Published online Jul 7, 2015. doi: 10.3748/wjg.v21.i25.7851
Peer-review started: December 31, 2014
First decision: March 26, 2015
Revised: April 7, 2015
Accepted: May 27, 2015
Article in press: May 27, 2015
Published online: July 7, 2015
Processing time: 192 Days and 10.3 Hours
AIM: To investigated the performance of the tissue resonance interaction method (TRIM) for the non-invasive detection of colon lesions.
METHODS: We performed a prospective single-center blinded pilot study of consecutive adults undergoing colonoscopy at the University Hospital in Sassari, Italy. Before patients underwent colonoscopy, they were examined by the TRIMprobe which detects differences in electromagnetic properties between pathological and normal tissues. All patients had completed the polyethylene glycol-containing bowel prep for the colonoscopy procedure before being screened. During the procedure the subjects remained fully dressed. A hand-held probe was moved over the abdomen and variations in electromagnetic signals were recorded for 3 spectral lines (462-465 MHz, 930 MHz, and 1395 MHz). A single investigator, blind to any clinical information, performed the test using the TRIMprob system. Abnormal signals were identified and recorded as malignant or benign (adenoma or hyperplastic polyps). Findings were compared with those from colonoscopy with histologic confirmation. Statistical analysis was performed by χ2 test.
RESULTS: A total of 305 consecutive patients fulfilling the inclusion criteria were enrolled over a period of 12 months. The most frequent indication for colonoscopy was abdominal pain (33%). The TRIMprob was well accepted by all patients; none spontaneously complained about the procedure, and no adverse effects were observed. TRIM proved inaccurate for polyp detection in patients with inflammatory bowel disease (IBD) and they were excluded leaving 281 subjects (mean age 59 ± 13 years; 107 males). The TRIM detected and accurately characterized all 12 adenocarcinomas and 135/137 polyps (98.5%) including 64 adenomatous (100%) found. The method identified cancers and polyps with 98.7% sensitivity, 96.2% specificity, and 97.5% diagnostic accuracy, compared to colonoscopy and histology analyses. The positive predictive value was 96.7% and the negative predictive value 98.4%. Among the 281 non-IBD subjects, there were 7 cases with discordant results (2.5%) between TRIMprob and the reference standard including 5 false positive results (1.8%) and 2 false negative (0.7%) results. The main limitation of the TRIMprob system is the need for trained operators.
CONCLUSION: The study confirmed that TRIM provides rapid, accurate, convenient and noninvasive means to identify individuals most likely to benefit from colonoscopy.
Core tip: In this study we evaluated the potential role of a non-invasive method: the tissue resonance interaction method or TRIMprob, for enriching the population for colonoscopy with patients most likely to benefit. The apparatus was initially developed by the Italian scientist Clarbruno Vedruccio for military purpose and is another example of technology originally developed for military purpose adapted for medical use. The method is designed to detect differences in electromagnetic properties of pathologic and normal tissues and is currently being used in cancer detection in a number of other organs. The sensitivity, specificity and accuracy of the TRIMprob for detecting and correctly identifying colon cancer or polyps compared to endoscopy with histological examination were greater than 95%.
- Citation: Dore MP, Tufano MO, Pes GM, Cuccu M, Farina V, Manca A, Graham DY. Tissue resonance interaction accurately detects colon lesions: A double-blind pilot study. World J Gastroenterol 2015; 21(25): 7851-7859
- URL: https://www.wjgnet.com/1007-9327/full/v21/i25/7851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i25.7851
Colon rectal cancer (CRC) is a common disease worldwide and is associated with a high morbidity and mortality[1]. Despite improvement in cancer detection and treatment, the American Cancer Society estimated that more than 50 thousand of Americans would die of CRC in 2013[2]. Clinical outcomes in CRC are closely related to the stage of the disease at presentation. Current colon cancer prevention programs are predicated on identifying and removing premalignant and malignant lesions curable by local resection. Population screening with fecal occult blood testing, endoscopy, or radiology are currently being used in an attempt to reduce both the incidence and mortality from CRC[3].
Colonoscopy is currently the single best diagnostic test since it can identify, biopsy, and remove lesions throughout the large bowel[4]. Because most CRC are thought to develop from adenomas, the detection and removal of premalignant polyps has the potential, and has been proven, to reduce deaths from CRC. Despite major programs for CRC screening in many countries, approximately 50% of the cases of colon cancer are still diagnosed at a late stage[5], and test utilization is largely influenced by demographic and social-cultural factors[6].
Ongoing research to identify methods to enrich the proportion of patients with positive findings among the population undergoing colonoscopy have included development of improved methods for detection of fecal occult bleeding and fecal DNA testing[7]. A potential alternate approach, as described here, is based on an electronic device employing frequency-selective (resonant) absorption of electromagnetic waves capable of detecting biological anomalies in tissue in vivo such as inflammation, fibrosis, and malignant solid tumors developed by the Italian physicist, Clarbruno Vedruccio[8,9]. This technology uses a non-linear radiofrequency oscillator probe emitting electromagnetic waves at 462-465, 930, and 1395 MHz, plus harmonics as previously described[10,11]. This non-linear resonance interaction provides a selective characterization, that can be likened to an “electronic biopsy” of the tissues. The biophysical mechanisms responsible for differences in electromagnetic waves absorption have not yet been entirely elucidated. In the case of cancer, Pokorný et al[12] proposed that the effect is related to a mitochondrial malfunction (the Warburg effect) associated with increased damping of microtubule-based cellular elasto-electrical vibration states[13,14]. The principle of detection lies in the resonance between the coupled active nonlinear oscillator (the probe) and the passive oscillator (the tissue) in the radiofrequency range of the electromagnetic spectrum. Tissue suspected of harboring disease is irradiated by means of a hand-held probe placed 1 to 2 m from the patient, captured by using a special antenna and analyzed through a spectrum analyzer (Figure 1). The device has a high dynamic range, in the order of 30 decibel (dBm) or more, and can thus detect small lesions[8,15,16]. Originally the apparatus was developed for military purposes and is another example of technology being adapted for medical use.
Prior clinical experience with the TRIMprob has proven the method to be simple and reliable with high diagnostic yield when used for detection of prostate[9,15-17], breast[18], and bladder cancers[19], thyroid carcinoma in patients with multinodular goiter[20], gastric cancer[21], and rectal cancer[22]. The aim of this study was to extend the use of the TRIMprob to the non-invasive detection of colon lesions. We therefore compared the TRIMprob method for detection of colonic cancer and polyps to the results of colonoscopy with histology.
This was a prospective single-center, operator-blinded, pilot study. The clinicians, endoscopists and TRIMprob operator remained blinded to the results of the alternate method until the study was completed.
Consecutive patients aged 18 years and older attending the general gastroenterology section at the University Hospital in Sassari, Italy (Clinica Medica), with an indication for colonoscopy for any reason, were invited to participate.
Patients with an implanted pacemaker and pregnant women.
Written informed consent was obtained from each participant. The study protocol was approved by the Local Ethics Committee, more specifically by the “Comitato di Bioetica dell’Azienda Sanitaria Locale (A.S.L.) n° 1 di Sassari” (supplemental material, Appendix 1). There was no device company or commercial sponsor for this study.
The system consists of a work-station plus a battery powered hand-held probe 30 cm long with a tunable, autonomous, non linear oscillator that emits low intensity electromagnetic waves (similar to that experienced during the use of a cordless telephone). The work station is composed by a personal computer assisted spectrum analyzer-receiver to process and display the interaction between the radiofrequency probe emission and the diseased tissue (Figure 2).
In this study, a hand-held computer (PSA2701T-2.7 GHz RF Spectrum Analyzer Thurlby Thandar Instruments, Ltd, Huntingdon, United Kingdom), was added to the system; wave variations were displayed on the personal computer screen using a logarithmic scale and expressed in arbitrary units of 0-255 and also in decibel (dBm) by the hand-held computer based spectrum analyzer to facilitate signal interpretation by the operator (Figure 1).
A single investigator (MOT), blind to any clinical information, performed the test using the TRIMprob system (Galileo Avionica, Turin, Italy) approved by the “Ministero della Salute” (corresponding to the Italian FDA) as a medical device, and certified by the European Union (supplemental material, Appendix 2-4). The operator is a physician radiologist with expertise in ultrasound and specifically trained on the TRIMprob system with a multi-organ experience of more than 10 thousand TRIMprob examinations.
The test was performed approximately 15 min before the scheduled colonoscopy. All patients had completed the polyethylene glycol (PEG)-containing bowel prep for the colonoscopy procedure before being screened by the TRIM system. During the procedure the subjects remained fully dressed. The probe was moved over the surface of the abdomen (Figure 1), from the upper to the lower right and left quadrants of the abdomen, including the pelvic area in order to perform a complete examination of the colon segments and rectum. In addition, the perineal area was screened in order to exclude malignancies from the uterus or prostate.
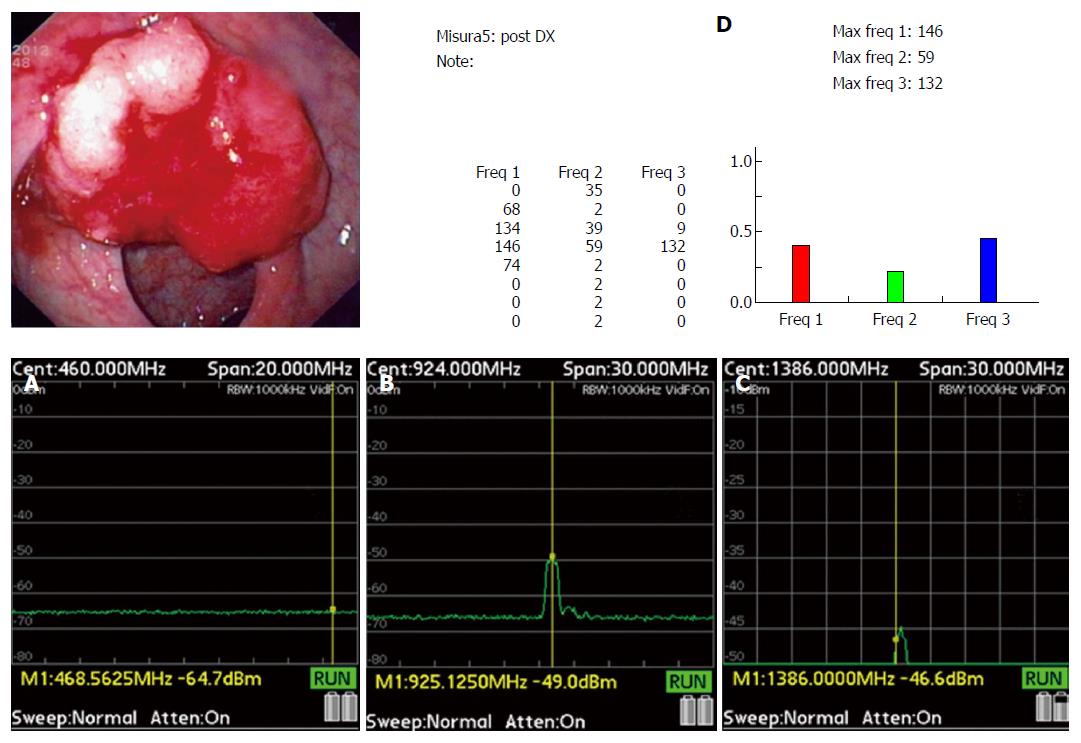
The colon was irradiated through the abdomen wall by the field resulting from the TRIM antenna. Nonlinear resonance interactions between the nonlinear oscillator and the tissue reduce the emitted wave energy at distinct frequencies on the basis of the pathological state of the tissue being examined and a reduction in signal amplitude indicates the presence of abnormal tissues or structures. Amplitude changes of the emitted signals at the established frequencies of 465, 930, and 1395 MHz were recorded in an electronic file as a value of the corresponding spectral line expressed in decibel, for each position. The entire patient examination took 8 ± 1.5 min.
Patients were provided written and verbal instructions about bowel preparation. The conventional four liter-dose PEG regimen combined with a strict liquid diet for a full day was used (supplemental material, Appendix 5).
Polyp size was assessed by comparing the polyp dimensions with the maximum jaw width of a fully open biopsy forceps (9 mm). Polyps equal to or smaller than one-half of the distance between the tips of the open biopsy forceps were termed diminutive polyps (≤ 5 mm), polyps between 6-9 mm small polyps, and those greater in size were considered as clinically significant polyps. Attempts were made to remove and retrieve all polyps irrespective of size and these, as well as biopsies from suspected cancers and other mucosal abnormalities, were sent for histological evaluation. Diminutive and small polyps were also recorded and biopsied or cold snared.
Historically, in our endoscopic unit cecal intubation had ranged from 95% to 97%. The proportion of polyps of all types identified in 100 screening colonoscopies in our population, for which there is no regional screening program, is generally typically greater than 60%.
A preliminary study was done prior to starting the blinded study and included 100 patients and 10 stool samples which were examined in vitro to determine the best threshold values for each of the three spectral lines using Receiver Operator Characteristics curves. The best cut-off for each frequency was determined by maximizing the sum of sensitivity + specificity (supplemental material, Appendix 6). Results of the interactions between the electromagnetic field emitted by the probe and normal/pathological tissue are shown in Table 1.
Sessile serrated adenoma/polyps were not found in this cohort of patients independently evaluated. The presence of stool appears as a decrease of 25 dBm in the first spectral line.
Analysis of the data from the blinded trial: The results of TRIMprob analysis were compared to that of the reference gold standard in a 2 × 2 contingency table, the absolute number of true positives (TPs), false positives (FPs), true negatives (TNs), and false negatives (FNs) was determined. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the TRIM procedure compared to the gold standard (colonoscopy with histologic confirmation) were calculated and 95% confidence intervals (CI) were estimated with the Wilson score method[23]. The Matthews correlation coefficient (MCC) was calculated[24], as a balanced measure of agreement between the two methods under comparison. For each study participant the most advanced colon lesion was considered in order to assign the patient to a diagnostic category. All analyses were performed with the SPSS statistical package (v. 16.0, Chicago, United States) and P-values lower or equal than 0.05 were considered statistically significant. The statistical review of the study was performed by a biomedical statistician. This report was done following STARD guidelines[25].
A total of 305 consecutive patients fulfilling the inclusion criteria were enrolled over a period of 12 mo. Patients were scheduled for colonoscopy for a variety of reasons (supplemental material Appendix 7).
The TRIMprob was well accepted by all patients, none spontaneously complained about the procedure, and no adverse effects were observed. Cecal intubation was performed in all patients, however in 10 cecum visualization was suboptimal because of a poor bowel prep.
TRIM proved inaccurate for polyp detection in patients with inflammatory bowel disease (IBD) likely because mucosal inflammation produced false positive results for polyps. Because IBD patients are not candidates for routine screening colonoscopy of normal patients, the analysis was performed both on all patients examined and separately without the 24 IBD patients to provide a better approximation of the intended population (Table 2) (i.e., as an adjunct to screening colonoscopy). Overall, the results were similar whether the IBD patients were included or excluded. Additional material about IBD patients is provided in the online supplemental material (Appendix 8).
| Histology | Colonoscopy(No. of positive cases/total No. of cases) | TRIMProb(No. of TP, FP, FN, TN) | Sensitivity, % (95%CI) | Specificity, % (95%CI) | Positive predictive value, % (95%CI) | Negative predictive value, % (95%CI) | |||
| All cases | 156/305 | 154 | 12 | 2 | 137 | 98.7 | 91.9 | 92.8 | 98.6 |
| (IBD + Non IBD) | (95.4-99.6) | (86.4-95.3) | (87.8-95.8) | (94.9-99.6) | |||||
| Non IBD cases | 149/281 | 147 | 5 | 2 | 127 | 98.7 | 96.2 | 96.7 | 98.4 |
| (95.2-99.6) | (91.4-98.4) | (92.5-98.6) | (94.5-99.6) | ||||||
| Hyperplastic1 | 73/281 | 71 | 4 | 2 | 204 | 97.3 | 98.1 | 94.7 | 99 |
| (90.5-99.2) | (95.2-99.2) | (87.0-97.9) | (96.5-99.7) | ||||||
| Adenomas2 | 47/281 | 40 | 4 | 7 | 230 | 85.1 | 98.3 | 90.9 | 97 |
| (72.3-92.6) | (95.7-99.3) | (78.8-96.4) | (94.0-98.6) | ||||||
| Advanced adenomas3 | 17/281 | 14 | 1 | 3 | 263 | 82.4 | 99.6 | 93.3 | 98.9 |
| (58.9-93.8) | (97.9-99.9) | (70.2-98.8) | (96.7-99.6) | ||||||
| Cancer | 12/281 | 12 | 0 | 0 | 269 | 100 | 100 | 100 | 100 |
| (75.7-100.0) | (98.5-100.0) | (75.7-100.0) | (98.6-100.0) | ||||||
In the 281 non-IBD patients TRIM was able to detected all adenocarcinomas (12: 3 were Tis; 4 were T1 N0 M0; 2 were T2 N0 M0; 2 Any T N M0; Any T Any N M1b respectively) (Figure 3), and 135 of 137 polyps (98.5%) (including 100% of the 64 adenomas) found at colonoscopy (supplemental material, Appendix 9). Among the 135 polyps detected, TRIMprob was able to correctly categorize 125 (Table 2). Ten TRIMprob diagnoses of the polyp histology were incorrect: 5 adenomas were thought to be hyperplastic polyps and 2 adenomas were recognized as adenomas but thought to be advanced adenomas. Among the advanced adenomas 2 were falsely characterized as hyperplastic polyps and 1 as an adenoma.
Assuming colonoscopy/histology as the gold standard for detecting adenomas, the overall sensitivity, specificity, positive and negative predictive values of TRIMprob among the non-IBD patients were 98.7%, 96.2%, 96.7%, and 98.4%. For the entire group examined the results were: sensitivity 98.7%; specificity 91.9%, positive and negative predictive value 92.8% and 98.6% respectively (Table 2).
Among the 281 non-IBD subjects, there were 7 cases with discordant results (2.5%) between TRIMprob and the reference standard including 5 false positive results (1.8%) and 2 false negative (0.7%) results (Table 3).
| 281 non-IBD patients | Normal | Hyperplasia | LGD | HGD | Cancer | Total |
| Colonoscopy | ||||||
| Normal | 127 | 2 | 0 | 0 | 0 | 129 |
| Hyperplasia | 0 | 73 | 5 | 2 | 0 | 80 |
| LGD | 4 | 0 | 40 | 1 | 0 | 45 |
| TRIM | ||||||
| HGD | 1 | 0 | 0 | 14 | 0 | 15 |
| Cancer | 0 | 0 | 0 | 0 | 12 | 12 |
| Total | 132 | 75 | 45 | 17 | 12 | 281 |
The 5 false positives that were not confirmed by the colonoscopy included one thought to be an advanced adenoma in the transverse and 4 thought to be hyperplastic polyps. There were no false positives for the presence of cancer. Importantly, second look endoscopy was not done to confirm that the false positive results were truly false positive results.
Two patients had hyperplastic polyps that went undetected. Both were between 6 and 9 mm and located in the sigmoid colon. One possible speculation could be a difficult niche, or the small size of the polyp and subsequent studies will need to look specifically at this region of the colon.
Patients with poor bowel prep: In the 10 patients with a poor bowel prep there were no false positive or false negative TRIMprob results. All patients with poor bowel preps had the colonoscopy repeated without additional finding.
The TRIMprob test displayed good performance distinguishing the number of polyps (supplemental material, Appendix 10). The consistency between the TRIM assay and the colonoscopy/histology was high as reflected by a Matthews correlation coefficient (MCC) of 0.897.
Adenoma detection and removal is one of the primary targets for prevention of CRC[4]. Currently, colonoscopy is the best available screening tool because it can both detect and remove precancerous lesions[4]. However, colonoscopy is labor intensive, expensive, and its availability is considerably less than the size of the population at risk. Attempts have been made to enrich the screening population in terms of significant abnormalities by the use of fecal occult blood testing (FOBT), fecal DNA testing, or computed tomographic (CT) colonography[26]. However, FOBT has a relatively poor sensitivity for adenoma detection and CT colonography is associated with significant radiation[27-30].
This pilot study evaluated a non-invasive detection method to identify premalignant and malignant colon lesions. Compared with colonoscopy/histology TRIMprob yielded a sensitivity as high as 98.7% with a high concordance (up to 90%) between the two methods. These results are similar to those reported for use of the TRIMprob method in prostate cancer (95.5%-86%)[9,15-17], breast cancer (84%)[18], for carcinomas detection in patients with multinodular goiter (100%)[20], gastric cancer (100%)[21], and for rectal malignant lesions (94%)[22]. More specifically in that pilot study, Vannelli et al[22] reported a diagnostic accuracy of the TRIMprob of 89.5% when a cutoff of 50 arbitrary units was chosen for the 465-MHz frequency. The specificity was 85.1% and lower than what was observed in our study (97.5%) suggesting a lower proportion of false positive attributable to a more expert operator[22].
For bladder cancer the level of agreement between TRIMprob and cystoscopy was also high (Cohen’s K = 0.77, P < 0.001)[19].
In this preliminary study, false-negative results among the non-IBD patients consisted of 2 cases thought to have hyperplastic polyps (0.7%). There was 100% TRIM-endoscopy concordance for actual cancers. One other case had a false positive diagnosis of an advanced adenoma that was not confirmed at the blinded endoscopy. Endoscopy was however not repeated to ensure that it was a true false positive result. In ten cases TRIM was able to detect benign lesions but failed to correctly categorize the type of benign polyp histologically.
This study was designed to evaluate the accuracy of the TRIM approach for identifying and correctly categorizing colonic lesions in a mixed population scheduled for endoscopy for any reason with the goal to detect polyps or cancer and thus potentially reduce the incidence of negative colons. The study was not restricted to those meeting the criteria for screening and contained patients with indications for colonoscopy which is likely responsible for the higher prevalence of CRC detected.
The TRIMprob demonstrated to provide excellent results except in patients with ulcerative colitis or Crohn’s disease where 7 of the 24 subjects had false positive results for polyps. IBD patients have marked mucosal inflammation and those with Crohn’s disease have full bowel wall thickness inflammation. As this group of patients is markedly different from healthy subjects who would participate in colon cancer screening in retrospect, they should not have been included. However, the data with and without IBD patients is given. Future studies will be required to determine what is responsible for the TRIMprob findings in IBD patients and include comparisons of the histology and radiologic findings in the areas with TRIMprob abnormalities.
One limitation of the TRIMprob system is the requirement for trained operators but this requirement is no different from any diagnostic technique including colonoscopy. Those who are already expert in interpreting ultrasound, computer tomography or magnetic resonance images should be able to quickly become proficient in this technology. Interobserver evaluations of trained TRIMprob operators for other indications have shown excellent correlations close to 100%[9,17]. These data suggest that developing a cadre of individuals with expertise in colon screening should not be a major problem.
The probe oscillations of biological tissues produce the phenomenon of “non linear resonance interaction’’ which is detected by the TRIM receiver. Because the required intensities of the electromagnetic waves are very low, there is thought to be no health hazard. The current price of the TRIMprob system is about € 60000. For the single patients we can hypothesize, at least in Europe, a cost no more expensive than an abdominal ultrasound (€ 100). An additional side benefit of the device is the possibility to detect other potential lesions located in the scanned areas, regardless of their origin.
Our results should encourage additional studies with different designs to confirm these results and explore other parameters. For example, to examine the need for the colon prep one might examine patients scheduled for screening endoscopy (e.g., one might perform TRIM before colon prep, immediately following the colon prep, and then perform the screening endoscopy). Those with positive TRIM but negative colonoscopy could be identified immediately after the procedure such as by opening a sealed opaque envelope to allow immediate comparison prompting the endoscopist to reevaluate a certain segment of the colon where lesions were identified but not seen on endoscopy. Alternate designs would couple TRIM with iFOBT to ask whether the combination would be complementary for identification of those most likely to benefit from colonoscopy. Other areas of research include evaluation of methods to automate the interpretation of the TRIMprob results and thus reduce the need for highly trained operators.
Overall our results are consistent with the notion that the non-invasive TRIMprob method is a novel, highly effective method for identifying which patients would likely benefit most from colonoscopy. The TRIMprob examination has the potential to become a first line tool in the armamentarium for CRC prevention. TRIMprob colon screening virtually offers an inexpensive, noninvasive approach that could make colonoscopic screening both more efficient and cost effective. Future research includes development of software to analyze the images to reduce the need for highly trained operators as well testing whether a bowel prep is needed prior to TRIM screening.
Colorectal cancer is a common and lethal disease. Colonoscopy is currently the preferred modality of screening, although associated with high costs and low patient compliance. Recently, a non invasive device was developed for detecting differences in electromagnetic properties of cancerous and normal tissues, using a non linear tuneable oscillator, the tissue resonance interaction method (TRIM) that proved to accurately identify those patients who would likely benefit from colonoscopy.
The diagnosis of cancer in humans is mainly based on morphological changes in cells and irregularities in tissues confirmed using cytological and histological methods. The TRIMprob focuses on differences in the biochemical metabolism between cancer and normal cells which produce electromagnetic fields inducing alignment in dipole movements. Most of the molecules in the body are electrical dipoles which electronically function like transducers in that they are able to transform acoustic waves into electrical waves and electrical waves into acoustic waves. The natural properties of biomolecular structures enable cell components and whole cells to oscillate and interact resonantly with other cells. The cells of the body and cellular components possess the ability to function as electrical resonators. A dipole movement is a function of polarization processes and the strength of the electric field. When cell membranes in the biological tissue are exposed to an electric field in the right frequency and amplitude windows a preferential alignment of dipoles occurs. TRIMprob utilizes frequency selective (resonant) absorption of electromagnetic waves in malignant tumors. The biochemical interactions are dislodged from electromagnetic fields in this way the TRIMprob represents a revolution in devising a new way of screening. This method has previously been proven to be highly accurate in detection of other cancers such as breast and prostate.
Colorectal cancer is one of the most common malignancies. Current colon cancer prevention programs focus on polyp detection and removal and detection of early cancers using colonoscopy to reduce cancer incidence and mortality. However, colonoscopy is associated with high costs, poor patient acceptability, and the majority of examinations are negative making the method very inefficient. A method to non-invasively detect lesions would allow to target colonoscopy to those who would most likely benefit. Here, the authors show that using a non-linear tunable oscillator to detect differences in electromagnetic properties of biological abnormal and normal tissues (i.e., TRIM) can achieve that goal.
This operator-blinded pilot study shows that TRIM is a rapid, highly accurate, and noninvasive method to identify individuals most likely to have polyps or cancers and thus to benefit from colonoscopy. If confirmed by subsequent studies, TRIM is likely to revolutionize colon cancer prevention programs.
This article reported the TRIMprob used to detect colon polys and cancers. TRIM present highly sensitivity and specificity. TRIMprob scanning is a valuable tool in diagnosing carcinoma of prostate, breast, gastric, rectal and so on. In this study, the authors compared TRIMprob with colonoscopy. It’s of clinical significance and convincing.
P- Reviewer: Kim TI, Li YY, Tan KY, Yao HR S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25518] [Article Influence: 1822.7] [Reference Citation Analysis (7)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9853] [Article Influence: 821.1] [Reference Citation Analysis (4)] |
| 3. | Centers for Disease Control and Prevention (CDC). Use of colorectal cancer tests--United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57:253-258. [PubMed] |
| 4. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1455] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 5. | Henley SJ, King JB, German RR, Richardson LC, Plescia M; Centers for Disease Control and Prevention (CDC). Surveillance of screening-detected cancers (colon and rectum, breast, and cervix) - United States, 2004-2006. MMWR Surveill Summ. 2010;59:1-25. [PubMed] |
| 6. | Stock C, Brenner H. Utilization of lower gastrointestinal endoscopy and fecal occult blood test in 11 European countries: evidence from the Survey of Health, Aging and Retirement in Europe (SHARE). Endoscopy. 2010;42:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, Ahnen DJ, Knigge K, Lance MP, Burgart LJ, Hamilton SR. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441-450, W81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Vedruccio C, Meessen A. EM cancer detection by means of non linear resonance interaction. Italy: Pisa 2004; 909-912. |
| 9. | Bellorofonte C, Vedruccio C, Tombolini P, Ruoppolo M, Tubaro A. Non-invasive detection of prostate cancer by electromagnetic interaction. Eur Urol. 2005;47:29-37; discussion 37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Brenan KE, Campbell SL, Campbell SLV, Petzold LR. Numerical solution of initial-value problems in differential algebraic equations. Philadelphia: Society for Industrial Mathematics 1996; . |
| 11. | Dormand JR, Prince PJ. A family of embedded Runge-Kutta formulae. J Comput Appl Math. 1980;6:19-26. [DOI] [Full Text] |
| 12. | Pokorný J, Vedruccio C, Cifra M, Kučera O. Cancer physics: diagnostics based on damped cellular elastoelectrical vibrations in microtubules. Eur Biophys J. 2011;40:747-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Purdom L, Ambrose EJ, Klein G. A correlation between electrical surface charge and some biological characteristics during the stepwise progression of a mouse sarcoma. Nature. 1958;181:1586-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Van Lamsweerde-Gallez D, Meessen A. The role of proteins in a dipole model for steady-state ionic transport through biological membranes. J Membr Biol. 1975;23:103-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Da Pozzo L, Scattoni V, Mazzoccoli B, Rigatti P, Manferrari F, Martorana G, Pietropaolo F, Belgrano E, Prezioso D, Lotti T. Tissue-resonance interaction method for the noninvasive diagnosis of prostate cancer: analysis of a multicentre clinical evaluation. BJU Int. 2007;100:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Tubaro A, De Nunzio C, Trucchi A, Stoppacciaro A, Miano L. The electromagnetic detection of prostatic cancer: evaluation of diagnostic accuracy. Urology. 2008;72:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Gokce O, Sanli O, Salmaslioglu A, Tunaci A, Ozsoy C, Ozcan F. Tissue Resonance Interaction Method (TRIMprob) has the potential to be used alongside the recognized tests in the screening protocols for prostate cancer. Int J Urol. 2009;16:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | De Cicco C, Mariani L, Vedruccio C, Ricci C, Balma M, Rotmensz N, Ferrari ME, Autino E, Trifirò G, Sacchini V. Clinical application of spectral electromagnetic interaction in breast cancer: diagnostic results of a pilot study. Tumori. 2006;92:207-212. [PubMed] |
| 19. | Gervino G, Autino E, Kolomoets E, Leucci G, Balma M. Diagnosis of bladder cancer at 465 MHz. Electromagn Biol Med. 2007;26:119-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Sacco R, Innaro N, Pata F, Lucisano AM, Talarico C, Aversa S. [Preoperative diagnosis of incidental carcinoma in multinodular goitre by means of electromagnetic interactions]. Chir Ital. 2007;59:247-251. [PubMed] |
| 21. | Sacco R, Sammarco G, De Vinci R, Vescio G, Scarpelli A, Lucisano AM, Pata F, Mascia E, Martines V. [Relief of gastric cancer with an electromagnetic interaction system (TRIMprob) in outpatients]. Chir Ital. 2007;59:823-828. [PubMed] |
| 22. | Vannelli A, Leo E, Battaglia L, Poiasina E. Diagnosis of rectal cancer by electromagnetic interactions: preliminary results. Dis Colon Rectum. 2009;52:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Gardner MJ, Altman DG. Calculating confidence intervals for proportions and their differences. Statistics with confidence. London: BMJ Publishing Group 1989; 28-33. |
| 24. | Baldi P, Brunak S, Chauvin Y, Andersen CA, Nielsen H. Assessing the accuracy of prediction algorithms for classification: an overview. Bioinformatics. 2000;16:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1097] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 25. | Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138:W1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 842] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 26. | Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85:1311-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 250] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Guittet L, Bouvier V, Mariotte N, Vallee JP, Arsène D, Boutreux S, Tichet J, Launoy G. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut. 2007;56:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Zalis ME, Blake MA, Cai W, Hahn PF, Halpern EF, Kazam IG, Keroack M, Magee C, Näppi JJ, Perez-Johnston R. Diagnostic accuracy of laxative-free computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: a prospective evaluation. Ann Intern Med. 2012;156:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Muñoz R, Lau C, Somsouk M, El-Nachef N. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |