Published online Jul 21, 2015. doi: 10.3748/wjg.v21.i27.8352
Peer-review started: January 25, 2015
First decision: February 10, 2015
Revised: March 9, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: July 21, 2015
Processing time: 178 Days and 19.7 Hours
AIM: To evaluate human pancreatic carcinoma cell line (PANC-1) cells apoptosis and Bcl-2 and Bax expression induced by Yin Chen Hao Decoction (YCHD).
METHODS: The cell growth inhibitory rate was determined by MTT assay. Apoptosis of PANC-1 cells before and after treatment with YCHD was determined by TUNEL staining. Expression of the apoptosis-associated genes, Bcl-2 and Bax, was detected by immunohistochemical staining and reverse transcription -PCR.
RESULTS: YCHD inhibited the growth of PANC-1 cells. Following treatment with YCHD for 24-96 h, the apoptotic rate of PANC-1 cells increased with time. In addition, the positive rate of Bcl-2 protein expression decreased in a time-dependent manner, whereas the positive rate of Bax protein expression increased in a time-dependent manner. Following treatment of with YCHD for 24-96h, expression of BAX mRNA increased gradually and BCL-2 mRNA reduced gradually with time.
CONCLUSION: YCHD induces apoptosis of PANC-1 cells mediated in part via up-regulation of BAX and down-regulation of BCL-2.
Core tip: Yin Chen Hao Decoction (YCHD) inhibits the growth of pancreatic carcinoma cells. Following treatment with YCHD for 24-96 h, the apoptotic rates in these cells is increased by YCHD, which is accompanied by time-dependent increased expression of Bax and decreased expression of Bcl-2. YCHD-induced apoptosis of these cells may be mediated by upregulation of BAX and downregulation of BCL-2.
- Citation: Zhou HB, Chen JM, Shao LM, Chen ZG. Apoptosis of human pancreatic carcinoma cell-1 cells induced by Yin Chen Hao Decoction. World J Gastroenterol 2015; 21(27): 8352-8357
- URL: https://www.wjgnet.com/1007-9327/full/v21/i27/8352.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i27.8352
Yin Chen Hao Decoction (YCHD) is a classic Chinese medicine formula consisting of three herbal drugs Rheum officinale Baill, Artemisia capillaries Thunb and Gardenia iasminoides Ellis, and has long been used to treat cholestasis, hepatitis C[1], primary biliary cirrhosis[2], and liver fibrosis[3]. Previous research reported that YCHD is effective for choleresis and has anti-inflammatory and anti-asthmatic activity to relax bronchial smooth muscle[4,5]. Moreover, YCHD is a potent inhibitor of carcinoma[6]. The anti-cancer activity of YCHD may induce apoptosis of carcinoma cells.
The Bcl-2 family plays a key role in the apoptosis. The Bcl-2 family includes proapoptotic members, such as Bax and Bad, and antiapoptotic members such as Bcl-2 and Bcl-xL[7-10]. Overexpression of Bax can quicken cell death[11-20], whereas overexpression of Bcl-2 can delay cell death[20-29]. The critical determinant of cell apoptosis is the ratio of Bcl-2/Bax[30].
In this study, the cell growth inhibitory rate was determined by MTT assay. The apoptosis status in pancreatic carcinoma PANC-1 cells after YCHD treatment was determined by the TUNEL staining method. Gene and protein expression of Bcl-2 and Bax was detected by immunohistochemical staining and reverse transcription (RT)-PCR, respectively.
Rheum officinale Baill, Artemisia capillaries Thunb and Gardenia iasminoides Ellis were purchased from the Second Affiliated Hospital of Zhejiang University, China. MTT was obtained from Sigma-Aldrich (St. Louis, MO, Unite States). The in situ cell detection kit and anti-Bcl-2 and anti-Bax monoclonal antibodies were purchased from Beijing Zhongshan Biotechnology Co, Ltd., (Beijing, China). Human pancreatic carcinoma PANC-1 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
For the preparation of YCHD, Artemisia capillaries Thunb (200 g), Gardenia iasminoides Ellis (100 g) and Rheum officinale Baill (60 g) were placed in a round-bottomed flask and boiled for 1 h in distilled water was added and then boiled for 1 h. The decoction was then percolated to obtain the filtrate and the residue was reboiled twice. The collected filtrates were then added together and concentrated under reduced pressure. The concentrated liquid was then vacuum dried. The yield of YCHD extract was 28.6% and the concentration was 40 μg/mL. The extract was stored in a desiccator until use.
The PANC-1 cells were incubated in Dulbecco’s modified Eagle’s medium (HyClone of Thermo Fisher Scientific, Waltham, MA, United States) supplemented with 2 mmol/L glutamine, 0.05 g/L penicillin, 0.1 g/L streptomycin, and 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was replaced every two days.
Cells were seeded at 1 × 105 cells/well in 96-well plate overnight and treated with various concentrations of YCHD (5 μg/mL, 10 μg/mL, 20 μg/mL and 40 μg/mL) for different times for 24 h, 48 h and 72 h. After treatment, an MTT assay was used to determine cell densities. All experiments were performed in triplicate three separate times.
A MEBSTAIN Apoptosis Kit Direct (MBL International, Woburn, MA, United States) was used to perform TUNNEL staining according to the manufacturer’s protocol. Nucleosome-sized DNA fragments are detected by tailing their 3′-OH ends with digoxigenin nucleotides using terminal deoxynucleotidyl transferase (TdT). After treatment, the cells were combined with POD-horseradish peroxidase and incubated with the TdT buffer. The numbers of TUNNEL-positive cells was counted under a light microscope.
Pancreatic carcinoma PANC-1 cells were exposed to YCHD (20 μg/mL) for various times(24 h, 48 h, 72 h and 96 h). After treatment, the cells were grown on six-well glass plates and were fixed with acetone. After washing in PBS, The cells were washed with PBS and incubated in 3 mL/L H2O2 solution at room temperature for 5 min. Then, anti-Bcl-2 or anti-Bax monoclonal antibodies at a 1:300 dilution were added and incubated at 4 °C overnight. The cells were washed with PBS and incubated with the secondary antibody(biotinylated anti-rat IgG) at room temperature for 1 h. Then the cells were washed with PBS and incubated with ABC compound (Vector Laboratories Inc., Burlingame, CA, United States) at room temperature for 10 min. DAB was used as the chromagen. After 10 min, the brown color signifying the presence of antigen bound to antibodies was detected by light microscopy and photographed at magnification ×200.
PANC-1 cells were exposed to YCHD (20 μg/mL) for various times (24 h, 48 h, 72 h and 96 h) and total RNA was extracted. The primers for Bcl-2, Bax, and β-actin in were as follows: β-actin (500 bp): 5’-GTGGGGCGCCCCAGGCACCA-3’ and 5’-CTCCTTAATGTCACGCACGATTTC-3’; Bcl-2 (716 bp): 5’-GGAAATATGGCGCACGCT-3’ and 5-’TCACTTGTGGCCCAGAT-3’; Bax (508 bp): 5’-CCAGCTCTGAGCAGATCAT-3’ and 5-’TATCAGCCCA TCTTCTTCC-3’. PCR was performed using the following procedures: 94 °C for 7 min followed 35 (Bax) or 30 (Bcl-2 and β-actin) cycles of 94 °C for 1 min, 60 °C for 45 s, and 72 °C for 45 s, and completed with one cycle at 72 °C for 7 min. For visualization, 10 μL of PCR product was loaded onto a 15 g/L agarose gel.
Statistical differences of data were analyzed by the paired two-tailed Student’s t-test. P < 0.05 was considered significant.
PANC-1 cells were exposed to various concentrations of YCHD (5 μg/mL, 10 μg/mL, 20 μg/mL, and 40 μg/mL) for 24 h, 48 h, and 72 h. Apoptosis of PANC-1 cells was increased with dose and time (Table 1).
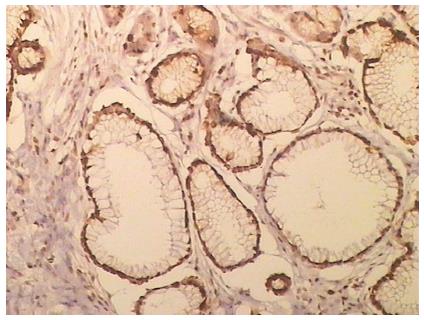
A TUNEL assay detected apoptotic cells according to the manufacturer’s instructions. Positive staining was located in the nucleus (Figure 1). The apoptotic index of PANC-1 cells was increased with time (Table 2).
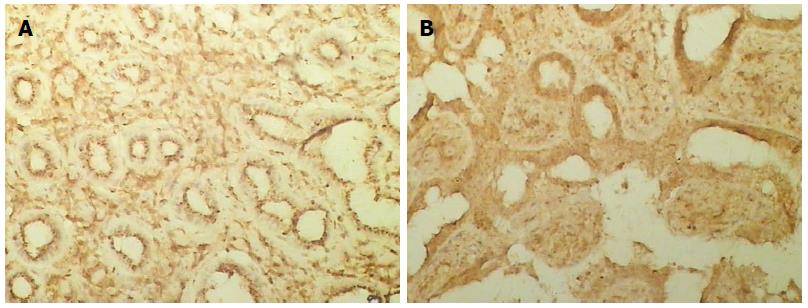
Positive staining was located in the cytoplasm (Figure 2A). PANC-1 cells were exposed to YCHD (20 μg/mL) for various times (24 h, 48 h, 72 h, and 96 h). The positive rate of Bcl-2 expression in PANC-1 cells was reduced with time (P < 0.05) (Table 3).
Positive staining was located in the cytoplasm (Figure 2B). PANC-1 cells were exposed to YCHD (20 μg/mL) for various times (24 h, 48 h, 72 h, and 96 h). The positive rate of Bax expression increased with time (P < 0.05) (Table 4).
When PANC-1 cells were exposed to YCHD (20 μg/mL) for various times (24 h, 48 h, 72 h, and 96 h), the expression of Bcl-2 mRNA decreased and Bax mRNA increased with time.
Cancer involves dysregulation of the cell cycle and uncontrolled growth due to the combined effects of hereditary and environmental factors. The mechanism of cell cycle dysregulation is an important cause of cell proliferation, which leads to cancer. Normally, each period of cell division and proliferation are strictly regulated by a variety of specific proteins.
The results of the present study show that apoptosis of PANC-1 cells is time- and dose-dependent. This suggests that YCHD inhibits the growth of pancreatic carcinoma cells. Following treatment with 20 μg/mL YCHD, the positive rate of Bax protein expression was significantly increased, whereas the rate of Bcl-2 protein expression was significantly reduced with time. Furthermore, the expression of Bcl-2 mRNA decreased and the density of Bax mRNA increased with time. Thus, the ratio of Bcl-2/Bax decreased with YCHD treatment to result in apoptosis of these cells.
In conclusion, this study demonstrates that YCHD induces apoptosis of pancreatic carcinoma cells. Decreased expression of the BCL2 and increased expression of BAX may induce apoptosis. YCHD may be a new drug for pancreatic cancer chemotherapy.
Yin Chen Hao Decoction (YCHD) is a classic Chinese medicine formula consisting of three herbal drugs (Rheum officinale Baill, Artemisia capillaris Thunb, and Gardenia jasminoides Ellis), and has long been used to treat cholestasis, hepatitis C, primary biliary cirrhosis, and liver fibrosis. Previous research reported that YCHD is effective for choleresis and has anti-inflammatory and antiasthmatic activity to relax bronchial smooth muscle. Moreover, reports show that YCHD is a potent inhibitor of carcinoma. The anti-cancer activity of YCHD may induce apoptosis of carcinoma cells.
YCHD has antitumor effects. The research hotspot related to YCHD is how it affects the progression of human cancer.
The present study demonstrates that YCHD is able to induce apoptosis of pancreatic carcinoma cells. Decreased expression of BCL2 and increased expression of BAX may induce apoptosis.
In understanding the role and mechanism of YCHD against pancreatic carcinoma, this study is expected to suggest a way of improving clinical treatment.
This study is a very enjoyable and valuable work. The results may represent a molecular mechanism of YCHD for treatment of pancreatic carcinoma.
P- Reviewer: Nakano H S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Itoh T, Shibahara N, Mantani N, Tahara E, Shimada Y, Terasawa K. Effect of Kampo treatment on chronic viral hepatitis on the basis of traditional diagnosis. J Tradit Med. 1997;14:204-210. |
| 2. | Onji M, Kikuchi T, Michitaka K, Yamashita K, OhtaY . Combined use of ursodeo × ycholic acid and Inchinko-to in jaundice patients with primary bjliaryclrrhosis. J Med Pharmaceut. 1990;7:161-167. |
| 3. | Arai M, Yokosuka O, Fukai K, Kanda T, Kojima H, Kawai S, Imazeki F, Hirasawa H, Saisho H. A case of severe acute hepatitis of unknown etiology treated with the Chinese herbal medicine Inchinko-to. Hepatol Res. 2004;28:161-165. [PubMed] |
| 4. | Liu YJ, Li Z, Lin ZB. Scoparone’s effects on the airway smooth muscle of asthmatic guinea pigs. Zhongguo Yike Daxue Xuebao. 2001;30:12-13. |
| 5. | Yuan X, Li Z, Liu HR, Yu XH, Han LW. Inhibitory effect of scoparone on airway inflammation and enhanced ROS of asthmatic models of guinea pigs. Zhongguo Yike Daxue Xuebao. 2002;31:404-406. |
| 6. | Chen SD, Zhou HH, Li XM, Lin JP, Fan Y, Xu WJ. Inhibitory effects of Yinchenhao Decoction Inhibitory on fatty deposition and TNF- α secretion in HepG2 cells inducedby free fatty acid. Zhonghua Zhongyiyao Zazhi. 2010;9:1381-1384. |
| 7. | Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | van der Woude CJ, Jansen PL, Tiebosch AT, Beuving A, Homan M, Kleibeuker JH, Moshage H. Expression of apoptosis-related proteins in Barrett’s metaplasia-dysplasia-carcinoma sequence: a switch to a more resistant phenotype. Hum Pathol. 2002;33:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Panaretakis T, Pokrovskaja K, Shoshan MC, Grandér D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem. 2002;277:44317-44326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood. 2002;100:1810-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Qian W, Salamoun J, Wang J, Roginskaya V, Van Houten B, Wipf P. The combination of thioxodihydroquinazolinones and platinum drugs reverses platinum resistance in tumor cells by inducing mitochondrial apoptosis independent of Bax and Bak. Bioorg Med Chem Lett. 2015;25:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Yan H. [Effect of simvastatin on retinal Bcl-2/Bax expression and cell apoptosis in rats with ischemia-reperfusion injury]. Zhonghua Yanke Zazhi. 2014;50:826-832. [PubMed] |
| 13. | Azova MM, Blagonravov ML, Frolov VA. Effect of phosphocreatine and ethylmethylhydroxypyridine succinate on the expression of Bax and Bcl-2 proteins in left-ventricular cardiomyocytes of spontaneously hypertensive rats. Bull Exp Biol Med. 2015;158:313-314. [PubMed] |
| 14. | Fan W, Du F, Liu X. Effects of hepatitis C virus gene NS2 on the expressions of Bcl-2 and Bax in HepG2 cells. J Pak Med Assoc. 2014;64:1127-1131. [PubMed] |
| 15. | Peng X, Chen K, Chen J, Fang J, Cui H, Zuo Z, Deng J, Chen Z, Geng Y, Lai W. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of fabricius in broiler chickens. Environ Toxicol. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Ghasemian M, Mahdavi M, Zare P, Ali Hosseinpour Feizi M. Spiroquinazolinone-induced cytotoxicity and apoptosis in K562 human leukemia cells: alteration in expression levels of Bcl-2 and Bax. J Toxicol Sci. 2015;40:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Fickova M, Macho L, Brtko J. A comparison of the effects of tributyltin chloride and triphenyltin chloride on cell proliferation, proapoptotic p53, Bax, and antiapoptotic Bcl-2 protein levels in human breast cancer MCF-7 cell line. Toxicol In Vitro. 2015;29:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Abarikwu SO, Farombi EO. Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway. Pestic Biochem Physiol. 2015;118:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Yin RF, Teng JS. Wogonoside induces cell cycle arrest and mitochondrial mediated apoptosis by modulation of Bcl-2 and Bax in osteosarcoma cancer cells. Int J Clin Exp Pathol. 2015;8:63-72. [PubMed] |
| 20. | Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y, Na LX. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol Med Rep. 2015;12:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Hao C, Gao L, Zhang Y, Wang W, Yu G, Guan H, Zhang L, Li C. Acetylated chitosan oligosaccharides act as antagonists against glutamate-induced PC12 cell death via Bcl-2/Bax signal pathway. Mar Drugs. 2015;13:1267-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Zhang G, Wan Y, Zhang Y, Lan S, Jia R, Wang Z, Fan Y, Wang F. Expression of Mitochondria-Associated Genes (PPARGC1A, NRF-1, BCL-2 and BAX) in Follicular Development and Atresia of Goat Ovaries. Reprod Domest Anim. 2015;50:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Qu Y, Zhang X, Wu R. Knockdown of NF-κB p65 subunit expression suppresses growth of nude mouse lung tumor cell xenografts by activation of Bax apoptotic pathway. Neoplasma. 2015;62:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Jung KJ, Min KJ, Bae JH, Kwon TK. Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma caki cells. Oncotarget. 2015;6:1556-1568. [PubMed] |
| 25. | Choi JE, Woo SM, Min KJ, Kang SH, Lee SJ, Kwon TK. Combined treatment with ABT-737 and VX-680 induces apoptosis in Bcl-2- and c-FLIP-overexpressing breast carcinoma cells. Oncol Rep. 2015;33:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Fu H, Wang QS, Luo Q, Tan S, Su H, Tang SL, Zhao ZL, Huang LP. Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. World J Emerg Med. 2014;5:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ye L, Yuan G, Xu F, Sun Y, Chen Z, Chen M, Li T, Sun P, Li S, Sun J. The small-molecule compound BM-1197 inhibits the antiapoptotic regulators Bcl-2/Bcl-xL and triggers apoptotic cell death in human colorectal cancer cells. Tumour Biol. 2015;36:3447-3455. [PubMed] |
| 28. | EI-Emshaty HM, Saad EA, Toson EA, Abdel Malak CA, Gadelhak NA. Apoptosis and cell proliferation: correlation with BCL-2 and P53 oncoprotein expression in human hepatocellular carcinoma. Hepatogastroenterology. 2014;61:1393-1401. [PubMed] |
| 29. | Song H, Han IY, Kim Y, Kim YH, Choi IW, Seo SK, Jung SY, Park S, Kang MS. The NADPH oxidase inhibitor DPI can abolish hypoxia-induced apoptosis of human kidney proximal tubular epithelial cells through Bcl2 up-regulation via ERK activation without ROS reduction. Life Sci. 2015;126:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |