||
| Histologic grading | |||||||||||||||||||
| I | 15 | 0.795 ± 0.056 | 7 | 8 | 53.3 | 7 | 8 | 53.3 | 8 | 7 | 46.7 | ||||||||
| II | 25 | 0.898 ± 0.086 | 22.535 | 0.000 | 3 | 22 | 88.0 | 9.520 | 0.009 | 2 | 23 | 92.0 | 13.744 | 0.001 | 7 | 18 | 72.0 | 4.014 | 0.134 |
| III | 22 | 0.979 ± 0.128 | 2 | 20 | 90.9 | 1 | 21 | 95.5 | 5 | 17 | 77.3 | ||||||||
| Invasion depth | |||||||||||||||||||
| Shallow group | 7 | 0.751 ± 0.036 | 4 | 3 | 42.9 | 4 | 3 | 42.9 | 6 | 1 | 14.3 | ||||||||
| Deep group | 55 | 0.924 ± 0.111 | 8.716 | 0.000 | 8 | 47 | 85.5 | 7.219 | 0.007 | 6 | 49 | 89.1 | 9.812 | 0.002 | 14 | 41 | 74.5 | 10.319 | 0.001 |
| Lymphatic metastasis | |||||||||||||||||||
| Without | 42 | 0.840 ± 0.074 | 9.042 | 0.000 | 11 | 31 | 73.8 | 3.898 | 0.048 | 10 | 32 | 76.2 | 5.678 | 0.017 | 18 | 24 | 57.1 | 6.693 | 0.010 |
| With | 20 | 1.032 ± 0.086 | 1 | 19 | 95.0 | 0 | 20 | 100.0 | 2 | 18 | 90.0 | ||||||||
RhoC protein showed as pale to dark yellow particles, mainly located in the cytoplasm (Figure 1B). The differences in the expression of RhoC protein among the three type of tissue sample were significant (P < 0.05) (Table 1). The lower the degree of differentiation, the higher the expression of RhoC protein, and the difference was significant (P < 0.05). The positive expression of RhoC protein in cancer tissues with lymph node metastasis was significantly higher than in the tissues without lymph node metastasis (P < 0.05). Sex, age and other factors of patients with esophageal squamous cell carcinoma were unrelated to positive expression of RhoC protein (P > 0.05) (Table 2).
The differences in VEGF protein expression in the three groups were significant (P < 0.05) (Table 1, Figure 1C). Positive expression of VEGF protein in esophageal squamous cell carcinoma of different histologic grading did not differ significantly (P > 0.05) (Table 2). Positive expression of VEGF protein in carcinoma tissues of the deep infiltrating groups was significantly higher than that in the superficial infiltrating group (P < 0.05). The positive expression of VEGF protein in cancer tissues with lymph node metastasis was significantly higher than in the tissues without lymph node metastasis (P < 0.05). Sex, age and other factors of patients with esophageal squamous cell carcinoma were independent of positive expression of VEGF protein (P > 0.05) (Table 2).
Detection of MVD by CD105 showed that positive protein expression signals of CD105 were mainly located in the vascular endothelial cell cytoplasm within the tumor stroma, or in cancer nests, with pale to dark yellow particles (Figure 1D). CD105-antibody-labeled MVD gradually increased in Grade I, II and III esophageal squamous cell carcinoma, but the differences among the groups were not significant (F = 0.298, P > 0.05). MVD in the cancer tissues of the deep group was higher than that of the superficial group (34.47 ± 11.69). MVD (39.00 ± 8.84 vs 41.0 ± 3.26) in esophageal squamous cell carcinoma with lymph node metastasis was significantly higher than in the group without lymph node metastasis (39.00 ± 8.84 vs 36.33 ± 3.76), and the differences among groups were significant (Table 2, Figure 1D).
Among the 52 cases with positive RhoC protein expression, 46 had positive mRNA expression, and in 10 cases with negative RhoC protein expression, six had negative mRNA expression. RhoC protein expression showed a positive correlation with RhoC mRNA expression in esophageal squamous cell carcinoma (P < 0.01) (Table 3). In the cases with positive RhoC protein expression, positive expression of VEGF protein was 50.0% (13/26), whereas in cases with negative RhoC expression, the rate was 82.6% (19/23), showing a positive correlation between the two (P < 0.05). In the cases with positive RhoC protein expression, MVD was 41.99 ± 9.83, whereas in cases with negative RhoC protein expression, MVD was 31.07 ± 6.38, and the difference was significant (t = 25.52, P < 0.01). MVD of 42 cases with positive VEGF protein expression was 38.88 ± 0.041, of which, 20 cases had negative VEGF protein expression with MVD of 36.45 ± 0.036. The difference was significant (t = 25.52, P < 0.05), indicating that VEGF protein expression was consistent with MVD.
| RhoC protein expression | n | RhoC mRNA expression | r | P value | VEGFexpression | r | P value | MVDCD105(mean ± SD) | r | P value | VEGF protein expression | n | MVDCD105(mean ± SD) | t | P value | ||
| + | - | + | - | ||||||||||||||
| + | 52 | 46 | 6 | 0.451 | 0.000 | 40 | 12 | 0.448 | 0.000 | 41.99 ± 9.83 | 0.451 | 0.000 | + | 42 | 38.88 ± 0.041 | 5.147 | 0.027 |
| - | 10 | 4 | 6 | 2 | 8 | 31.07 ± 6.38 | - | 20 | 36.45 ± 0.036 | ||||||||
RhoC is a member of the Rho family[8] and is a GTP-binding protein that hydrolyses GTP to inactivated GDP, acting as a signal converter or a molecular switch in cell signal transduction pathways to produce a variety of biological effects that act on the cytoskeleton or target proteins[9,10]. The most important function of RhoC is regulating cell motility and migration by regulating the actin cytoskeleton, and inducing aggregation of actin, integrins and associated proteins such as fibronectin[11,12]. The actin cytoskeleton plays an important role in cell shape changes, cell movement and phagocytosis, but also in various activities such as cell proliferation, growth and apoptosis[13,14]. It has been shown that RhoC overexpression is related to the development of a variety of malignancies. Clark et al[15] found that RhoC acts as a molecular switch in tumor metastasis in their study of the invasive and transfer phenotype of melanoma. Ikoma et al[16] found that RhoC overexpression leads to significantly increased mRNA expression of matrix metalloprotein (MMP)-2, MMP-9, and tissue inhibitor of matrix metalloprotein. RhoC increases the expression and activity of MMPs, which lead to enhanced tumor aggressiveness. RhoC gene overexpression also leads to increased expression of angiogenic factors (particularly VEGF) and then activation of mitogen-activated protein kinase, phospholipase C, and phosphatidylinositol 3-kinase to promote tumor invasion and metastasis. Van Golen et al[3] suggested that RhoC overexpression could increase the expression of VEGF and other angiogenic factors in breast epithelial cells. The extracellular C3 transferase enzyme of Clostridium inhibits RhoC function, which can significantly reduce the yield of angiogenic factors. Current studies evaluating RhoC gene expression in esophageal squamous cell carcinoma are limited to a few observational reports. Joint detection of RhoC, VEGF and CD105 in esophageal squamous cell carcinoma has not been reported.
The relative content of RhoC protein and mRNA suggest that RhoC overexpression may be involved in malignant transformation of esophageal epithelial cells, which may play an important role in the incidence and development of esophageal cancer. Protein and mRNA expression and the relative content of RhoC were significantly increased with the invasion depth of carcinoma, and were significantly higher in the tissues of patients with lymph node metastasis than those without metastasis. These results indicate that abnormal RhoC expression may be closely associated with invasion and lymph node metastasis in esophageal squamous cell carcinoma, which is consistent with the findings of Faried et al[17]. RhoC mRNA and protein expression and their relative content were significantly reduced with decreased degree of differentiation of esophageal cancer tissues. This suggests that RhoC gene plays an important role in the induction of tumor cell differentiation, and its overexpression may be associated with histologic grading and degree of malignancy in esophageal squamous cell carcinoma. Zhang et al[7] have reported that the degree of RhoC protein expression is unrelated to the degree of differentiation of esophageal cancer, and the cause of the discrepancy may be related to the different sources and numbers of specimens and antibodies.
As one of the most critical angiogenic growth factors, VEGF provides favorable conditions for the growth and metastasis of the majority of malignant tumors[18]. On the one hand, VEGF causes capillary lumen formation by acting directly on the proliferation and migration of the receptor-mediated capillary endothelial cells. On the other hand, vascular permeability is increased, which changes the tumor interstitium and plays an important role in cancer invasion and metastasis, through its autocrine or paracrine activity[19-23]. The results of the present study showed that VEGF protein expression in the lymph node metastasis group was significantly higher than in the groups without metastasis. Positive VEGF expression in the deep infiltration group was significantly higher than in the shallow infiltration group, indicating that high expression of VEGF protein can induce invasion and metastasis of esophageal squamous cell cancer. In esophageal squamous cell carcinoma with varying degrees of differentiation, the positive rate of VEGF protein expression did not differ significantly, suggesting that VEGF protein expression had nothing to do with histologic grade.
Research has shown that vascular endothelial markers such as CD34 and CD31 are expressed in tumor blood vessels, whereas the CD105 antibody is preferentially bound to the activated endothelial cells in tumor tissue. DC34 and CD31 are not expressed or only weakly expressed in the vascular endothelial cells of normal tissues, thus CD105 is a more specific marker for assessing the proliferation of vascular endothelial cells[24]. MVD in esophageal squamous cell the carcinoma tissues increased significantly with the depth of invasion. MVD in the group with lymph node metastasis was significantly higher than in the group without metastasis. CD105 expression in esophageal squamous cell carcinoma with different stages had no significant differences, which was similar to previous results[25,26]. Newborn capillaries can promote metastasis of tumor cells several ways: (1) amount of neovascularization; (2) high permeability of the new blood capillaries; and (3) degradative enzymes changing the tumor extracellular matrix[27]. Therefore, the number of blood vessels in the tumor stroma is an important factor affecting cancer prognosis and metastasis. The extent of angiogenesis in tumor tissues can be determined by assessing MVD[28]. Determination of MVD in esophageal squamous cell carcinoma could become one of the indicators to determine invasive and metastatic potential.
RhoA and RhoC can promote tumor angiogenesis by the Rho-associated kinase signaling pathway, as well as assisting distant metastasis of tumor cells through the vascular endothelium[29,30]. The results of our study showed that expression of RhoC protein was positively correlated with VEGF protein expression and MVD. The differences in MVD and expression of VEGF in patients with RhoC protein expression were significant compared with patients with negative RhoC protein expression. These findings indicate that RhoC overexpression promotes tumor angiogenesis by increasing expression of VEGF protein. We also found that MVD increased with high levels of VEGF protein expression. VEGF and CD105 staining in tumor tissues is similar, which confirms that VEGF specifically promotes tumor angiogenesis, which is consistent with previous findings in gastric cancer[31].
In summary, our results indicate that RhoC plays an important role in esophageal carcinogenesis and development. RhoC overexpression may be able to increase the expression of VEGF, thereby promoting tumor angiogenesis. VEGF protein overexpression and MVDCD105 were closely related to the invasion and metastasis of esophageal squamous cell carcinoma, which may be considered as an indicator for determining the lymph node metastatic potential of esophageal squamous cell carcinoma.
The physiological and pathological roles of Ras homolog (Rho)C, vascular endothelial growth factor (VEGF) and microvascular density (MVD) have attracted considerable attention in recent years. However, expression of RhoC, VEGF and MVD in esophageal squamous cell carcinoma and the relationship with metastasis and invasion have not been reported.
This study was performed to explore the expression of RhoC, VEGF and MVD in esophageal squamous cell carcinoma and the relationship with metastasis and invasion. The study implied that the expression of RhoC, VEGF and MVD play an important role in esophageal squamous cell carcinoma.
The results of this study suggested that RhoC protein may upregulate the expression of VEGF, thereby promote tumor angiogenesis. RhoC mRNA and protein expression was related to metastasis.
The results imply that the RhoC gene plays an important role in esophageal carcinogenesis and development. VEGF protein overexpression and MVDCD105 were closely related with the invasion and metastasis of esophageal squamous cell carcinoma, which may be considered as an indicator for judging the lymph node metastatic potential in esophageal squamous cell carcinoma.
This is a well-presented article on relationship among the expression of RhoC, VEGF and MVD in esophageal squamous cell carcinoma, which has not been reported domestically or abroad. The study is innovative, the results are convincing, and the findings are presented clearly.
P- Reviewer: Kim SM, Li BS S- Editor: Gou SX L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832-5838. [PubMed] |
| 4. | van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Du BL. Esophageal squamous cell carcinoma. 1st ed. Beijing: China Science and Technology Press 1994; 62-108. |
| 6. | Li SL, Liu ZW, Zhao QM, Yu JX, Zhao ZH, Gao DL, Pang X, Chen KS, Zhang YH. RECK mRNA and protein expression and significance in ESCC. Zhongguo Zhongliu Linchuang. 2007;34:1280-1286. |
| 7. | Zhang HZ, Liu JG, Wei YP, Wu C, Cao YK, Wang M. [Expressions of RhoC and osteopontin in esophageal squamous carcinoma and association with the patients’ prognosis]. Nanfang Yike Daxue Xuebao. 2006;26:1612-1615. [PubMed] |
| 8. | Aspenström P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 250] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Wang H, Li J, Liu Y, Ding Y. Overexpression of Rho GDP-dissociation inhibitor alpha is associated with tumor progression and poor prognosis of colorectal cancer. J Proteome Res. 2008;7:3994-4003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Morris SW, Valentine MB, Kirstein MN, Huebner K. Reassignment of the human ARH9 RAS-related gene to chromosome 1p13-p21. Genomics. 1993;15:677-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Dutt P, Nguyen N, Toksoz D. Role of Lbc RhoGEF in Galpha12/13-induced signals to Rho GTPase. Cell Signal. 2004;16:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Iwaki N, Karatsu K, Miyamoto M. Role of guanine nucleotide exchange factors for Rho family GTPases in the regulation of cell morphology and actin cytoskeleton in fission yeast. Biochem Biophys Res Commun. 2003;312:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Durkin ME, Avner MR, Huh CG, Yuan BZ, Thorgeirsson SS, Popescu NC. DLC-1, a Rho GTPase-activating protein with tumor suppressor function, is essential for embryonic development. FEBS Lett. 2005;579:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1476] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 15. | Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1083] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 16. | Ikoma T, Takahashi T, Nagano S, Li YM, Ohno Y, Ando K, Fujiwara T, Fujiwara H, Kosai K. A definitive role of RhoC in metastasis of orthotopic lung cancer in mice. Clin Cancer Res. 2004;10:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Faried A, Faried LS, Usman N, Kato H, Kuwano H. Clinical and prognostic significance of RhoA and RhoC gene expression in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3593-3601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Chung J, Yoon S, Datta K, Bachelder RE, Mercurio AM. Hypoxia-induced vascular endothelial growth factor transcription and protection from apoptosis are dependent on alpha6beta1 integrin in breast carcinoma cells. Cancer Res. 2004;64:4711-4716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9-19. [PubMed] |
| 20. | Bosari S, Lee AK, DeLellis RA, Wiley BD, Heatley GJ, Silverman ML. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992;23:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 407] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Wang FL, Wei LX, Chen YZ. Relationship between vascular endothelial growth factor, microvessel density and metastasis and prognosis of breast cancer lymph node. Zhonghua Binglixue Zazhi. 2000;29:172-175. |
| 22. | Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898-2905. [PubMed] |
| 23. | Li YP. Relationship between angiogenesis, microvessel counts and metastasis and prognosis of breast cancer. Guowai Yixue Zhongliu Fence. 1999;26:356-357. |
| 24. | Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031-1044. [PubMed] |
| 25. | Liu HN, Cao HC, Wang ZM, Wang JJ, Zhang YX, Su YP, Huo JA. VEGF expression in esophageal tissues on prognosis. Zhongguo Shiyong Yiyao. 1999;2:450-452. |
| 26. | Liu DB, Chen KN, Cao XZ, Wang T. [Expression of p53 and vascular endothelial growth factor in esophageal squamous cell carcinoma and their clinical significance]. Ai Zheng. 2002;21:989-993. [PubMed] |
| 27. | Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032:89-118. [PubMed] |
| 28. | Fox SH, Whalen GF, Sanders MM, Burleson JA, Jennings K, Kurtzman S, Kreutzer D. Angiogenesis in normal tissue adjacent to colon cancer. J Surg Oncol. 1998;69:230-234. [PubMed] |
| 29. | Croft DR, Sahai E, Mavria G, Li S, Tsai J, Lee WM, Marshall CJ, Olson MF. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 2004;64:8994-9001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Pillé JY, Denoyelle C, Varet J, Bertrand JR, Soria J, Opolon P, Lu H, Pritchard LL, Vannier JP, Malvy C. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol Ther. 2005;11:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Jing YM, Zhou SF. Expression of CD105 marker microvessel density and vascular endothelial growth factor in gastric cancer. Hebei Yiyao. 2004;10:1-5. |