Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4946
Peer-review started: October 18, 2014
First decision: December 2, 2014
Revised: December 31, 2014
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: April 28, 2015
Processing time: 193 Days and 21.5 Hours
AIM: To identify criteria for predicting successful drainage of unresectable malignant hilar biliary strictures (UMHBS) because no ideal strategy currently exists.
METHODS: We examined 78 patients with UMHBS who underwent biliary drainage. Drainage was considered effective when the serum bilirubin level decreased by ≥ 50% from the value before stent placement within 2 wk after drainage, without additional intervention. Complications that occurred within 7 d after stent placement were considered as early complications. Before drainage, the liver volume of each section (lateral and medial sections of the left liver and anterior and posterior sections of the right liver) was measured using computed tomography (CT) volumetry. Drained liver volume was calculated based on the volume of each liver section and the type of bile duct stricture (according to the Bismuth classification). Tumor volume, which was calculated by using CT volumetry, was excluded from the volume of each section. Receiver operating characteristic (ROC) analysis was performed to identify the optimal cutoff values for drained liver volume. In addition, factors associated with the effectiveness of drainage and early complications were evaluated.
RESULTS: Multivariate analysis showed that drained liver volume [odds ratio (OR) = 2.92, 95%CI: 1.648-5.197; P < 0.001] and impaired liver function (with decompensated liver cirrhosis) (OR = 0.06, 95%CI: 0.009-0.426; P = 0.005) were independent factors contributing to the effectiveness of drainage. ROC analysis for effective drainage showed cutoff values of 33% of liver volume for patients with preserved liver function (with normal liver or compensated liver cirrhosis) and 50% for patients with impaired liver function (with decompensated liver cirrhosis). The sensitivity and specificity of these cutoff values were 82% and 80% for preserved liver function, and 100% and 67% for impaired liver function, respectively. Among patients who met these criteria, the rate of effective drainage among those with preserved liver function and impaired liver function was 90% and 80%, respectively. The rates of effective drainage in both groups were significantly higher than in those who did not fulfill these criteria (P < 0.001 and P = 0.02, respectively). Drainage-associated cholangitis occurred in 9 patients (12%). A smaller drained liver volume was associated with drainage-associated cholangitis (P < 0.01).
CONCLUSION: Liver volume drainage ≥ 33% in patients with preserved liver function and ≥ 50% in patients with impaired liver function correlates with effective biliary drainage in UMHBS.
Core tip: An ideal biliary drainage strategy for unresectable malignant hilar biliary strictures (UMHBS) has not been defined. The aim of our study was to identify useful criteria for predicting successful drainage of UMHBS. In the present study, multivariate analysis revealed that liver function and drained liver volume calculated by computed tomography volumetry (CTV) are independent factors of drainage effectiveness for UMHBS. Receiver operating characteristic analysis showed cutoff values of 33% of liver volume for patients with preserved liver function and 50% for patients with impaired liver function. Before attempting biliary drainage procedures, establishing an appropriate drainage strategy using CTV is important.
- Citation: Takahashi E, Fukasawa M, Sato T, Takano S, Kadokura M, Shindo H, Yokota Y, Enomoto N. Biliary drainage strategy of unresectable malignant hilar strictures by computed tomography volumetry. World J Gastroenterol 2015; 21(16): 4946-4953
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4946.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4946
Effective treatment of obstructive jaundice is essential for improving the quality of life of patients with unresectable malignant hilar biliary strictures (UMHBS). These strictures may be caused by malignancies of the bile ducts, pancreas, or liver (primary or metastatic) or by metastases to lymph nodes around the bile ducts. In biliary obstruction in the hepatic hilum, effective drainage is difficult to achieve because of the anatomical complexity of the bile ducts. Various procedures of biliary drainage of hilar biliary obstruction have been performed: percutaneous or endoscopic routes[1-5], plastic or metallic stents[6-11], and unilateral or bilateral hepatic duct drainage[11-21]. However, no consensus has been reached on the optimal drainage strategy for treating biliary obstruction.
In recent years, advances in diagnostic imaging, such as multi-detector computed tomography (CT) and magnetic resonance cholangiopancreatography, have facilitated improved preoperative identification of an aberrant hepatic duct and determination of hilar tumor progression. In this study, we estimated the volumes of the lateral and medial sections of the left hepatic liver and the anterior and posterior sections of the right liver by using CT volumetry in patients who required drainage for UMHBS. We then measured the drained liver volume after stenting according to the type of bile duct stricture (bismuth classification) and assessed the effects of drainage and early complications related to drainage procedures.
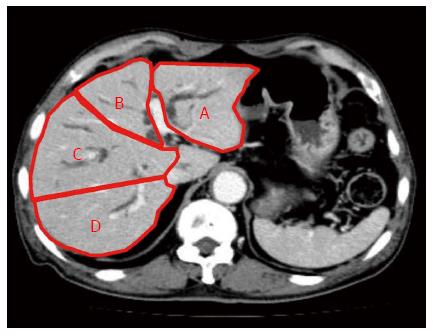
We retrospectively reviewed data on 78 patients who underwent biliary drainage procedures for UMHBS between March 2004 and April 2013. The inclusion criteria were as follows: obstructive jaundice caused by hilar malignancy (Bismuth type II or higher); abdominal CT scan performed within 2 wk before drainage; and clinical biochemical tests performed before and 14 d after stent placement. The exclusion criteria were as follows: benign stenosis; Bismuth type I obstruction; history of hepatectomy; or the presence of UMHBS without jaundice before stent placement, for which a stent was prophylactically placed. The presence of distant metastasis, locally far-advanced tumors, and/or poor liver function would preclude resection based on the consensus of gastroenterologists and surgeons. Figure 1 shows a CT volumetric image of a patient with UMHBS.
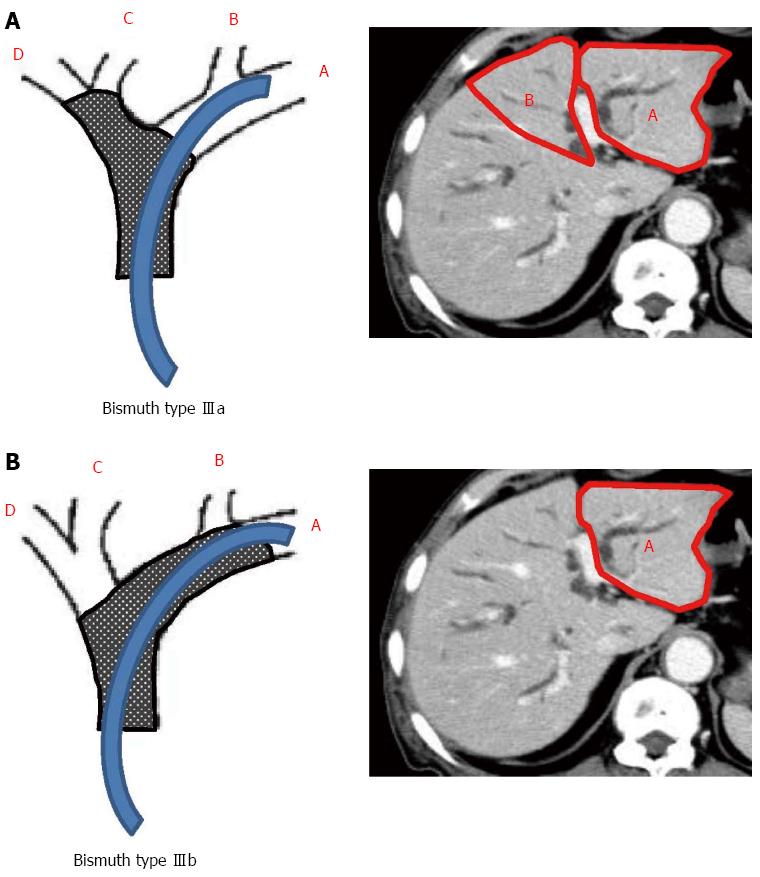
The 4 sections of the liver were defined according to the distribution of the portal vein branches: lateral and medial sections of the left liver, and anterior and posterior sections of the right liver. The drained liver volume was evaluated as follows. The area of each section was calculated by manual tracing using CT scans (axial view) with a 5 mm slice thickness. The volume of each section was calculated as the summed area of the comprising sections. Thereafter, drained liver volume was calculated based on the volume of each liver section and the type of bile duct stricture (according to the Bismuth classification). Figure 2A and B show the valid drainage calculation method. Tumor volume, which was calculated by using CT volumetry, was excluded from the volume of each section. Drainage was considered effective when the serum bilirubin level decreased by ≥ 50% of the value before stent placement within 2 wk after drainage, without additional intervention. Complications that occurred within 7 d after stent placement were considered as the early complications.
Receiver operating characteristic (ROC) analysis was performed to determine the cutoff value for drained liver volume in the group with preserved liver function (with normal liver or compensated liver cirrhosis) and the group with impaired liver function (with decompensated liver cirrhosis). Patients with hepatic encephalopathy and/or cytologically negative ascites in addition to chronic liver disease were considered to have decompensated liver cirrhosis.
The χ2 test or Fisher’s exact test of the contingency table was used for univariate analysis of the categorical data. Student’s t-test was used for analysis of the quantitative data. Multivariate analysis of the factors contributing to the initial drainage effect was performed using multiple logistic regression analysis. A statistically significant difference was defined as P < 0.05. The statistical methods of this study were reviewed by Dr. Kohta Suzuki from the Department of Health Sciences, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi.
Table 1 shows patient characteristics and morphological data. The subject cohort had a mean age of 74.8 ± 10.3 years (53 men and 25 women) and included 50 patients (64%) with cholangiocarcinoma, 10 (13%) with hepatocellular carcinoma, 9 (12%) with gallbladder carcinoma, 5 (6%) with metastatic liver carcinoma, and 4 (5%) with lymph node metastasis. According to Bismuth classification, 11 patients were classified as type II, 24 as type IIIa, 7 as type IIIb, and 36 as type IV. The median total bilirubin level was 9.0 mg/dL (3.0-31.0 mg/dL). Sixty-four patients (82%) had preserved liver function (without decompensated liver cirrhosis), and 14 (18%) had impaired liver function (with decompensated liver cirrhosis). With regard to the drainage procedure, endoscopic biliary stenting (metal stent/plastic stent), endoscopic nasobiliary drainage, and percutaneous transhepatic biliary drainage were performed in 49 (11/38), 21, and 8 patients, respectively. Unilateral and bilateral drainage were performed in 72 patients (92%) and 6 patients (8%), respectively. The average drained liver volume was 44.6% ± 18.6%.
| Characteristic | |
| Age (yr), mean ± SD | 74.8 ± 10.3 |
| Gender, male/female | 53/25 |
| Etiology | |
| Cholangiocarcinoma | 50 (64) |
| Hepatocellular carcinoma | 10 (13) |
| Gallbladder carcinoma | 9 (12) |
| Liver metastasis | 5 (6) |
| Lymph node metastasis | 4 (5) |
| Bismuth type (II/IIIa/IIIb/IV) | 11/24/7/36 |
| Total bilirubin (mg/dL), median (range) | 9.0 (3.0-31.0) |
| Cholangitis | 13 (17) |
| Liver function | |
| Preserved liver function (without decompensated liver cirrhosis) | 64 (82) |
| Impaired liver function (with decompensated liver cirrhosis) | 14 (18) |
| Drainage method (MS/PS/ENBD/PTBD) | 11/38/21/8 |
| Drainage areas | |
| Unilateral/bilateral | 72/6 |
| Drained liver volume (%), mean ± SD | 44.6 ± 18.6 |
Drainage procedures were successfully performed in 78 patients (procedure success rate, 100%). Effective drainage was achieved in 49 of the patients (63%). Of the 21 patients who underwent additional drainage because of ineffective initial drainage, successful drainage was achieved in 17. Thus, eventually, 66 patients (85%) had effective drainage. Univariate analysis for each factor potentially influencing the effectiveness of initial drainage showed that there were significant differences in Bismuth type IV (P = 0.02), impaired liver function (P = 0.03), and drained liver volume (%) (P < 0.01). There was no significant difference between unilateral and bilateral drainage (Table 2). Multivariate analysis (multiple logistic analysis) of the 3 factors with significant differences in univariate analysis indicated that the independent factors contributing to the effectiveness of drainage were impaired liver function (OR = 0.06, 95%CI: 0.009-0.426; P = 0.005) and drained liver volume (OR = 2.92, 95%CI: 1.648-5.197; P < 0.001) (Table 3).
| Effective (n = 49) | Ineffective (n = 29) | P value | |
| Age (yr), mean ± SD | 74.0 ± 11.6 | 76.0 ± 7.5 | 0.39 |
| Gender, male/female | 32/17 | 21/8 | 0.78 |
| Etiology, cholangiocarcinoma | 32 (65.3) | 18 (62.1) | 0.81 |
| Bismuth type IV | 17 (34.6) | 19 (65.5) | 0.02 |
| Total bilirubin (mg/dL), mean ± SD | 9.9 ± 6.3 | 10.0 ± 4.7 | 0.93 |
| Cholangitis | 8 (16.3) | 5 (17.2) | 0.91 |
| Impaired liver function | 5 (10.2) | 9 (31.0) | 0.03 |
| Drainage method, endoscopic drainage | 43 (87.8) | 27 (93.1) | 0.71 |
| Type of stent, metal stent | 7 (14.3) | 4 (13.8) | 1.00 |
| Drainage areas | |||
| Unilateral/bilateral | 43/6 | 29/0 | 0.16 |
| Drained liver volume (%), mean ± SD | 51.1 ± 18.6 | 33.5 ± 12.5 | < 0.01 |
| Factors | OR | 95%CI | P value |
| Bismuth type IV | 0.92 | 0.245-3.418 | 0.896 |
| Impaired liver function | 0.06 | 0.009-0.426 | 0.005 |
| Drained liver volume | 2.92 | 1.648-5.197 | < 0.001 |
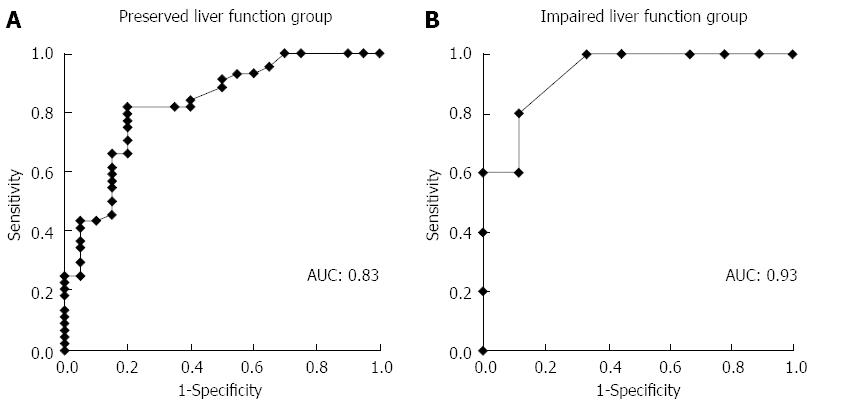
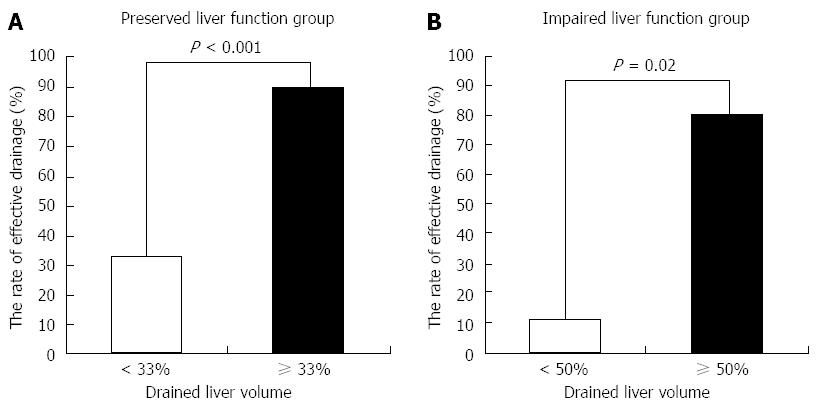
In ROC analysis of the drained liver volume required for effective drainage, the area under the curve with preserved liver function and with impaired liver function was 0.83 and 0.93, respectively (Figure 3). The optimal cutoff (%), 33% of liver volume for subjects with preserved liver function and 50% of liver volume for subjects with impaired liver function, was calculated by determining the smallest distance between the ROC curve and the upper left corner of the graph. The sensitivity and specificity of these cutoff values were 82% and 80% for preserved liver function, and 100% and 67% for impaired liver function, respectively. When the cutoff value was set at 33% in patients with preserved liver function, the rate of effective drainage was 90% (≥ 33% of liver volume) and 33% (< 33% of liver volume) (P < 0.001) (Figure 4A). When the cutoff value was set at 50% in patients with impaired liver function, the rate of effective drainage was 80% (≥ 50% of liver volume) and 11% (< 50% of liver volume; P = 0.02) (Figure 4B).
Early complications occurring within 7 d after stent placement were found in 14 patients (18%) and included pancreatitis in 5 (6%) and drainage-associated cholangitis in 9 (12%). All pancreatitis episodes were mild and resolved with conservative management alone. Among the 9 patients with cholangitis, 5 patients required additional stenting and the remaining 4 patients were managed with antibiotics alone.
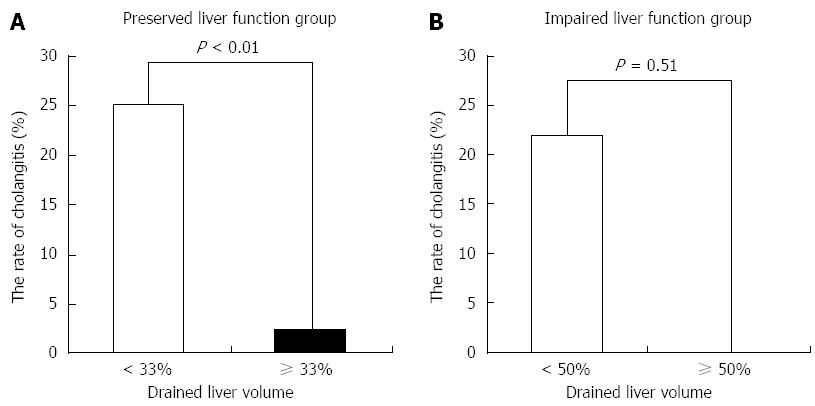
Factors associated with cholangitis are shown in Table 4. Univariate analysis for each factor associated with cholangitis showed that there was a significant difference in drained liver volume. Smaller drained liver volume was associated with drainage-associated cholangitis (P < 0.01). In the preserved liver function group, the incidence of cholangitis was 25% in patients with < 33% of liver volume vs 2.5% in patients with ≥ 33% of liver volume (P < 0.01) (Figure 5A). In the impaired liver function group, the incidence of cholangitis was 22% in patients with < 50% of liver volume vs 0% in patients with ≥ 50% of liver volume (P = 0.51) (Figure 5B).
| Cholangitis | Non-cholangitis | P value | |
| n = 9 | n = 69 | ||
| Age (yr), mean ± SD | 76.9 ± 8.3 | 74.5 ± 10.5 | 0.51 |
| Gender, male/female | 5/4 | 48/21 | 0.46 |
| Etiology, cholangiocarcinoma | 6 (66.7) | 44 (63.8) | 1.00 |
| Bismuth type IV) | 6 (66.7) | 30 (43.5) | 0.29 |
| Total bilirubin (mg/dL) | 8.3 ± 3.5 | 10.6 ± 6.1 | 0.12 |
| Impaired liver function | 2 (22.2) | 7 (10.1) | 0.66 |
| Drainage method, endoscopic drainage | 7 (77.8) | 63 (91.3) | 0.23 |
| Type of stent, metal stent | 1 (11.1) | 10 (14.5) | 1.00 |
| Drainage areas | |||
| Unilateral/bilateral | 9/0 | 63/6 | 1.00 |
| Drained liver volume (%), mean ± SD | 29.3 ± 4.4 | 46.6 ± 18.9 | < 0.01 |
To establish a more effective strategy for biliary drainage, a number of studies concerning the drainage route, the number, size, and material of the stent have been reported[1-23]. In recent years, advances in diagnostic imaging have provided detailed information about bile duct strictures and liver volume. The surgical indications and extent of resection for hepatectomy are determined based on the results of preoperative liver function and remnant liver volume[24-26]. However, few research studies are available on the significance of individual liver function and liver volume in biliary drainage.
In the present study, multivariate analysis revealed that liver function and drained liver volume were independent factors of drainage effectiveness for UMHBS. Vienne et al[27] reported that effective drainage could be attained by draining ≥ 50% of the liver volume based on examination of bile duct stricture type and volume of each section. The required drained liver volume is expected to be variable depending on liver function; however, no previous reports are available on the role of liver function in determining the drainage method. Our study revealed that effective drainage could be expected by draining ≥ 33% of the liver volume in those with preserved liver function and ≥ 50% of the liver volume in those with impaired liver function. The overall drainage response rate in this study was 63%, but the response rate was 89% for the subjects who met the abovementioned criteria. These subjects had a better response rate than those in earlier studies (49%-87%)[3,13-19]. In addition, of the 29 patients who did not respond to the initial drainage, 24 (83%) did not meet the above criteria. Inadequate drained volume is a possible reason for drainage failure. This study is the first report to determine the drainage method of UMHBS by using both drained liver volume and liver function. When unilateral drainage achieves the required drained liver volume, which occurred in 83% of the subjects, unilateral drainage is sufficient. Bilateral drainage should be considered only when the estimated drained liver volume by unilateral stenting does not reach the abovementioned criteria.
Cholangitis is an important complication after stenting for drainage of hilar biliary strictures. Contrast injection into the undrained sector and stent placement within a small area were risk factors for cholangitis[5,6,13,16,27], which may be attributed to the decreased bile excretory function in an atrophied area[27]. In our series, 3 of 9 patients (33%) who underwent cholangiography of the undrained section developed cholangitis (data not shown). In addition, our study found that drainage of a small area was a risk factor contributing to post-drainage cholangitis. This finding supports those of previous studies. Although there was no statistical difference in cholangitis in impaired liver function because of a lack of power, cholangitis was not observed in patients with the obtained cutoff values or more.
Our study has 2 limitations. First, because it was a retrospective study, the drainage procedures were not uniform; the procedures were chosen by the patient’s attending physician, which might have led to bias. Second, because of the sample size, statistical subgroup analyses were not possible (e.g., analysis by the type of disease and Bismuth classification). Our observations should be confirmed in a multicenter prospective study with a large number of patients.
In conclusion, liver function and drained liver volume are important factors in the drainage of unresectable malignant hilar biliary strictures. Assembling an appropriate drainage strategy using CT volumetry before ERCP is important.
We thank Dr. Kohta Suzuki, from the Department of Health Sciences, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, for reviewing our statistical analysis.
The effective treatment of obstructive jaundice is essential for improving the quality of life of patients with unresectable malignant hilar biliary strictures (UMHBS). In cases of biliary obstruction in the hepatic hilum, effective drainage is difficult to achieve because of the anatomical complexity of the bile ducts. Various procedures of biliary drainage for hilar biliary obstruction have been performed, involving percutaneous or endoscopic routes, plastic or metallic stents, and unilateral or bilateral hepatic duct drainage. However, no consensus has been reached on the optimal drainage strategy for treating biliary obstruction.
The appropriate treatment for patients with UMHBS has been attempted through the development of stents of various shapes and materials. Computed tomography (CT) volumetry enables the accurate estimation of drained liver volume.
In the present study, multivariate analysis revealed that liver function and drained liver volume calculated by computed tomography volumetry are independent factors of drainage effectiveness for UMHBS. Receiver operating characteristics analysis for effective drainage showed cutoff values of 33% for the liver volume of patients with preserved liver function (without decompensated liver cirrhosis) and 50% for the liver volume of patients with impaired liver function (with decompensated liver cirrhosis). This study is the first report to assess the drainage method of UMHBS by using both drained liver volume calculated by using CT volumetry and liver function.
By using this cutoff value, appropriate drainage may be possible for individual cases.
CT volumetry involves the measurement of volume using CT.
To estimate whether the accurate drained liver volume using CT volumetry is superior as an objective evaluation. To choose an appropriate drainage method for individual cases by using the cutoff determined in the present study before stating that biliary drainage procedures are useful.
P- Reviewer: Kato H, Laukkarinen J, Liu YL, Morioka D, Sharma SS S- Editor: Yu J L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Saluja SS, Gulati M, Garg PK, Pal H, Pal S, Sahni P, Chattopadhyay TK. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6:944-950.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Paik WH, Park YS, Hwang JH, Lee SH, Yoon CJ, Kang SG, Lee JK, Ryu JK, Kim YT, Yoon YB. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Ducreux M, Liguory C, Lefebvre JF, Ink O, Choury A, Fritsch J, Bonnel D, Derhy S, Etienne JP. Management of malignant hilar biliary obstruction by endoscopy. Results and prognostic factors. Dig Dis Sci. 1992;37:778-783. [PubMed] |
| 4. | Cheng JL, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002;56:33-39. [PubMed] |
| 5. | Rerknimitr R, Kladcharoen N, Mahachai V, Kullavanijaya P. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38:518-523. [PubMed] |
| 6. | Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25:213-218. [PubMed] |
| 7. | Perdue DG, Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Overby CS, Ryan ME, Bochna GS, Snady HW. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: a prospective multicenter observational cohort study. J Clin Gastroenterol. 2008;42:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Raju RP, Jaganmohan SR, Ross WA, Davila ML, Javle M, Raju GS, Lee JH. Optimum palliation of inoperable hilar cholangiocarcinoma: comparative assessment of the efficacy of plastic and self-expanding metal stents. Dig Dis Sci. 2011;56:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Hong W, Sun X, Zhu Q. Endoscopic stenting for malignant hilar biliary obstruction: should it be metal or plastic and unilateral or bilateral? Eur J Gastroenterol Hepatol. 2013;25:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Iwano H, Ryozawa S, Ishigaki N, Taba K, Senyo M, Yoshida K, Sakaida I. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Dig Endosc. 2011;23:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547-553. [PubMed] |
| 14. | De Palma GD, Pezzullo A, Rega M, Persico M, Patrone F, Mastantuono L, Persico G. Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc. 2003;58:50-53. [PubMed] |
| 15. | Polydorou AA, Cairns SR, Dowsett JF, Hatfield AR, Salmon PR, Cotton PB, Russell RC. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32:685-689. [PubMed] |
| 16. | Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47:354-362. [PubMed] |
| 17. | Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34:95-101. [PubMed] |
| 18. | Dowsett JF, Vaira D, Hatfield AR, Cairns SR, Polydorou A, Frost R, Croker J, Cotton PB, Russell RC, Mason RR. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96:1180-1186. [PubMed] |
| 19. | Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J, Hauss J. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230-239. [PubMed] |
| 20. | Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, Okayama Y, Sano H, Kitajima Y, Hirai M. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Bulajic M, Panic N, Radunovic M, Scepanovic R, Perunovic R, Stevanovic P, Ille T, Zilli M, Bulajic M. Clinical outcome in patients with hilar malignant strictures type II Bismuth-Corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage. Hepatobiliary Pancreat Dis Int. 2012;11:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Hintze RE, Abou-Rebyeh H, Adler A, Veltzke-Schlieker W, Felix R, Wiedenmann B. Magnetic resonance cholangiopancreatography-guided unilateral endoscopic stent placement for Klatskin tumors. Gastrointest Endosc. 2001;53:40-46. [PubMed] |
| 23. | Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self-expanding metallic stents. Gastrointest Endosc. 2003;58:41-49. [PubMed] |
| 24. | Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155-162. [PubMed] |
| 25. | Nanashima A, Yamaguchi H, Shibasaki S, Morino S, Ide N, Takeshita H, Tsuji T, Sawai T, Nakagoe T, Nagayasu T. Relationship between CT volumetry and functional liver volume using technetium-99m galactosyl serum albumin scintigraphy in patients undergoing preoperative portal vein embolization before major hepatectomy: a preliminary study. Dig Dis Sci. 2006;51:1190-1195. [PubMed] |
| 26. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [PubMed] |
| 27. | Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |