Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4499
Peer-review started: October 18, 2014
First decision: November 14, 2014
Revised: December 2, 2014
Accepted: January 30, 2015
Article in press: January 30, 2015
Published online: April 21, 2015
Processing time: 186 Days and 16.6 Hours
AIM: To verify the hypothesis that caspase-8 (Casp8), which regulates cellular apoptosis and necroptosis, is critically involved in enterocyte migration.
METHODS: Casp8-silenced Caco2 cells were used in migration assays. In addition, enterocyte-specific Casp8 heterozygous (Casp8+/∆int) or homozygous knockout mice (Casp8∆int) were generated by crossing genetically modified mice carrying loxP recombination sites in intron 2 and 4 of the murine Casp8 gene with transgenic animals expressing a cre-transgene under control of the villin promoter in a pure C57/BL6 genetic background. The nucleoside analog BrdU was injected i.p. in male Casp8+/∆int and Casp8∆int animals 4 h, 20 h, or 40 h before performing morphometric studies. Locations of anti-BrdU-immunostained cells (cellmax) in at least 50 hemi-crypts of 6 histoanatomically distinct intestinal mucosal regions were numbered and extracted for statistical procedures. For the mice cohort (n = 28), the walking distance of enterocytes was evaluated from cellmax within crypt (n = 57), plateau (n = 19), and villus (n = 172) positions, resulting in a total of 6838 observations. Data analysis was performed by fitting a three-level mixed effects model to the data.
RESULTS: In cell culture experiments with Caco2 cells, Casp8 knockdown efficiency mediated by RNA interference on Casp8 transcripts was 80% controlled as determined by Western blotting. In the scratch assay, migration of Casp8-deleted Caco2 cells was significantly diminished when compared with controls (Casp8∆scramble and Caco2). In BrdU-labeled Casp8∆int mice, cellmax locations were found along the hemi-crypts in a lower position than it was for Casp8+/∆int or control (cre-negative) animals. Statistical data analysis with a three-level mixed effects model revealed that in the six different intestinal locations (distinct segments of the small and large intestine), cell movement between the three mice groups differed widely. Especially in duodenal hemi-crypts, enterocyte movement was different between the groups. At 20 h, duodenal cellmax location was significantly lower in Casp8∆int (25.67 ± 2.49) than in Casp8+/∆int (35.67 ± 4.78; P < 0.05) or control littermates (44.33 ± 0.94; P < 0.01).
CONCLUSION: Casp8-dependent migration of enterocytes is likely involved in intestinal physiology and inflammation-related pathophysiology.
Core tip: Caspase 8 (Casp8) is involved in necroptosis and apoptosis of enterocytes. In addition, Casp8 is thought to be a mediator of cellular migration. Using cell culture experiments, migration of Casp8-deleted Caco2 cells is diminished. In vivo, enterocyte migration is significantly impaired in Casp8-deficient mice. The phenomenon is especially found along the duodenal crypt-villus axis. It is suggested that Casp8-dependent enterocyte migration could be involved in intestinal physiology and pathophysiology.
- Citation: Kaemmerer E, Kuhn P, Schneider U, Jeon MK, Klaus C, Schiffer M, Weisner D, Liedtke C, Jäkel J, Kennes LN, Hilgers RD, Wagner N, Gassler N. Intestinal genetic inactivation of caspase-8 diminishes migration of enterocytes. World J Gastroenterol 2015; 21(15): 4499-4508
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4499
The intestinal mucosa includes a highly specialized surface epithelium with enterocytes, goblet cells, enterochromaffin cells, and Paneth cells. The continuous renewal of surface lining epithelia is associated with cellular migration along the crypt-villus axis (CVA) in the small intestine or crypt-plateau axis (CPA) in the large intestine[1,2]. In a previous study, migration and time course of migration/proliferation in intestinal mucosa were characterized and visualized using the bromodeoxyuridine (BrdU) approach[3]. It is assumed that about 1400 cells per villus are peeled within 24 h[4]. Enterocyte migration is essential in intestinal homeostasis and intestinal restitution, which is known to play a pivotal role in intestinal healing after inflammation-related or non-inflammation-related mucosa[5,6].
Cellular migration is a complex phenomenon with diverse types of cell movements ranging from single cell to collective cell migration[7]. Individual cell movement is categorized as mesenchymal or amoeboid, with a continuum between these extremes[8]. Molecular mechanisms, best investigated in fibroblasts, driving cellular migration include cellular polarization with G-protein-mediated local increase in phosphatidylinositol-3,4,5-triphosphate, and activation of Rho GTPases, e.g., Rho, Rac, and Cdc42[9,10]. Rho GTPases are active in actin polymerization together with WASP/WAVE and Arp2/3-complex forming lamellipodia, filopodia, podosomes, or invadopodia[11,12]. In addition, formation of cellular protrusions is associated with establishment and reconfiguration of cell matrix adhesions and intercellular contacts[8]. Migration-related re-configuration of adhesions includes lysis of contacts by myosin II contraction, protein dephosphorylation by calcineurin and others, re-organization of microtubules, and finally adhesiolysis by calpain-2 (Cpn2)[13]. Activation of small GTPases and Cpn2 by Caspase-8 (Casp8) results in disruption of the Cpn2/calpastatin complex and promotes cell migration[14,15]. In turn, Cpn2 may trigger activation of Casp8 through proteolytic cleavage of immature pro-caspase-8 molecules via the amyloid-beta-peptide and CD95 pathways, along with degradation of FLICE-inhibitory protein-small[16].
Casp8, a protease with a cysteine residue in its active side, is critically involved in diverse forms of cell death. Predominantly, Casp8 acts as the apical initiator caspase driving extrinsic, death-receptor-mediated apoptosis, and also prevents an alternative mode of cell death termed necroptosis[6]. In addition, Casp8 was found to promote cell migration and cell-matrix adhesion[17]. The processing of Casp8, which is controlled in part by tyrosine phosphorylation, is considered an important switch deciding between migration/adhesion and cell death mechanisms[18]. Recently, development of severe intestinal inflammation similar to Crohn’s disease with depletion of Paneth cells and a reduced number of goblet cells has been described in intestinal Casp8-knockout mice[19]. In this study, the inflammatory intestinal phenotype of Casp8-knockout mice was due to impaired antimicrobial immune functions of the intestinal epithelium, but migration of intestinal epithelia was not investigated. However, migration of enterocytes is assumed as crucial in the pathogenesis of intestinal epithelial wound healing and inflammatory bowel disease[20,21].
We hypothesized that enterocyte migration is hampered in intestinal Casp8-knockout mice, which could promote development of the inflammatory phenotype.
For cell culture experiments, the established cell line Caco2 (ATCC: HTB-37), derived from a colorectal adenocarcinoma (TP53 and APC mutated), was cultured as previously described[22]. For RNA interference on Casp8 transcripts, small interfering RNAs (siRNAs) and negative siRNAs as non-silencing control (for sequences see Table 1) were used (both from Qiagen, Hilden, Germany). Cells were transfected with 5 nM Lipofectamine (Invitrogen of Thermo Fisher Scientific, Waltham, MA, United States) following manufacturer’s recommendations. Knockdown efficiency was evaluated by quantitative real-time (qRT)-PCR and Western blot analysis.
| Gene | Forward Primer | Reverse Primer |
| Human caspase-8 | 5’-GGATGAGGCTGACTTTCTGC-3’ | 5’-CACTTCAGTCAGGATGGTGAGA-3’ |
| Mouse tumor necrosis factor-α | 5’-CAGTCTGCAGGGAGTGTGAA-3 | 5’-CACGCACTGGAAGTGTGTCT’-3 |
| Mouse interleukin-1β | 5’-GCCCATCCTCTGTGACTCAT-3’ | 5’-AGGCCACAGGTATTTTGTCG-3’ |
| Human caspase-8 small interfering RNA | Sense 5’-GUUCCUGAGCCUGGACUACTT-3’ | |
| Antisense 5’-GUAGUCCCAGGCUCAGGAACTT-3’ | ||
The Chomczynski procedure was used for isolation of total RNAs and proteins[23]. RNA integrity was determined using standard procedures. Superscript III reverse transcription reagents (Invitrogen) were used for cDNA synthesis. Expression of specific mRNA, including Casp8, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β (for primers see Table 1), was analyzed by qRT-PCR using an IQ 5 detection system (Bio-Rad Laboratories, Hercules, CA, United States) followed by data analysis according to the ∆∆ct method[24].
Extracted proteins were solubilized in 2 × Laemmli buffer, separated with SDS-PAGE, and electrotransferred to a PVDF Immobilon-P membrane (Millipore Corp., Billerica, MA, United States). The following primary antibodies were used: anti-human Casp8 (Alexis/Enzo, Lörrach, Germany), anti-human beta actin (A5441; Sigma-Aldrich, St. Louis, MO, United States), and anti-proliferating cell nuclear antigen (PCNA) (Invitrogen). Secondary HRP-conjugated antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, United States) (1:10000) and visualized with enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL, United States).
Confluent Caco2 mono-layers in 6-well plates either treated with siRNAs to knockdown Casp8 or negative siRNA were subjected for the scratch-based migration assay[25]. Random migration of cells in the scratch area (about 0.5 mm) was documented by imaging at three intervals (0 h, 6 h, and 24 h) with a 10 × objective. Measurements of scratch sizes were performed three times and independent triplicates were used.
For the present study, genetically modified mice carrying loxP recombination sites in intron 2 and 4 of the murine Casp8 in a pure C57/BL6 genetic background as described recently were used[26]. These animals were crossed with transgenic animals expressing a cre-transgene under control of the villin promoter, which is expressed in enterocytes[27], to generate enterocyte-specific Casp8 heterozygous (Casp8+/∆int) or homozygous knockout (Casp8∆int) mice. Animal experiments were performed in male Casp8∆int mice. As controls, heterozygous mice (Casp8+/∆int) and cre-negative littermates (Casp8f/f) were used. All animals were maintained in a temperature-controlled room with 12-h light/dark cycle at the core facility of the University Hospital Aachen. Induction of Casp8∆int was confirmed by genotyping as well as measurement of Casp8 mRNA and protein following standard protocols[26]. For each condition, a minimum of three mice per group were included in the study. The mice received a 30 μg/g single i.p. injection of the nucleoside analog BrdU (Applichem, Cheshire, CT, United Kingdom) 2 h, 20 h, or 40 h before sacrificing. All procedures were approved by the Authority for Environment Conservation and Consumer Protection of the State North Rhine-Westfalia (LANUV, Germany).
After sacrificing, small and large intestines were isolated and the different parts of the small (duodenum, jejunum, and proximal and distal ileum) and large intestine (proximal and distal colon) were dissected. The tissues were fixed for 24 h in neutral buffered formalin and automatically processed to paraffin-embedded tissue blocks following routine procedures. Orthogonal orientation of tissues in paraffin was visually controlled under a binocular loupe. From each tissue, sections of 3-5 μm were cut and stained with hematoxylin eosin and examined under a Nikon Eclipse 80i (Nikon Corp., Tokyo, Japan) for suitability in morphometric procedures.
The definitions of mucosal parameters for the small or large intestine were adapted from a previously published study[28]. In all tissues, 50 hemi-crypts were identified for further morphometric analysis. Criteria for a small intestinal hemi-crypt were defined as following: (1) single epithelial layer is visible from crypt basis to villus tip; (2) crypt basis without distension; (3) open crypt lumen; (4) plateau is visible between crypt and villus; (5) villus height 3/4 to 2/3 of the total CVA; and (6) lamina propria mucosae is visible in each villus. Morphologic criteria for the definition of large intestinal hemi-crypts were: (1) nuclear maturation with round nuclei of crypt lining epithelial cells and elongated nuclei in epithelial cells at the mucosal plateau; (2) mitotic figures only in the lower 1/4 to 1/5 of crypts; and (3) crypt basis without distension.
Serial sections of intestinal mice tissues were immunostained either for BrdU or Ki67 protein. Non-serial sections of intestinal tissues were used for anti-CD11 immunostaining (550282; BD Biosciences; Franklin Lakes, NY, United States). After heat-induced antigen retrieval with citrate buffer (pH 6) and blocking of endogenous peroxidase, anti-Ki67 immunostaining (RM9106; Thermo Fisher Scientific) was performed following manufacturer’s recommendations. For detection, a horseradish peroxidase-labeled anti-rabbit polymer (ImmPress anti-rabbit; Vector Laboratories Inc., Burlingame, CA, United States) with diaminobenzidine as a chromogen (Vector) were used. In mice tissues, DNA-incorporated BrdU was detected with an anti-BrdU antibody (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). For detection, the mouse-on-mouse kit and diaminobenzidine (both from Vector) were applied. CD11 immunostaining was visualized with streptavidin-conjugated Cy3 (016 160 084; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, United States).
Immunostained cells were numbered in at least 50 hemi-crypts of each intestinal segment according to previous studies[4]. All positive immunostained cells were characterized with “1”, and all negative cells with “0”. Blinded numbering was independently performed using a Nikon Eclipse 80i (Nikon) by two morphologically experienced investigators (Kuhn P and Gassler N).
In the in vivo experiments, a total of 28 mice were subdivided into a minimum of three mice per group (Casp8+/∆int, Casp8∆int, and Casp8f/f) and the three incubation times (2 h, 20 h, or 40 h); four mice were included in the Casp8+/∆int group at 20 h of incubation. In all other conditions, mice died during the experiments and an increase in the minimal number of three per group and incubation time was not possible. For the mice cohort (n = 28) three statistical levels were evaluated. Up to 56 hemi-crypts (minimum 50 hemi-crypts; level one) at six locations of the bowel (duodenum, jejunum, proximal and distal ileum, proximal and distal colon; level two) per mouse (level three) were investigated. The “walking distance of enterocytes” was evaluated from the maximal position of BrdU-stained cells within crypt (57 positions), plateau (19 positions), and villus (172 positions), resulting in a total of 6838 observations. Final data analysis was performed by fitting a three level mixed effects model to the data using SAS 9.3 (TSM12, 64 bit; SAS Insitute Inc., Cary, NC, United States). Data were illustrated by dot plots. Calculated data from densitometry or cell imaging were processed with the ImageJ Quant 5.1 software (National Institutes of Health, United States; http://rsb.info.nih.gov/ij). Two-tailed paired Student’s t tests were used for further data analysis, and P < 0.05 was considered as significant for all analyses.
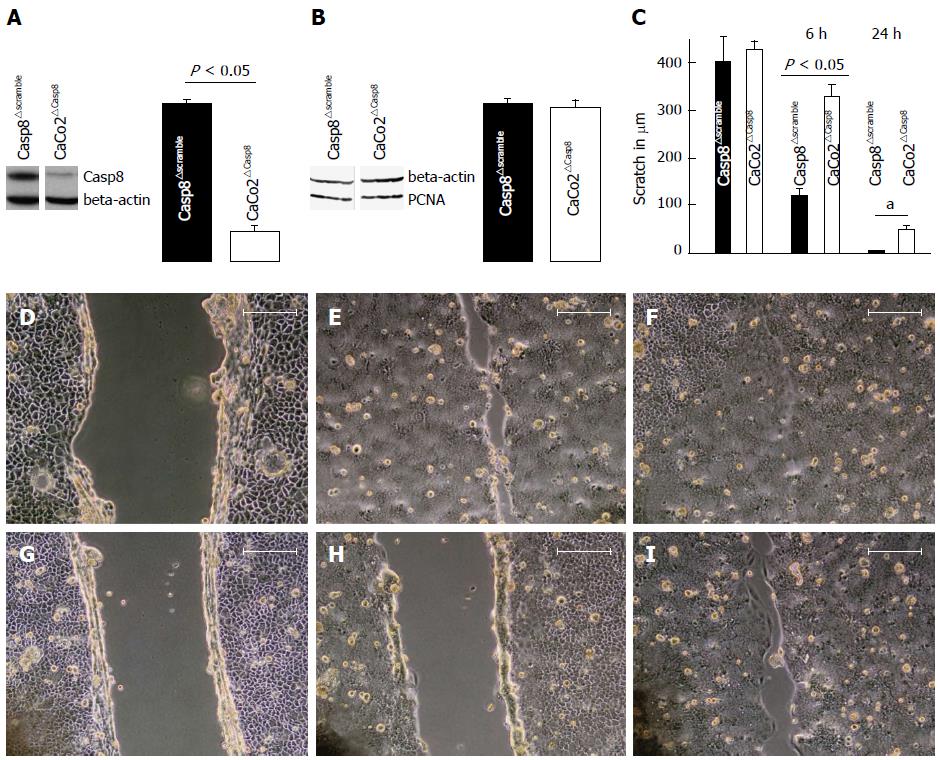
In Caco2 cells, Casp8 knockdown was performed using RNA interference against Casp8 transcripts. Knockdown efficiency was about 80% as determined by Western blotting (Figure 1A). Loss of Casp8 was not associated with differences in cell proliferation as determined by analysis of PCNA, a cell cycle-related protein essential for cellular DNA synthesis (Figure 1B). Migration of Casp8 siRNA-treated Caco2 cells was significantly diminished when compared with controls (P < 0.05) (Figure 1C). Six hours after scratching, 80% of the primary scratch was open in Caco2∆Casp8, but only 32% in Casp8∆scramble. Twenty-four hours after scratching, the scratch was closed in control cells (Casp8∆scramble), but persisted to 29% of the primary scratch in the knockout. Examples are illustrated in Figure 1D-I.
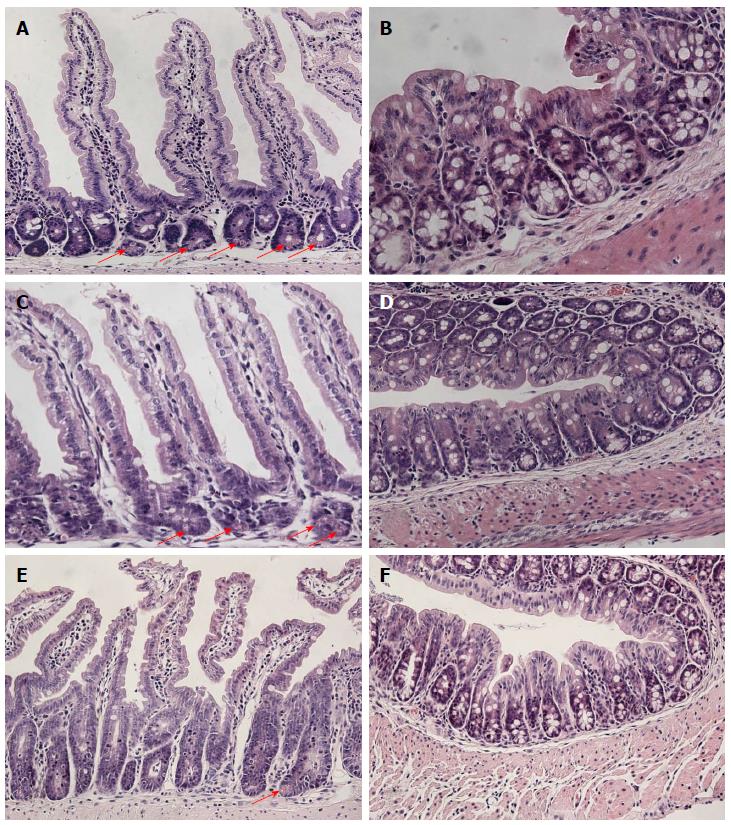
At four weeks of age, the body weight of Casp8∆int mice was reduced compared to control littermates (Casp8+/∆int and Casp8f/f). Histologic analysis of Casp8∆int intestinal tissues showed inflammation in the small intestine with disturbances of the CVA. Hallmarks of the Casp8∆int-related tissue damage included marked reduction of Paneth cells with storage of nuclear remnants in crypts and sometimes crypt elongation. The changes were most prominent in the jejunum and ileum. In contrast to the small intestine, histomorphologic findings in the large intestine were uncharacteristic and not sufficient to define a strong colorectal Casp8∆int phenotype (Figure 2). The inflammatory intestinal phenotype of Casp8∆int mice was paralleled by an increased expression of TNF-α and IL-1β mRNA in small and large intestine of when compared with Casp8+/∆int and Casp8f/f mice (all P < 0.05). In addition, stronger anti-CD11 immunostaining was found in the small intestine of Casp8∆int mice when compared with control mice. Essentials of the Casp8∆int phenotype were identical to already published data[19].
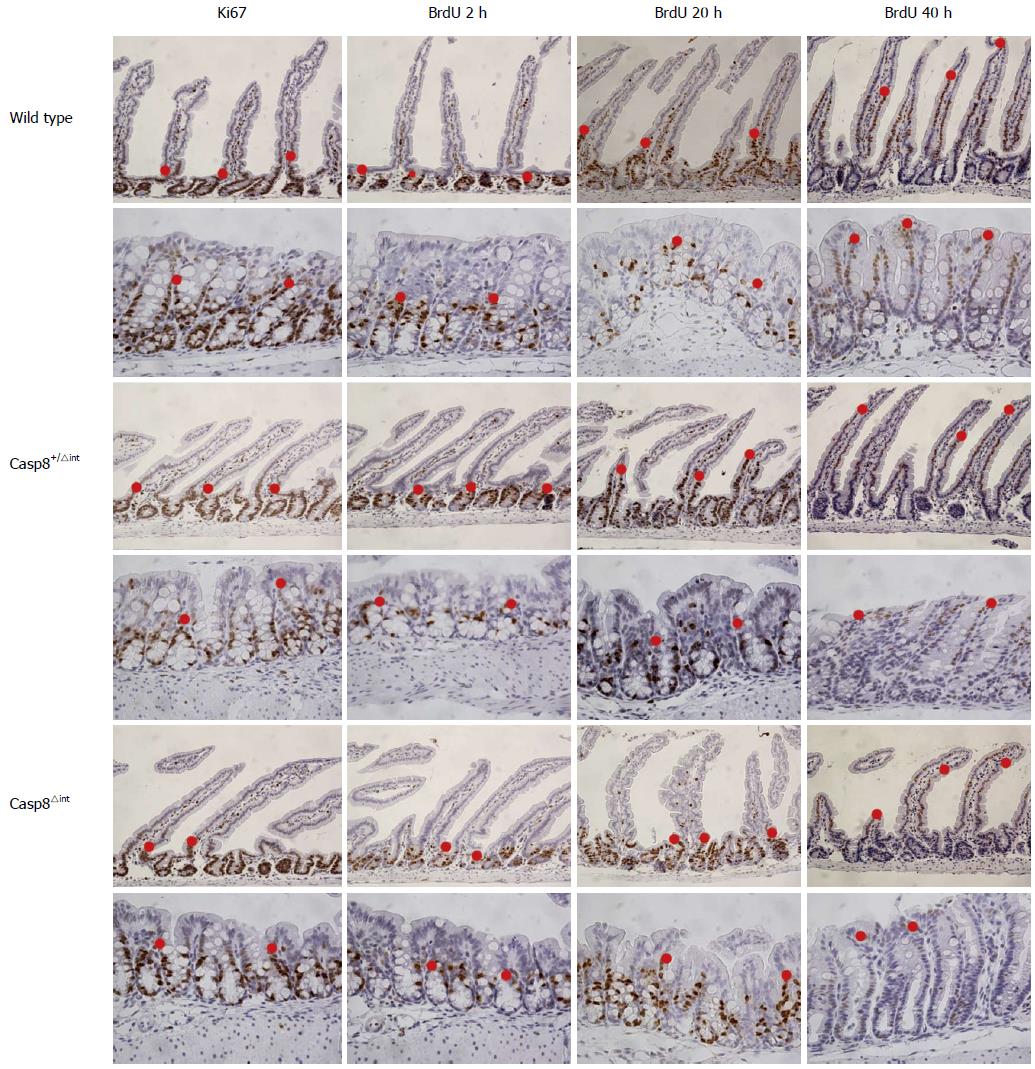
Migration of enterocytes in vivo was evaluated with the BrdU assay. Casp8∆int, Casp8+/∆int, and Casp8f/f mice were sacrificed 2 h, 20 h, or 40 h after BrdU application. The outermost BrdU-labelled/stained cells (cellmax) in the hemi-crypts of different segments of small as well as large intestine were numbered (Figure 3).
In the intestinal mucosa of Casp8∆int mice, cellmax location was found along the CVA/ CPA, sometimes in a lower position than it was in Casp8+/∆int or Casp8f/f controls. Fitting the three-level mixed effects model to the data showed that 39% was due to the variation between mice, but 44% was dependent on the six intestinal locations. Cell movement between the three groups of mice (28 mice valid model) was significantly different concerning the incubation time (df = 15; DDF = 74; F = 4.03; P < 0.01) and depended on the locations from the incubation time (df = 10; DDF = 74; F = 44.20; P < 0.01). In addition, the cell movement differed in the six locations among the groups (df = 10; DDF = 74; F = 2.88; P < 0.01). Among the groups, cellular migration was dependent on the incubation time (df = 3; DDF = 15; F = 13.79; P < 0.01). In a full model view, cell movement was different among the groups (df = 2; DDF = 15; F = 7.21; P < 0.01), the incubation time (df = 2; DDF = 15; F = 302.43; P < 0.01), and the location (df = 5; DDF = 74; F = 158.6; P < 0.01), indicating group, incubation time, and location as different variables. At 20 h, duodenal cellmax location was significantly lower in Casp8∆int than in Casp8+/∆int (P < 0.05) or Casp8f/f (P < 0.01) mice. In this position, the difference between both controls was not significant (Figure 4).
At 2 h after BrdU injection, no differences in cellmax location were found among the groups throughout the intestine. In order to further characterize this finding, anti-Ki67 immunostaining was performed. Distribution of anti-Ki67-immunostained cells was quite similar to the 2 h anti-BrdU immunostaining.
Enterocyte migration, which is essential for integrity and maintenance of the intestinal mucosal border, is a complex phenomenon established and regulated by a plethora of molecular pathways and structural components[5,6,8]. In previous studies, apoptosis/necroptosis-independent functions of Casp8 have been demonstrated, indicating a role for this protease in cell migration[18]. In a recent study, intestinal loss of Casp8 was shown to be crucial for the development of terminal ileitis resembling Crohn’s disease[19]. In the present study, we hypothesized that Casp8 knockdown would be associated with disturbed enterocyte migration, which could be tested in cell culture experiments and a Casp8∆int mouse model.
We demonstrate that siRNA-mediated inhibition of Casp8 in Caco2 cells results in diminished cell migration. This finding is in line with previous observations that Casp8 is able to promote cell migration and stabilizes cell-matrix adhesions[15,17]. In these studies, several types of established cell lines were investigated, including epithelial cells (e.g., A549 lung carcinoma cells)[15]. In A549 as well as Caco2 cells, loss of Casp8 diminished cell migration, indicating that the epithelial character does not contradict a Casp8-related function in cellular migration. For the present studies, a sufficient small intestinal cell line was not available. Therefore the studies were conducted with the permanent cell line Caco2.
The cell culture-based finding that loss of Casp8 diminishes cellular migration, was further substantiated in vivo by characterization of Casp8∆int mice with the BrdU assay. This technique is well established for measurement of epithelial cell migration and proliferation[3]. In addition to the BrdU assay, anti-Ki67 immunostaining was used to determine cellular proliferation. Our Casp8∆int animal model demonstrates a histomorphologic phenotype as previously described[19]. In the study by Günther et al[19], loss of Paneth cells was demonstrated as essential for phenotypic expression, but Casp8∆int-related disorders in enterocyte migration were not addressed.
Cellmax location 2 h after BrdU injection was not different among the groups and was identical to anti-Ki67 immunostaining. This indicates that assessment of epithelial cell migration with the BrdU approach within 2 h after BrdU injection is not possible. In Casp8∆int mice, migration of enterocytes was reduced 20 h and 40 h after BrdU application. The phenomenon was preferentially visible in proximal small intestinal segments, i.e. duodenal mucosa, but not significantly expressed in the colon. Migration distance in the CVA is longer than in the CPA, which could be crucial to explain why the absence of Casp8 only significantly affects small bowel epithelial cell migration. A breakthrough to understand the underlying molecular mechanisms could be an expression study of Casp8-related targets in enterocytes isolated from the small or large intestine.
In general, defective epithelial maturation, migration, and intercellular junctions, as well as disturbed host-microbiota homeostasis, are essential in the pathogenesis of intestinal barrier defects and inflammatory bowel diseases[29,30]. In Casp8∆int mice, loss of Paneth cells is suggested to be essential in the pathogenesis of Casp8∆int-related intestinal inflammation[19]. In normal tissues, long villi and only few Paneth cells are found in the proximal small intestine, but short villi and an increased number of Paneth cells are normally seen in the terminal ileum. Accordingly, it is reasonable to assume that the inflammatory intestinal phenotype of Casp8∆int mice in the duodenum is mainly evoked by migration defects, whereas ileal inflammation is preferentially determined by loss of Paneth cells. The study by Günther et al[19] supports this assumption because in the terminal ileum, increased Paneth cell death and severe intestinal inflammation were found. It could be hypothesized that Casp8∆int-related migration defects are mostly involved in the pathogenesis of mucosal inflammation in the proximal small intestine, but not in the colon.
In conclusion, our study demonstrates that Casp8 is partially involved in migration of enterocytes along the CVA/CPA. In the proximal small intestine, the Casp8∆int inflammatory phenotype is more likely determined by an injured epithelial migration than Paneth cell loss.
Caspase-8 (Casp8), a cysteine protease, is critically involved in several cellular functions, including apoptosis and necroptosis. In the intestinal mucosa, Casp8 is strongly expressed by non-epithelial and epithelial cells including enterocytes.
Intestinal Casp8-deficient mice demonstrate an inflammatory intestinal phenotype with reduction of Paneth cells, but preserved villus morphogenesis. In this study, the authors demonstrate in vitro and in vivo that enterocyte migration is likely mediated by Casp8.
Intestinal Casp8 expression is a focus of research due to its role in the apoptosis and necroptosis of enterocytes. In the present study, enterocyte migration as a further intestinal Casp8 function is addressed using cell culture and Casp8-knockout mice. Loss of Casp8 is crucial to decelerate migration along the crypt-villus and crypt-plateau axis.
The finding that Casp8 affects enterocyte migration could be of interest in pathophysiologic concepts of intestinal barrier configuration and chronic inflammatory bowel disease.
Casp8 is a cysteine protease involved in several forms of cell death, including apoptosis and necroptosis. It has been suggested that disturbed intestinal Casp8 expression is involved in the pathogenesis of chronic inflammatory bowel disease.
Using cell culture and Casp8-knockout mice, the authors demonstrate that loss of Casp8 decelerates migration in the crypt-villus and crypt-plateau axis and reduces cell migration of Casp8-deficient Caco2 cells. The work is well conducted and the results are clear and convincing.
P- Reviewer: Bettaieb A S- Editor: Yu J L- Editor: AmEditor E- Editor: Liu XM
| 1. | van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1231] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 2. | Stange DE, Clevers H. Concise review: the yin and yang of intestinal (cancer) stem cells and their progenitors. Stem Cells. 2013;31:2287-2295. [PubMed] |
| 3. | Potten CS, Kellett M, Roberts SA, Rew DA, Wilson GD. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992;33:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001-1020. [PubMed] |
| 5. | Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Becker C, Watson AJ, Neurath MF. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology. 2013;144:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 976] [Cited by in RCA: 1015] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 8. | Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 9. | Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 284] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell. 2009;101:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Cory GO, Ridley AJ. Cell motility: braking WAVEs. Nature. 2002;418:732-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917-4919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 325] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 14. | Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829-3838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 15. | Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, Bogyo M, Barilà D, Lahti JM, Schlaepfer D. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755-3763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Vaisid T, Barnoy S, Kosower NS. Calpain activates caspase-8 in neuron-like differentiated PC12 cells via the amyloid-beta-peptide and CD95 pathways. Int J Biochem Cell Biol. 2009;41:2450-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, Bong Kang T, Wallach D, Dorfleutner A, Lahti JM, Flynn DC. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273-4278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491-4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 809] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 20. | Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827-G838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 237] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 22. | Gassler N, Roth W, Funke B, Schneider A, Herzog F, Tischendorf JJ, Grund K, Penzel R, Bravo IG, Mariadason J. Regulation of enterocyte apoptosis by acyl-CoA synthetase 5 splicing. Gastroenterology. 2007;133:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532-534, 536-537. [PubMed] |
| 24. | Fink L, Seeger W, Ermert L, Hänze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 448] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 25. | Hou A, Toh LX, Gan KH, Lee KJ, Manser E, Tong L. Rho GTPases and regulation of cell migration and polarization in human corneal epithelial cells. PLoS One. 2013;8:e77107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Liedtke C, Bangen JM, Freimuth J, Beraza N, Lambertz D, Cubero FJ, Hatting M, Karlmark KR, Streetz KL, Krombach GA. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology. 2011;141:2176-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476-6482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Trbojević-Stanković JB, Milićević NM, Milosević DP, Despotović N, Davidović M, Erceg P, Bojić B, Bojić D, Svorcan P, Protić M. Morphometric study of healthy jejunal and ileal mucosa in adult and aged subjects. Histol Histopathol. 2010;25:153-158. [PubMed] |
| 29. | Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 472] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 30. | Bibiloni R, Schiffrin EJ. Intestinal Host-Microbe Interactions under Physiological and Pathological Conditions. Int J Inflam. 2010;2010:386956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |