Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4184
Peer-review started: September 29, 2014
First decision: October 29, 2014
Revised: December 16, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 14, 2015
Processing time: 200 Days and 12.6 Hours
AIM: To test whether hepatic stellate cells (HSCs) at different activation stages play different roles in acetaminophen (APAP)-induced acute liver injury (ALI).
METHODS: HSCs were isolated from mouse liver and cultured in vitro. Morphological changes of initiation HSCs [HSCs (5d)] and perpetuation HSCs [HSCs (p3)] were observed by immunofluorescence and transmission electron microscopy. The protective effects of HSC-derived molecules, cell lysates and HSC-conditioned medium (HSC-CM) were tested in vivo by survival and histopathological analyses. Liver injury was determined by measuring aminotransferase levels in the serum and by histologic examination of tissue sections under a light microscope. Additionally, to determine the molecular mediators of the observed protective effects of initiation HSCs, we examined HSC-CM using a high-density protein array.
RESULTS: HSCs (5d) and HSCs (p3) had different morphological and phenotypic traits. HSCs (5d) presented a star-shaped appearance with expressing α-SMA at non-uniform levels between cells. However, HSCs (p3) evolved into myofibroblast-like cells without lipid droplets and expressed a uniform and higher level of α-SMA. HSC-CM (5d), but not HSC-CM (p3), provided a significant survival benefit and showed a dramatic reduction of hepatocellular necrosis and panlobular leukocyte infiltrates in mice exposed to APAP. However, this protective effect was abrogated at higher cell masses, indicating a therapeutic window of effectiveness. Furthermore, the protein array screen revealed that HSC-CM (5d) was composed of many chemokines and growth factors that correlated with inflammatory inhibition and therapeutic activity. When compared with HSC-CM (p3), higher levels of monocyte chemoattractant protein-1, macrophage inflammatory protein-1γ, hepatocyte growth factor, interleukin-10, and matrix metalloproteinase-2, but lower levels of stem cell factor and Fas-Ligand were observed in HSC-CM (5d).
CONCLUSION: These data indicated that initiation HSCs and perpetuation HSCs were different in morphology and protein expression, and provided the first experimental evidence of the potential medical value of initiation HSC-derived molecules in the treatment of ALI.
Core tip: In this study, we isolated hepatic stellate cells (HSCs) from mice by in situ perfusion of the liver and created primary and secondary cultures in plastic tissue culture dishes. Then, we observed different morphologies and phenotypes between initiation HSCs and perpetuation HSCs and described the first use of molecules secreted from HSCs in acetaminophen-induced acute liver injury. Initiation HSC-derived molecules showed hepatocyte-protective effects. Our findings provide novel insight into the mechanisms of HSCs in liver injury therapy. Whether the potential value of initiation HSC-derived molecular therapy is derived from the effect of a single cytokine or a combination of cytokines should be explored in future.
- Citation: Chang WJ, Song LJ, Yi T, Shen KT, Wang HS, Gao XD, Li M, Xu JM, Niu WX, Qin XY. Early activated hepatic stellate cell-derived molecules reverse acute hepatic injury. World J Gastroenterol 2015; 21(14): 4184-4194
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4184.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4184
Hepatic stellate cells (HSCs), first described by Kupffer in 1876, have emerged in the past 30 years as remarkably versatile mesenchymal cells[1]. Previous studies have explored the importance of HSCs in liver fibrosis, because HSC activation into myofibroblasts is thought to be the major step in hepatic fibrogenesis associated with liver injury[2]. Beyond this well-known characteristic, however, many newly discovered activities have led to a greater understanding of this fascinating cell type and the complexity of cellular homeostasis in the liver[3].
The hepatocyte protecting effects of HSCs in acute liver injury (ALI) has ignited growing interest[4]. We previously performed loss-of-function studies by depleting activated HSCs with gliotoxin in acetaminophen (APAP)-induced ALI in mice[5]. We demonstrated that severe liver damage and decreased survival rate were correlated with depletion of activated HSCs. These data provided clear evidence that activated HSCs are involved in both hepatocyte death and proliferation of hepatocytes and hepatic progenitor cells (HPCs) in APAP-induced ALI.
Quiescent HSCs, characterized by retinoid droplets in the cytoplasm, are present in the space of Disse in close contact with hepatocytes and sinusoidal endothelial cells. When HSCs are activated, they lose retinoid, move from the space of Disse to sites of damage (where the activated HSCs differentiate into myofibroblasts), and secrete extracellular matrix and growth factors that are involved in liver regeneration[6]. Because of the close anatomic relationship between HSCs and epithelial cells (hepatocytes and HPCs), HSCs are part of the stem cell niche and directly contact epithelial cells to participate in the early phase of hepatocyte regeneration[7]. However, it is unclear whether the products of activated HSCs are required to attenuate acute hepatocyte injury. In addition, in the process of differentiation from quiescent HSCs to fully activated HSCs (myofibroblasts), the cells change in morphology and phenotype, but it is not known whether those different stages of cells have different effects on protecting hepatocytes from acute injury. To the best of our knowledge, no previous studies have tried to answer that question.
In this study, we isolated HSCs from mice by in situ perfusion of the liver and created primary and secondary cultures in plastic tissue culture dishes. Then, the differences in morphology and phenotypic features were observed between activated HSCs at early stage and later stage. Furthermore, we investigated whether molecules produced by activated HSCs would protect hepatocytes in APAP-induced ALI, and analyzed the difference in the HSC secretome between the early and late stages by a protein array screen.
Male C57BL/6J mice (6-8-wk-old, weighing 20 ± 2 g) were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences. All of the animals were maintained in the animal facility of Zhongshan Hospital, Fudan University. The mice were kept on a 12-h light/dark cycle with access to mouse chow and water ad libitum. All surgery was performed under a mixture anesthesia of ketamine (80 mg/kg, Hengrui Medicine, Lianyungang, China) and xylazine (30 mg/kg, Sigma-Aldrich, St. Louis, MO, United States) given intraperitoneally, and all efforts were made to minimize suffering. The experimental protocol was approved by the Animal Care and Use Committee of Fudan University. All animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
HSCs were isolated from mouse livers as we described before[8]. Briefly, C57BL/6J mice livers were perfused in situ, and the HSCs were isolated by an optimized density gradient centrifugation technique. HSCs were seeded in high-glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco, New York, United States) containing 10% fetal bovine serum (FBS, Sigma-Aldrich, Poole, United Kingdom). The cells were incubated at 37 °C in a humidified atmosphere with 5% carbon dioxide. The primary HSCs cultured for 5 d were defined as HSCs (5d), and after being passaged three generations, HSCs were defined as HSCs (P3).
Cellular lysates were prepared by sonication (VWR Scientific, West Chester, PA). The dose of cells administered was 2 × 106 per subject. Conditioned medium was prepared by collecting serum-free medium (high-glucose DMEM without FBS; supplemented with 0.05% bovine serum albumin (BSA) to prevent protein aggregation) after 24-h culture of different cell masses. The majority of experiments were performed with the optimal cell mass of 2 × 106 cells. Supernatants were centrifuged and filtered to eliminate potential cell bodies. The medium was then concentrated approximately 25-fold using ultrafiltration units (Amicon Ultra-PL 3, Millipore, Bedford, MA, United States) with a 3 kDa molecular weight cut-off. Finally, the collected medium, containing paracrine molecules (HSC-CM), was stored at -80 °C until use.
HSCs (5d) and HSCs (P3) grown in 35-mm tissue culture plates were fixed with ice-cold 2% methanol for 10 min at 4 °C. After blocking with 5% BSA (Sigma Aldrich, St. Louis, MO, United States) in PBS, cells were incubated with mouse monoclonal anti-α-SMA IgG (Abcam, Cambridge, MA; 1:100) or rabbit anti-desmin (Abcam, Cambridge, MA; 1:100) for 2 h at room temperature, followed by development with donkey anti-mouse Alexa Fluor 488 (Invitrogen, Carlsbad, California, United States; 1:500) or donkey anti-rabbit Alexa Fluor 594 (Invitrogen, Carlsbad, California, United States; 1:500) for 30 min at room temperature, respectively. All plates were examined under an Axiovert 200 (Carl-Zeiss, Jena, Germany) using a computer-assisted image analysis program (AxioVision Ver. 4.0; Carl-Zeiss).
HSCs (5d) and HSCs (P3) were harvested by trypsinization and centrifugation for 10 min at 1000 g at room temperature. Cells were fixed in 2.5% glutaraldehyde for 1 h at 4 °C and postfixed with 1% osmic acid for 30 min. Cells were then stained with lead-uranium, and the ultrastructural organization was observed with a transmission electron microscope (JEM-1200EX, Japan).
All animals were fasted overnight before APAP treatment (Sigma-Aldrich, St. Louis, MO, United States). ALI was induced by intraperitoneal injection of APAP in phosphate-buffered saline (PBS, Gibco, New York, United States) at a dose of 750 mg/kg. Blood samples were obtained at 12, 24, 36, and 48 h after injection by retro-orbital puncture for analysis of liver enzyme levels. Mice were sacrificed 24 h after injection by cervical dislocation under a mixture anesthesia of ketamine and xylazine, and liver tissues were harvested and fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, United States) for histological analysis.
To assess the protective effect of paracrine molecules (HSC-CM) on ALI, after one dose of APAP treatment, all animals were randomly divided into three groups: an HSC-CM (5d) group, an HSC-CM (P3) group and a control group. Two hours after APAP injection, the HSC-CM (5d) group and HSC-CM (P3) group were treated with one dose of paracrine molecules of HSCs (5d) (0.20 mL, about 2 × 106 HSCs) and paracrine molecules of HSCs (P3) (0.20 mL, about 2 × 106 HSCs), respectively, via tail vein injection. Control animals received the same volume of blank conditioned medium (0.2 mL, high-glucose DMEM). Four animals per group were sacrificed 24 h later to collect liver tissue, and 13 animals per group were used for survival analysis. Blood samples were collected 12, 24, 36, and 48 h after treatment by retro-orbital puncture for analysis of liver enzyme release levels. The survival analysis was set for 7 d and animal survival was monitored every 12 h. On 7 d after administration of paracrine molecules, the end-point for the survival experiment was reached and the survival rate was analyzed, and all survival mice were sacrificed by cervical dislocation under a mixture anesthesia of ketamine and xylazine.
Liver injury was determined by measuring aminotransferase levels in the serum and by histologic examination of tissue sections under a light microscope. Serum samples were stored at -80 °C until use. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using Infinity ALT reagent and Infinity AST reagent, respectively (Thermo Electron, Louisville, CO, United States) according to the manufacturer’s instructions. Liver tissues were fixed with 4% paraformaldehyde in PBS for 18 h and then embedded in paraffin. Sections of 5 µm thickness were cut and stained with hematoxylin and eosin (HE; Sigma-Aldrich, St. Louis, MO, United States). HE-stained liver sections were examined, and necrosis was graded using a previously described system[9].
For HSC-CM, HSCs were cultured in serum-free DMEM supplemented with 0.05% BSA. Supernatants were prepared by collecting serum-free medium after 24 h culture of approximately 2 × 106 HSCs (5d) or HSCs (P3). The media were then concentrated approximately 25-fold using ultrafiltration units (Millipore, Bedford, MA, United States) with a 3 kDa molecular weight cut-off. Finally, the collected medium, containing paracrine molecules (HSC-CM), was stored at -80 °C until use. These were analyzed for a panel of specified proteins using an antibody array (RayBio Mouse Cytokine Antibody Array c2000, RayBiotech Inc., Norcross, GA) as specified by the vendor.
The quantitative results are expressed as the mean ± SD. For multi-group comparison, one-way analysis of variance was applied. Comparisons between groups were performed using the non-parametric Mann-Whitney U-test, or one-way analysis of variance. The Kruskal-Wallis rank-sum test was applied when the samples were not normally distributed. Kaplan-Meier analysis was used for survival analysis. A P-value less than 0.05 were considered statistically significant. The statistical methods of this study were reviewed by Department of Biostatistics of Fudan University.
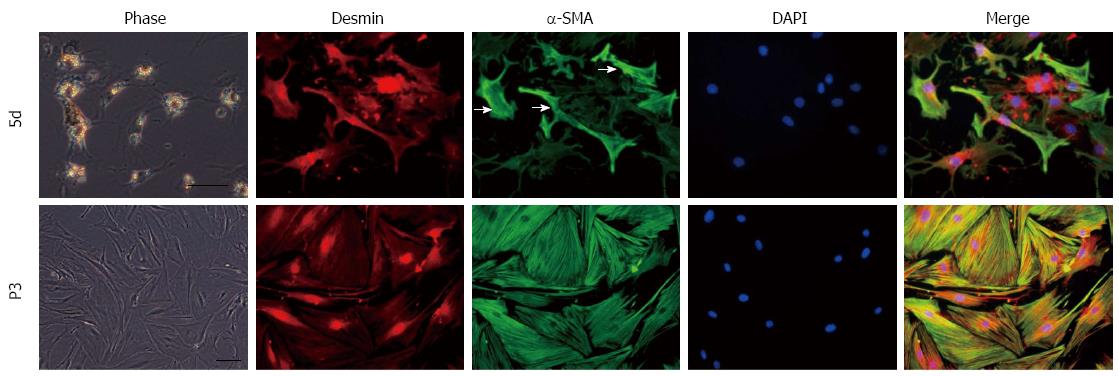
The isolated HSCs were cultured on uncoated plastic tissue culture plates and observed under a phase contrast microscope. After primary culture for 24 h, HSCs were quiescent, had a spherical shape and displayed a clear nuclear region surrounded by retinoid droplets in the cytoplasm. These cells expressed desmin and were negative for a-smooth muscle actin (α-SMA, data not shown). HSCs presented a star-shaped appearance with fewer and smaller lipid droplets in the cytoplasm after primary culture for five days, (Figure 1) and began to express α-SMA at non-uniform levels between cells (because the activation stage varied between cells) (Figure 1). These cells were at the early stage of activation and defined as “initiation” HSCs. During this activation period, HSCs developed the star-shaped pseudopodium branches with gradually fewer lipid droplets in the cytoplasm. Subcultured HSCs evolved into myofibroblast-like cells without lipid droplets (Figure 1) and expressed a uniform and higher level of α-SMA (Figure 1). These cells were persistently activated and defined as “perpetuation” HSCs.
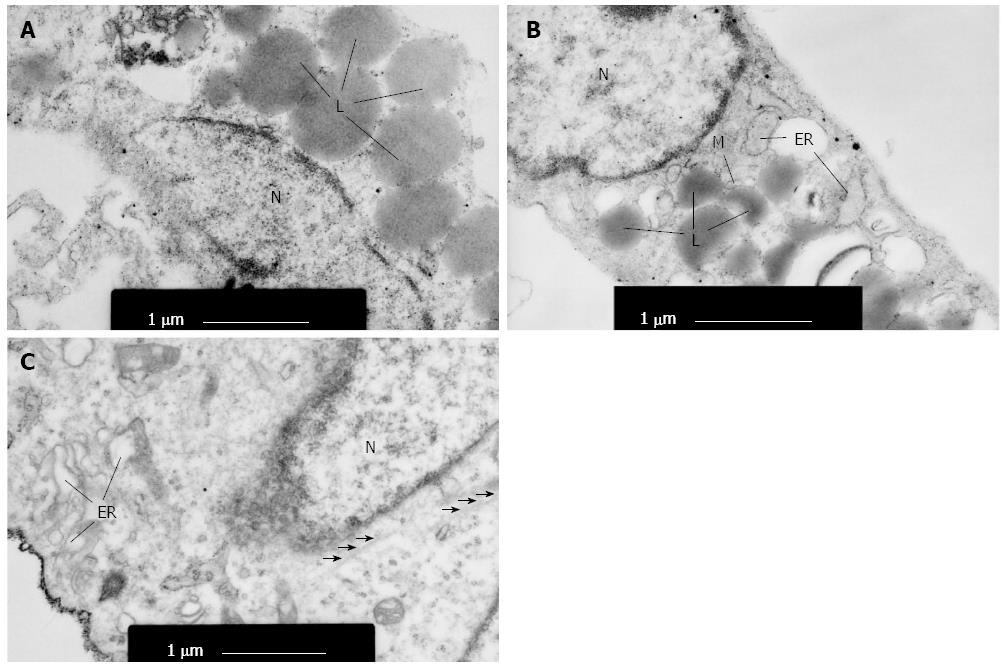
At the ultrastructural level, primary cultured HSCs contained a small amount of rough endoplasmic reticulum and a few mitochondria, without microfilaments in the cytoplasm (Figure 2A). HSCs (5d) were characterized with increased rough endoplasmic reticulum, Golgi apparatus and microfilaments (Figure 2B). HSCs (P3) exhibited marked hypertrophy of rough endoplasmic reticulum and Golgi apparatus. They also had extensive microfilaments (Figure 2C), suggesting that perpetuation HSCs exhibited active synthesis and secretion function.
In summary, initiation HSCs and perpetuation HSCs had different morphological and phenotypic traits, which indicated that the synthesis and secretion functions of HSCs changed during the activation process. We speculated that HSCs of these two stages of activation might play different roles in liver regeneration after ALI. Therefore, we assessed the therapeutic effects of HSCs in APAP-induced ALI in mice.
We previously established a mouse model of APAP-induced ALI[9]. Liver enzyme levels measured in the peripheral blood provide a good estimate of ongoing liver damage. In this study, serum ALT and AST started to rise 6 h after APAP treatment, rose rapidly 12 h later, and peaked at 24 h (data not shown).
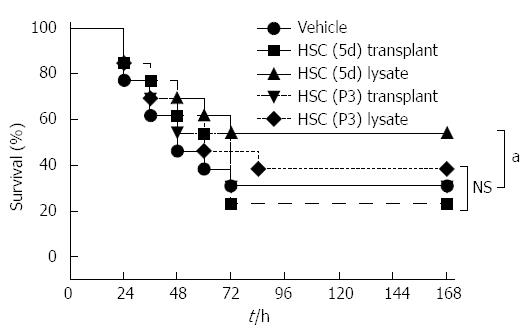
To reverse the APAP-induced ALI, we first assessed various HSC treatment modalities: cell transplantation, delivery of cellular lysates, and delivery of conditioned medium to assess the most efficacious therapy. Animals were treated 2 h after ALI induction by tail vein injections of wholes cells or cell lysates. No significant survival benefit was observed after the intravenous transplantation of HSCs (5d) or HSCs (P3), which was most likely due to poor engraftment and entrapment in the alveolar capillary (Figure 3). In contrast, treatment with cellular lysates of HSCs (5d), derived from the same cell mass as used for transplantation, resulted in an increased survival trend compared to other four groups (P < 0.05).
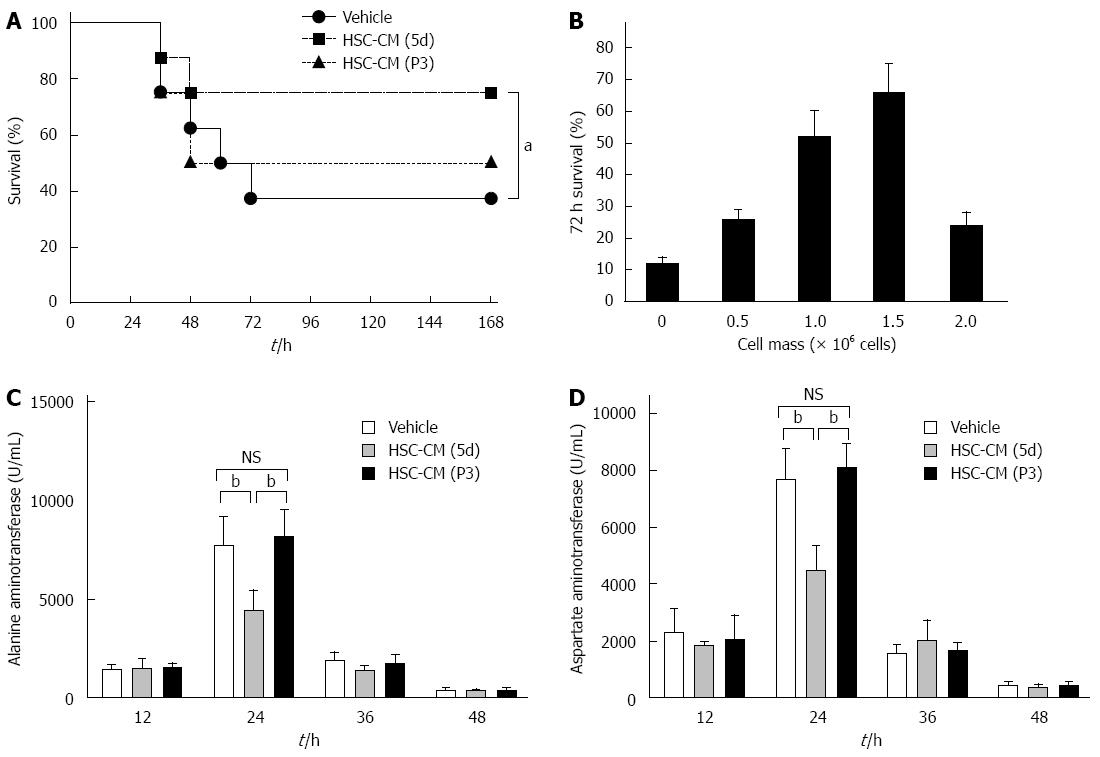
We then determined if the efficacy observed with lysates could be reproduced by using the secreted molecules from HSCs. A longitudinal analysis using HSC-CM (5d) from the same cell mass (i.e., 2 × 106 HSCs) revealed a distinct survival benefit compared to vehicle (P < 0.05) and HSC-CM (P3) (P < 0.05). There was no significant difference between the HSC-CM (P3) and vehicle groups in survival rate (Figure 4A). In addition, we monitored 72 h survival of ALI-induced mice as a function of the mass of HSCs (5d) from which the medium was conditioned (Figure 4B). Interestingly, the effect of HSC (5d) concentrate was abrogated at higher cell masses, indicating a therapeutic window of effectiveness. However, the HSC-CM (P3) could not improve mouse survival in all cell masses (data not show).
Based on these results, we assessed the protective effects of the molecules secreted from HSCs (5d). Animals were treated 2 h after ALI induction by injection of HSC-CM (5d) into the systemic circulation. HSC-CM (P3) and blank-CM (vehicle) served as controls. Serum was obtained every 12 h and analyzed for hepatocyte release. Liver enzyme levels in serum provide a good estimate of ongoing liver damage. The peak in liver damage was observed at 24 h after CM treatment in each group. However, ALT was decreased by 42% (P < 0.01) and 45% (P < 0.01), respectively, in HSC-CM (5d)-treated mice compared with HSC-CM (P3) and vehicle mice, and AST was decreased by 42% (P < 0.01) and 46% (P < 0.01), respectively (Figure 4C and D). Overall, these results show that the molecules secreted from initiation HSCs were associated with less severe liver damage and higher survival rate.
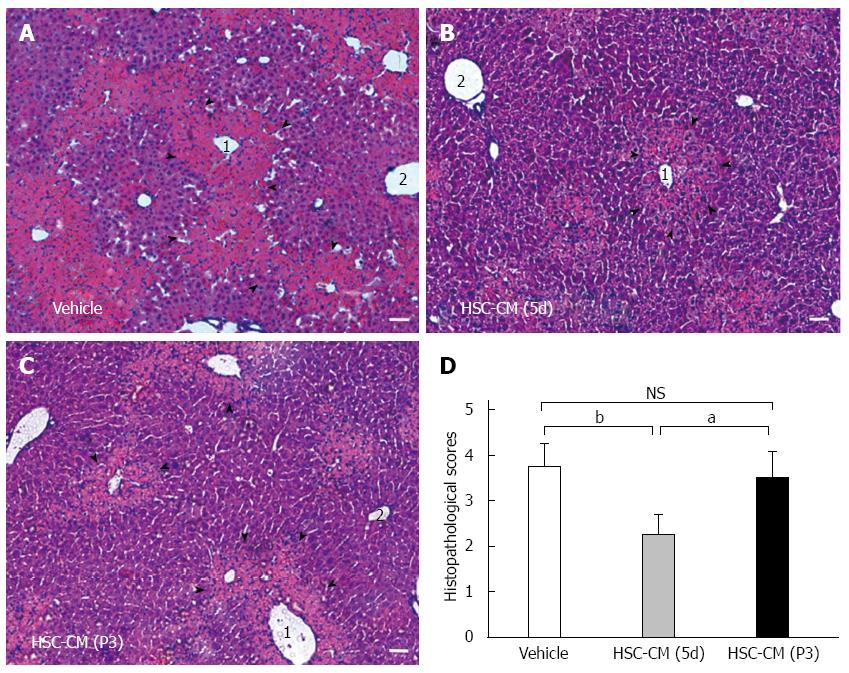
Microscopic evaluation of HE-stained liver tissue from HSC-CM (P3) and vehicle mice revealed profound hepatocellular necrosis and panlobular mononuclear leukocyte infiltration with cytoplasmic vacuolization and severe distortion of tissue architecture (Figure 5A and C). HSC-CM (5d)-treated mice showed no signs of disseminated inflammation, although minor pericentrilobular vein infiltration and hepatocellular death were observed (Figure 5B). Semi-quantitative histological examination of liver tissue confirmed the differences between the three groups (Figure 5D). The average score in the HSC-CM (5d) group was 2.25 ± 0.45, compared with 3.51 ± 0.57 in the HSC-CM (P3) group (P < 0.05) and 3.75 ± 0.51 in the vehicle group (P < 0.01). There was no difference between HSC-CM (P3) and vehicle. These results demonstrate that the molecules secreted from initiation HSCs correlated with less severe hepatocellular necrosis.
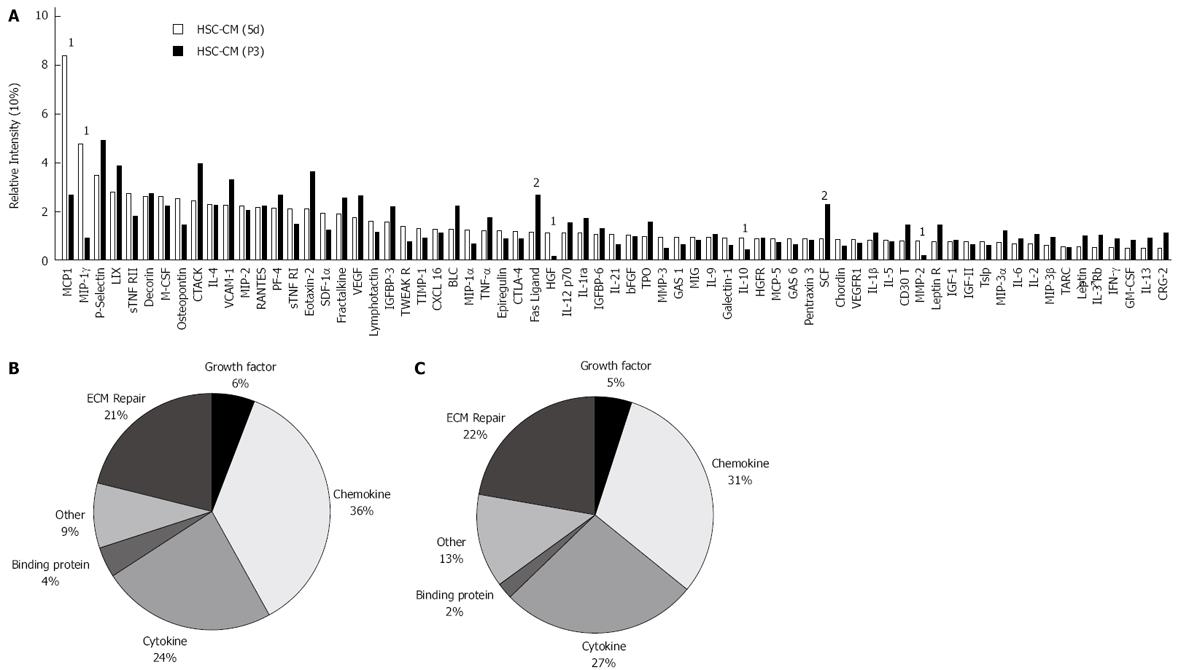
To determine the molecular mediators of the observed protective effects of initiation HSCs, we examined HSC-CM using a high-density protein array. HSC-CM contained 69 of the 144 assayed proteins (Figure 6A), which included a broad spectrum of molecules involved in immunomodulation and liver regeneration. Cluster analysis revealed that a large proportion of HSC-CM (5d) was composed of chemokines (36.29%) and growth factors (21.03%) (Figure 6B), many of which were expressed at high relative levels. There was no significant difference between HSC-CM (5d) and HSC-CM (P3) in the constituent ratios of proteins. Only 7 of the 69 proteins had a constituent ratio difference of more than 2-fold between the HSC-CM (5d) and the HSC-CM (P3) group: monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1γ (MIP-1γ), hepatocyte growth factor (HGF), interleukin-10 (IL-10), matrix metalloproteinase-2 (MMP-2), stem cell factor (SCF) and Fas-Ligand. HSC-CM (5d) contained significantly more MCP-1, MIP-1γ, HGF, IL-10 and MMP-2, which might correlate with the greater inflammatory inhibition and therapeutic activity of HSC-CM (5d) in ALI.
It is becoming increasingly clear that HSCs have a profound impact on the proliferation, differentiation, and morphogenesis of other hepatic cell types during liver development and regeneration. Inhibiting activated HSCs using gliotoxin[5] and L-cysteine[10] prevents normal regenerative responses of both hepatocytes and oval cells in APAP- and 2AAF/PH-induced ALI, respectively. In this study, we observed that initiation HSCs were different from perpetuation HSCs in morphology, phenotype and molecule secretion. Initiation HSC-derived molecules protected hepatocytes against death and increased the survival rate of mice subjected to APAP-induced ALI. The efficacy of initiation HSC-CM was a function of the cell mass from which the medium was conditioned, suggesting important pharmacological aspects of this treatment. These results are significant because we identified differences between initiation HSCs and perpetuation HSCs and provide clear evidence that delivery of HSC secretions has the potential to dramatically reduce cell death in the acutely injured liver.
The use of transgenic models has yielded information on how abnormal function of HSCs translates into regenerative defects. Foxf1+/- mice subjected to CCl4 injury show decreased HSC activation and more severe hepatocyte necrosis during the regenerative period[11]. These results suggest that the defect in HSC activation consecutive to haploinsufficiency of Foxf1 results in impaired regeneration. However, following CCl4 injury, Col-1a1r/r mice show persistent activation of HSCs and fail to regenerate properly[12]. In the context of progressive fibrosis, this inhibition of hepatocyte proliferation may represent a significant mechanism preventing the restoration of effective hepatocellular function. These two examples suggest that deficient as well as uncontrolled HSC activation impairs liver regeneration. Thus, a finely tuned HSC response may be an important factor to ensure adequate regeneration[13]. Based on the data, we speculate that perpetuation HSCs mainly take part in the process of liver fibrosis, but initiation HSCs are involved in hepatocyte protection.
In normal circumstances, the liver hardly proliferates and was therefore classified as a stable organ. After liver injury, however, proliferation of the main epithelial compartments (hepatocytes and cholangiocytes), followed by proliferation of the mesenchymal cells (HSCs and endothelial cells), quickly restores the liver. As the support cell for hepatocytes, HSCs interact in order to control the hepatocytes, and these interactions can often be broken down into one of two major mechanistic categories: physical contact and diffusible factors[14]. It has been showed that HSC-clusters frequently contained α-SMA expressing HSCs and were in close association with hepatocytes, indicating the possibility that activation of HSCs and HSC-hepatocyte interaction were related events during regeneration[7]. However, whether diffusible factors from HSCs have a protective effect on hepatocytes is not clear. In this study, we firstly showed that early activated HSC-derived molecules could reverse ALI. Evarts et al[15] used the HSC cell line to verify that naproxen stimulated VEGF and HGF expression was of particular relevance in improving survival of transplanted hepatocytes. Naproxen and celecoxib increased desmin expression in HSCs after hepatocytes transplantation. This desmin-positive phenotype of HSCs was similar to previous cell transplantation studies, where desmin was expressed without α-SMA, presumably because this stimulus was transient and nonfibrogenic.
Following liver injury, HSCs undergo “activation”, which consists of two major phases: initiation and perpetuation, followed by resolution of fibrosis if injury subsides[4]. In this study, regarding the morphological and phenotypic differences, initiation HSCs were spherical and expressed little α-SMA, while perpetuation HSCs were myofibroblast-like and expressed a uniform and higher level of α-SMA. Thus, we hypothesized that perpetuation HSCs exhibited active synthesis and secretion. Previous studies have shown that secretion of IL-10 and HGF was gradually reduced with the activation of HSCs, and these factors are confirmed to have a protective effect on hepatocytes in in vitro studies[15,16].
To determine the molecular mediators of the observed protective effects of initiation HSCs, we further used proteomic analysis to reveal a broad spectrum of molecules that are involved in immunomodulation and hepatocyte protection. Compared to perpetuation HSC-CM, initiation HSC-CM included higher levels of MCP-1, MIP-1γ, HGF, IL-10 and MMP-2, but less SCF and Fas-Ligand. MCP-1 (CCL2) is a C-C chemokine that attracts monocytes and memory T cells specifically during an inflammatory response via its specific receptor, CCR2[17]. MCP-1 is upregulated in a variety of diseases that are characterized by mononuclear cell infiltration[18]. Deficiency of MCP-1 protects mice against alcoholic liver injury[19], but there is also evidence that MCP-1 protects against hepatic injury by directly inhibiting NKT cell IL-4 production in T cell-mediated hepatitis[20]. MIP-1γ also belongs to a C-C chemokine family containing MIP-1α, MIP-1β, and MIP-1γ, which are produced by monocytes and other types of leukocytes. By binding to chemokine receptor 1 (CCR1), a specific receptor on neutrophils, MIP-1γ acts as a chemoattractant that induces the chemotaxis of CD4+ T cells, CD8+ T cells, and monocytes[21]. In this study, we detected high levels of MCP-1 and MIP-1γ in HSC-CM (5d) but observed relatively less monocyte infiltration in hepatic tissue of the HSC (5d) group, indicating that MCP-1 and MIP-1γ might not only affect monocyte trafficking. Further study is needed to determine the specific roles of MCP-1 and MIP-1γ in ALI. IL-10 is an anti-inflammatory pleiotropic cytokine that is secreted by monocytes, macrophages, and other leukocytes upon stimulation. Endogenous IL-10 protects hepatocytes by suppressing the ability of effector cells (e.g., Kupffer cells) to release multiple cytokines, including TNF-α and chemokines, thereby inhibiting cytokine-dependent hepatocyte injury[22]. We speculate that the anti-inflammation effects observed in this study are a comprehensive regulatory process achieved by MCP-1, MIP-1γ and IL-10. HGF is regarded as one of the most potent stimulants for hepatocyte regeneration. All biological effects of HGF are mediated by a single tyrosine kinase receptor, c-Met[23]. Gene-knockout studies have shown that both HGF and c-Met are absolutely required for liver development. HGF/c-Met signaling stimulates hepatocyte growth through paracrine and autocrine mechanisms, and these can initiate liver regeneration[24]. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) maintain hepatic ECM stability by regulating its formation and degradation[25]. Activated HSCs are responsible for the majority of ECM protein deposition in liver fibrosis[26]. A recent study reported the role of MMP-2 in reducing hepatic injury and enhancing liver regeneration[27]. MMP-2 promotes pericellular collagen deposition, creating a microenvironment supporting the growth of regenerative hepatocytes. In contrast, perpetuation HSC-CM contained more Fas-ligand and SCF than initiation HSC-CM. Fas promotes hepatocyte apoptosis and hepatic fibrogenesis[28]. SCF regulates the differentiation of CD34-positive stem cells and modulates the synthesis of more specific cell types[29]. SCF also plays an important role in liver-remodeling processes. SCF combined with GM-CSF affects cellular differentiation and proliferation in various types of cells besides hepatobiliary epithelial cells[30]. However, our data show that SCF may not protect hepatocytes from ALI. Thus, initiation HSCs attenuate acute hepatocyte injury, while perpetuation HSCs may take part in hepatic fibrosis. The specific mechanisms of action of these molecules may be pluralistic, and whether the molecules could promote liver regeneration after ALI merits further study.
In conclusion, we observed different morphologies and phenotypes between initiation HSCs and perpetuation HSCs, and provide the first experimental evidence of the potential medical value of initiation HSC-derived molecules in the treatment of ALI. Our findings provide novel insight into the mechanisms of HSCs in liver injury therapy. Whether the potential value of initiation HSC-derived molecular therapy is derived from the effect of a single cytokine or a combination of cytokines should be explored in future studies.
Hepatic stellate cells (HSCs) play a role in hepatic regeneration. The authors have previously demonstrated that activated HSCs are involved in the proliferation of hepatocytes in acetaminophen (APAP)-induced acute liver injury (ALI). The goal was to determine if HSCs at different activation stages had different effects on APAP-induced ALI.
Some studies directly supported the involvement of hepatocyte stellate cells (HSCs) in liver regeneration. Activated HSCs have been implicated in assisting liver regeneration by producing angiogenic factors as well as factors that modulate endothelial cell and hepatocyte proliferation and by remodeling the extracellular matrix. Inhibiting activated HSCs using gliotoxin and L-cysteine prevents normal regenerative responses of both hepatocytes and oval cells in APAP- and 2AAF/PH-induced ALI, respectively. In addition, Foxf1+/- mice subjected to CCl4 injury show decreased HSC activation and more severe hepatocyte necrosis during the regenerative period. Notably, the mechanisms by which activated HSCs help mediate liver regeneration in experimental animals and human patients remain to be determined and the relative importance of different subtypes of hepatic stellate cells/myofibroblasts is still not clear in liver injury.
In this study, the authors observed different morphologies and phenotypes between initiation HSCs and perpetuation HSCs and described the first use of molecules secreted from HSCs in APAP-induced ALI. Initiation HSC-derived molecules showed hepatocyte-protective effects. This findings provide novel insight into the mechanisms of HSCs in liver injury therapy.
In conclusion, the current study shows that systemic HSC-CM (5d) therapy has profoundly improved survival in mice undergoing APAP-induced ALI. This work validates that conditioned medium of early activated HSCs induces an integrated response to liver injury and creates potential new avenues for the treatment.
HSCs, first described by Kupffer in 1876, have emerged in the past 30 years as remarkably versatile mesenchymal cells. Previous studies have explored the importance of HSCs in liver fibrosis because HSC activation into myofibroblasts is thought to be the major pathway in hepatic fibrogenesis associated with liver injury. Beyond this well-known characteristic, however, many newly discovered activities have led to a greater understanding of this fascinating cell type and the complexity of cellular homeostasis in the liver.
This work by Chang et al describes the protective effect of conditioned medium from early activated HSCs on hepatocytes in injured liver from APAP injury. The work is novel and not described before. The findings point towards an in vivo protective component of HSCs during early activation.
P- Reviewer: Cappon A, Chao JCJ, Guo JS, Pan Q, Ramani K, Wu TJ, Xiao EH, Yu HP S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Lysy PA, Campard D, Smets F, Najimi M, Sokal EM. Stem cells for liver tissue repair: current knowledge and perspectives. World J Gastroenterol. 2008;14:864-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 406] [Article Influence: 36.9] [Reference Citation Analysis (10)] |
| 3. | Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 4. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2244] [Cited by in RCA: 2200] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 5. | Shen K, Chang W, Gao X, Wang H, Niu W, Song L, Qin X. Depletion of activated hepatic stellate cell correlates with severe liver damage and abnormal liver regeneration in acetaminophen-induced liver injury. Acta Biochim Biophys Sin (Shanghai). 2011;43:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Roskams T. Different types of liver progenitor cells and their niches. J Hepatol. 2006;45:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Mabuchi A, Mullaney I, Sheard PW, Hessian PA, Mallard BL, Tawadrous MN, Zimmermann A, Senoo H, Wheatley AM. Role of hepatic stellate cell/hepatocyte interaction and activation of hepatic stellate cells in the early phase of liver regeneration in the rat. J Hepatol. 2004;40:910-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Chang W, Yang M, Song L, Shen K, Wang H, Gao X, Li M, Niu W, Qin X. Isolation and culture of hepatic stellate cells from mouse liver. Acta Biochim Biophys Sin (Shanghai). 2014;46:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE. Hepatic stellate cells’ involvement in progenitor-mediated liver regeneration. Lab Invest. 2010;90:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Kalinichenko VV, Bhattacharyya D, Zhou Y, Gusarova GA, Kim W, Shin B, Costa RH. Foxf1 +/- mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology. 2003;37:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Balabaud C, Bioulac-Sage P, Desmoulière A. The role of hepatic stellate cells in liver regeneration. J Hepatol. 2004;40:1023-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Evarts RP, Hu Z, Fujio K, Marsden ER, Thorgeirsson SS. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993;4:555-561. [PubMed] |
| 16. | Wang SC, Ohata M, Schrum L, Rippe RA, Tsukamoto H. Expression of interleukin-10 by in vitro and in vivo activated hepatic stellate cells. J Biol Chem. 1998;273:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 20. | Ajuebor MN, Hogaboam CM, Le T, Swain MG. C-C chemokine ligand 2/monocyte chemoattractant protein-1 directly inhibits NKT cell IL-4 production and is hepatoprotective in T cell-mediated hepatitis in the mouse. J Immunol. 2003;170:5252-5259. [PubMed] |
| 21. | Shen PC, Wu CL, Jou IM, Lee CH, Juan HY, Lee PJ, Chen SH, Hsieh JL. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Dinant S, Veteläinen RL, Florquin S, van Vliet AK, van Gulik TM. IL-10 attenuates hepatic I/R injury and promotes hepatocyte proliferation. J Surg Res. 2007;141:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 790] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 24. | Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Zhou X, Hovell CJ, Pawley S, Hutchings MI, Arthur MJ, Iredale JP, Benyon RC. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Salguero Palacios R, Roderfeld M, Hemmann S, Rath T, Atanasova S, Tschuschner A, Gressner OA, Weiskirchen R, Graf J, Roeb E. Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab Invest. 2008;88:1192-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Padrissa-Altés S, Zaouali MA, Franco-Gou R, Bartrons R, Boillot O, Rimola A, Arroyo V, Rodés J, Peralta C, Roselló-Catafau J. Matrix metalloproteinase 2 in reduced-size liver transplantation: beyond the matrix. Am J Transplant. 2010;10:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis. 2008;28:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Akel S, Petrow-Sadowski C, Laughlin MJ, Ruscetti FW. Neutralization of autocrine transforming growth factor-beta in human cord blood CD34(+)CD38(-)Lin(-) cells promotes stem-cell-factor-mediated erythropoietin-independent early erythroid progenitor development and reduces terminal differentiation. Stem Cells. 2003;21:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |