Published online Apr 14, 2015. doi: 10.3748/wjg.v21.i14.4169
Peer-review started: July 3, 2014
First decision: July 21, 2014
Revised: October 25, 2014
Accepted: December 22, 2014
Article in press: December 22, 2014
Published online: April 14, 2015
Processing time: 286 Days and 16.7 Hours
AIM: To investigate the effect of metformin on silibinin-induced apoptosis in human colorectal cancer (COLO 205) cells.
METHODS: MTT assays were performed to quantify cell viability. Western blot assays were applied to identify the expression of signaling proteins.
RESULTS: The combined treatment of COLO 205 cells with metformin and silibinin decreased cell survival at a dose insufficient to influence the non-malignant cells [Human colonic epithelial cells (HCoEpiC)]. Silibinin and metformin increased phosphatase and tensin homolog and 5’-adenosine monophosphate-activated protein kinase expression in COLO 205 cells and inhibited the phosphorylation of mammol/Lalian target of rapamycin. This combined treatment resulted in an increase in the expression of activated caspase 3 and apoptosis inducing factor, indicating apoptosis.
CONCLUSION: The combined treatment of human colorectal cancer cells with silibinin and metformin may induce apoptosis at a dose that does not affect HCoEpiC. This finding reveals a potential therapeutic strategy for the treatment of colorectal cancer.
Core tip: Silibinin is known to provide protection against hepatotoxic stress. Silibinin has also been shown to have high efficacy against cancer cells through increased expression of the phosphatase and tensin homolog; Metformin, a well-known antidiabetic agent, has recently been reported to inhibit cancer by increasing adenosine monophosphate-activated protein kinase expression. We investigated the effect of metformin on silibinin-induced apoptosis in human colorectal cancer cells. The combined treatment of human colorectal cancer cells with silibinin and metformin may induce apoptosis at a dose that does not affect human colonic epithelial cells. This finding reveals a potential therapeutic strategy for the treatment of colorectal cancer.
- Citation: Tsai CC, Chuang TW, Chen LJ, Niu HS, Chung KM, Cheng JT, Lin KC. Increase in apoptosis by combination of metformin with silibinin in human colorectal cancer cells. World J Gastroenterol 2015; 21(14): 4169-4177
- URL: https://www.wjgnet.com/1007-9327/full/v21/i14/4169.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i14.4169
Colorectal cancer is one of the leading causes of cancer associated deaths globally[1]. The high mortality of patients with colorectal cancer is mainly attributed to its metastasis to distant organs[2]. The application of traditional radiotherapy and chemotherapy treatments is limited due to their high toxicity and damaging side effects. Therefore, the development of alternative treatments is a viable strategy that may help lower the side effects from the treatment of colorectal cancer. Interventions using nontoxic phytochemicals, which are aimed at multiple targets, have been shown to reduce carcinogenesis and overall cancer risk[3]. Numerous studies have reported strong anticancer properties of both dietary and non-dietary phytochemicals against multiple cancers, including colorectal cancer[4]. Thus, phytochemicals have received increased attention due to their potential uses in cancer therapy, and many phytochemicals have been investigated in various phases of clinical trials[5].
Milk thistle (Silybum marianum) has been widely utilized in the United States and Europe as a popular dietary supplement for treating liver diseases[6]. Silibinin, a polyphenolic flavonoid, is the major active compound in milk thistle[7]. Milk thistle is known to be safe and effective without side effects when administered to protect the liver against chemical or alcohol-related injury[8]. The inhibitory action of silibinin has been demonstrated in multiple cancer cell lines, including lung[9], liver[10], skin[11], colon[12] and prostate[13] cancer cells.
Metformin is an antidiabetic agent widely used to treat diabetic patients. Its action is mainly mediated by the activation of 5’-adenosine monophosphate (AMP)-activated protein kinase (AMPK), which inhibits hepatic gluconeogenesis and enhances glucose uptake in skeletal muscle[14]. In addition, metformin has been reported to reduce cell proliferation in several human tumors, including breast cancer[15], pancreatic cancer[16] and gastric cancer[17]. Metformin also inhibited the growth of tumors in xenograft mouse models of breast cancer[18], prostate cancer[19], ovarian cancer[20] and melanoma[21]. The administration of metformin to diabetic patients was associated with lower rates of cancer incidence and mortality[22]. Colorectal cancer patients with diabetes who were treated with metformin as part of their diabetic therapy exhibited a greater overall survival rate[23].
Metformin might inhibit mammol/Lalian target of rapamycin (mTOR) through regulation at multiple sites. The low energy charge in metformin-treated cancer cells activates AMPK, which can inhibit cell growth and proliferation. AMPK activates the tumor suppressor gene, resulting in inhibition of mTOR and an increase in apoptosis[24]. Silibinin was shown to be associated with increased phosphatase and tensin homolog (PTEN) activity and decreased phosphorylated protein kinase B (p-Akt) production, indicating a role for the PTEN/Akt pathway in the apoptosis of cancer cells[25].
Both metformin and silibinin exhibit anticancer activities. Thus, we administered silibinin and metformin in combination to investigate the anticancer efficacy of the combination therapy in human colorectal cancer cell line (COLO 205). In the present study, we determined an effective dose for this combination of two agents that was not nontoxic to non-malignant cells.
Antibodies against activated caspase 3 and apoptosis inducing factor (AIF) were purchased from Millipore (Millipore, Bedford, MA, United States). Antibodies against the PTEN, signal transducer and p-Akt, phosphorylated-AMPK (p-AMPK), phosphorylated mammol/Lalian target of rapamycin (p-mTOR) and β-actin (actin) were purchased from Abcam (Cambridge, MA, United States). Metformin and silibinin were purchased form Sigma-Aldrich (St Louis, MO, United States).
The human colorectal cancer cell line (COLO 205) was purchased from the Culture Collection and Research Center of the Food Industry Institute (Hsin-Chiu City, Taiwan). Human colonic epithelial cells (HCoEpiC) were purchased from ScienCell (CA, United States). COLO 205 cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO). HCoEpiC were cultured in Colonic Epithelial Cell Medium (CoEpiCM, Cat. No. 2951) purchased from ScienCell (CA, United States). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2. When cells reached approximately 60% confluence, the medium was replaced with serum-free cell medium containing various concentrations of silibinin (0, 50, 100, 150 or 200 μmol/L)[10] and metformin (0, 5, 10, 15 or 20 mmol/L) and the cells were cultured for 24 h as previously described[21]. The cells were trypsinized with 0.25% trypsin and 0.2 g/L EDTA and harvested for further studies.
The Loewe additivity model[26] was used as a second method of analyzing the interaction between silibinin and metformin. The interaction between the compounds is reported as the combination index (CI) in the following equation: CI = (d1/D×,1) + (d2/D×,2).
In the equation, d1 and d2 represent the concentrations of the compounds in combination required to achieve × effect. D×,1 and D×,2 represent the concentrations of the same compounds individually that would quantitatively achieve the same × effect. A CI < 1.0 indicates that the combination is synergistic, and a CI > 1.0 indicates an antagonistic interaction. The combination indexes for this study were determined by using concentrations corresponding to the ED50 of the dose response curves for silibinin, metformin and silibinin + metformin.
Apoptotic response was quantified by the detection of DNA histone complexes released from the nucleus to the cytosol of cells using the Apo-Glo Assay (Promega, WI, United States). The assay was performed to assess viability and caspase 3/7 activation within a single assay well. Briefly, 104 cells were seeded on 96-well plates in triplicate and treated with different combinations of varying concentrations of metformin and silibinin for 24 h. Each well contained a final volume of 0.2 mL of culture medium. Viability reagent was added to each well and mixed with culture medium using orbital shaking (500 rpm for 30 s), and the cells were incubated with the viability reagent for 1 h at 37 °C. Fluorescence was measured at two wavelengths: 400 nm/505 nm. Following the addition of caspase-Glo 3/7 (100 μL), we mixed the samples using orbital shaking (500 rpm for 30 s). After incubation for 30 min at room temperature, luminescence was measured using a microplate reader to assess apoptosis while the fluorescence was measured at 380Ex/510Em.
Protein was extracted from tissue homogenates and cell lysates using ice-cold radio-immol/Luno-precipitation assay (RIPA) buffer supplemented with phosphatase and protease inhibitors (50 mmol/L sodium vanadate, 0.5 mmol/L phenylmethylsulphonyl fluoride, 2 mg/mL aprotinin, and 0.5 mg/mL leupeptin). The protein concentrations were determined using a Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, United States). Total protein samples (30 μg) were separated by SDS/polyacrylamide gel electrophoresis (10% acrylamide gel) using the Bio-Rad Mini-Protein II system. The proteins were transferred to polyvinylidene difluoride membranes (PerkinElmer, Waltham, MA, United States) using a Bio-Rad Trans-Blot system. After the transfer, the membrane was blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) and then incubated for two hours. The membrane was then washed in TBS-T and hybridized with primary antibodies, which were diluted to a suitable concentration in TBS-T, for 16 h. Specific antibodies for activated caspase 3, AIF, PTEN, p-Akt, p-AMPK and p-mTOR (1:1000 dilution) were used. Additionally, the membranes were incubated with a goat polyclonal antibody specific for β-actin (Actin) (1:10000 dilution) to serve as an internal control. Incubation with secondary antibodies and detection of the antigen-antibody complex were performed using an ECL kit (Amersham Biosciences, United Kingdom). After comparing with the marker to determine specificity, the immol/Lunoblots of β-actin (43 kDa), activated caspase 3 (17 kDa), AIF (57 kDa), PTEN (42 kDa), p-Akt (60 kDa), p-AMPK (62 kDa) and p-mTOR (289 kDa) were quantified with a laser densitometer (Avegene Life Science, Taipei, Taiwan).
The data are expressed as the mean ± SEM for the indicated number (n) of samples in each group. Repeated measures analysis of variance (ANOVA) was used to analyze gene and protein expression level changes and other parameters. Differences resulting in a P-value of 0.05 or less were considered to be significant.
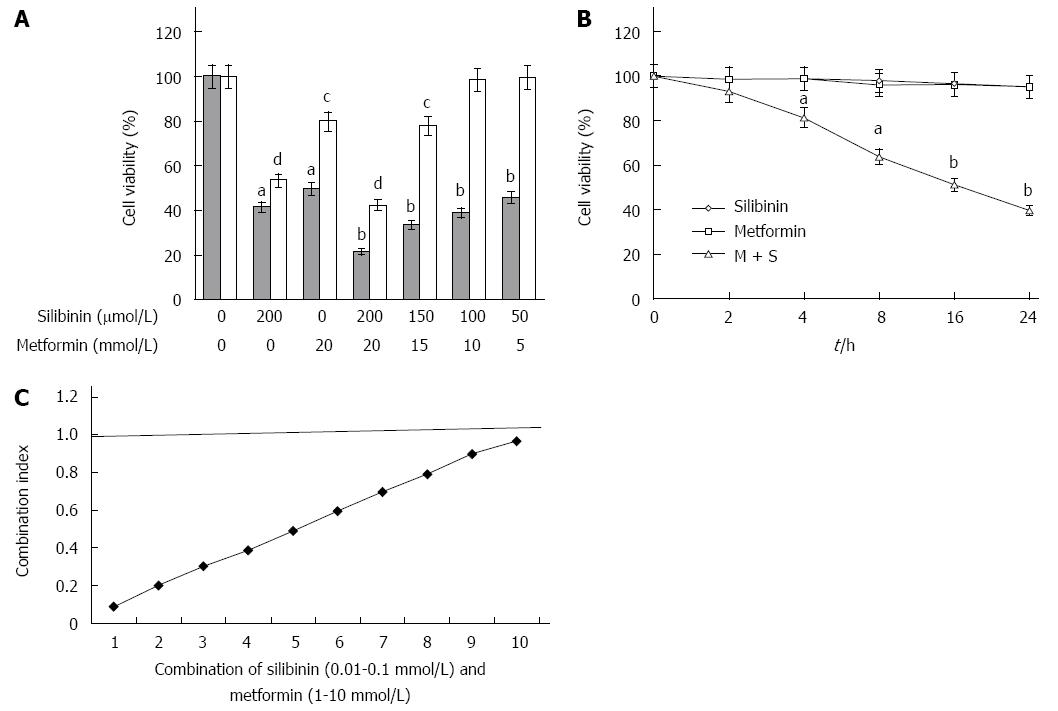
Figure 1A shows the survival rate of COLO 205 cells and HCoEpiC treated with a combination of silibinin and metformin at various concentrations for 24 h. Both silibinin and metformin at their highest dose, in combination or individually, exerted an inhibitory effect on the survival of COLO 205 cells. However, individual treatment with silibinin or metformin at their highest respective dose exerted a toxic effect on HCoEpiC, and the rate of cell death was even greater following the combination treatment. Furthermore, combined treatment with 100 μmol/L silibinin and 10 mmol/L metformin was more effective than treatment with 50 μmol/L silibinin and 5 mmol/L metformin without altering the survival of HCoEpiC. Figure 1B shows the survival curves of COLO 205 cells treated with silibinin (100 μmol/L) and metformin (10 mmol/L) in combination or individually at different exposure times. The survival curves shifted to the left after a longer exposure to the combined treatment, while the individual treatments did not affect the survival of COLO 205. The synergistic effect of silibinin + metformin on COLO 205 cells was further confirmed using an alternative approach of calculating the CI. The combination of silibinin at concentrations below 100 μmol/L + metformin at concentrations below 10 mmol/L metformin had a CI < 1, indicating synergy between the two compounds (Figure 1C). Thus, in the following experiments, we used 100 μmol/L silibinin with 10 mmol/L metformin in combination to compare the effects of this combination treatment with the individual treatments on COLO 205 cells after 24-h incubation.
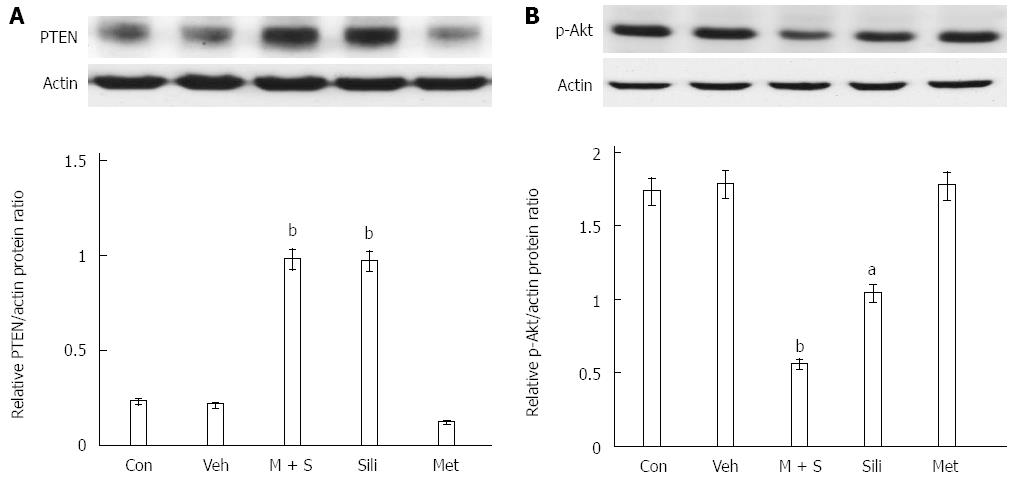
The effects of combined metformin and silibinin (M + S) treatment on the expression of PTEN were examined by Western blot. As shown in Figure 2, expression of phosphorylated AKT decreased while expression of PTEN increased in COLO 205 cells after silibinin treatment or M + S treatment for 24 h.
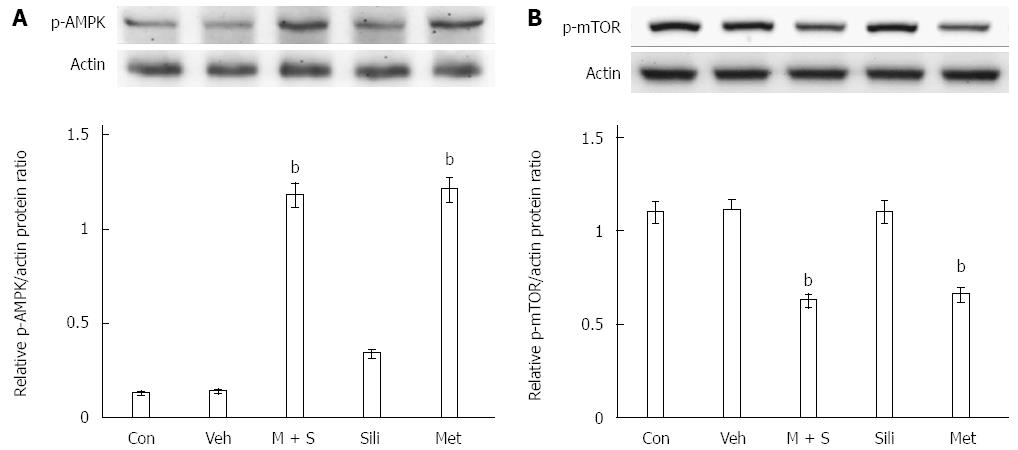
The effects of M + S treatment on the phosphorylation of AMPK were examined by Western blot. As shown in Figure 3A, expression of phosphorylated AMPK increased in COLO 205 cells after treatment with metformin or M + S for 24 h. Additionally, treatment with metformin or M + S inhibited the phosphorylation of mTOR in COLO 205 cells (Figure 3B).
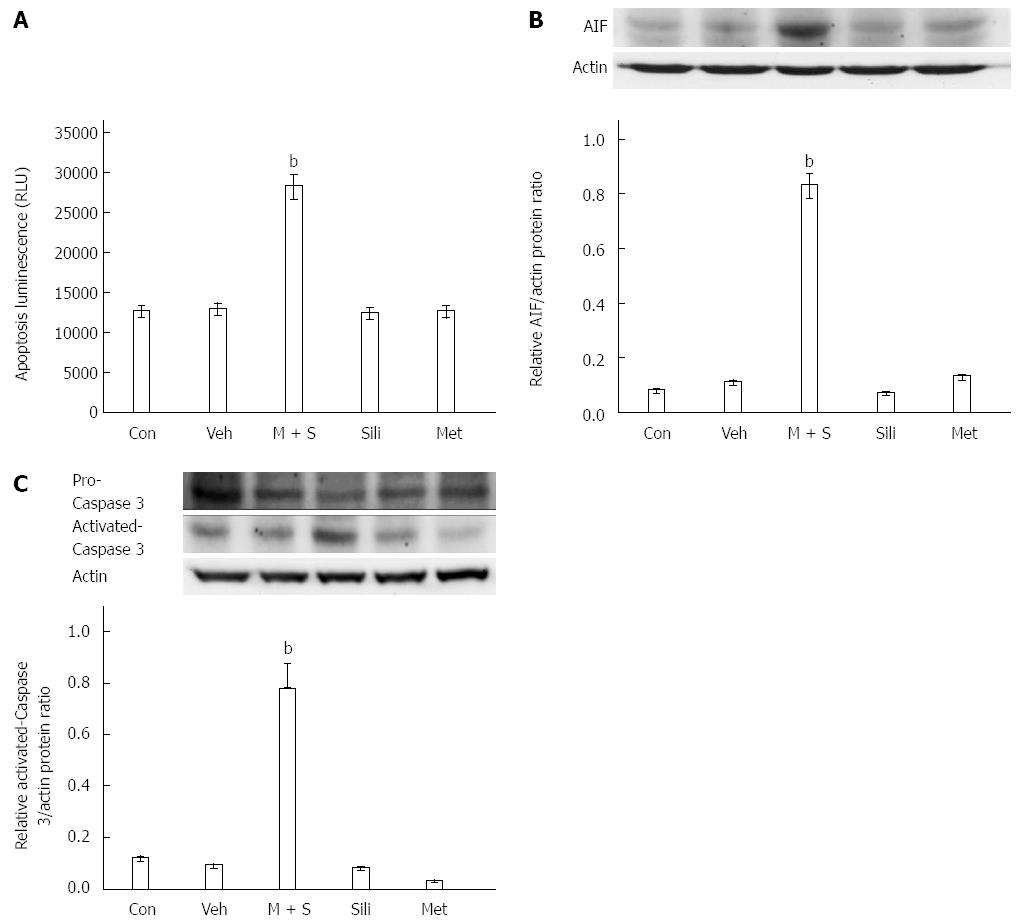
We measured COLO 205 cell apoptosis using an Apo-Tox Glo assay. As shown in Figure 4A, the combination treatment with 100 μmol/L silibinin and 10 mmol/L metformin induced cytotoxicity and apoptosis in COLO 205 cells, however, this effect was not seen when cells were treated with silibinin or metformin alone. The levels of activated caspase 3 and AIF were determined by Western blot, as described previously[27], to assess the apoptosis. M + S treatment increased the levels of activated caspase 3 and AIF in COLO 205 cells, whereas treatment with silibinin or metformin alone did not produce the same effects (Figure 4B and C). Thus, (M + S)-induced inhibition is primarily mediated by the induction of apoptosis in cancer cells.
In the present study, we found that the co-treatment of human colorectal cancer cell line (COLO 205 cells) with silibinin (100 μmol/L) and metformin (10 mmol/L) directly inhibits cell survival. In addition, the combination of silibinin (100 μmol/L) and metformin (10 mmol/L) at the doses that effectively inhibited COLO 205 cell survival did not affect normal HCoEpiC. There was considerable specificity in the inhibition of cancer cells using this combination therapy. Moreover, silibinin alone or in combination with metformin increased the level of PTEN expression in COLO 205 cells, resulting in a significant reduction in phosphorylated Akt (at Ser473). Moreover, metformin alone or in combination with silibinin increased the phosphorylation of AMPK and inhibited the phosphorylation of mTOR in COLO 205 cells. Furthermore, results of the Apo-Glo assay indicated that treatment of COLO 205 cells with 100 μmol/L silibinin and 10 mmol/Lol/Lol/L metformin in combination induced apoptosis, while this effect was not seen when cells were treated with silibinin or metformin alone. Finally, the combined treatment of cells with silibinin and metformin increased caspase 3 activation and AIF expression, both of which are widely used as indicators of apoptosis[27]. Therefore, our results suggest that the combination of silibinin and metformin more effectively induced apoptosis compared to treatment with silibinin or metformin alone in COLO 205 cells and at a dose that was nontoxic to normal cells.
Previous studies have identified the major roles of PTEN/Akt signaling in carcinogenesis and cancer progression[28]. PTEN is an upstream factor that inhibits p-Akt, and silibinin may increase PTEN expression to inhibit cell growth[10,27,29]. The PTEN/PI3K/Akt pathway is associated with silibinin-induced inhibition of growth in human hepatic cellular carcinoma (HCC) cells[25]. Many cellular events are associated with silibinin-induced apoptosis. Silibinin could cause the loss of mitochondrial membrane potential, resulting in an increased release of cytochrome c or Bax from mitochondria, as well as the decrease in expression of Mcl-1 protein, indicating that silibinin-induced apoptosis is mediated through caspase-dependent and/or caspase-independent mechanisms[30]. Additionally, our previous reports show that the silibinin-induced increase in PTEN expression and the resulting decrease in p-Akt expression are associated with decreased survival rate and enhanced apoptosis in FaDu oral cancer cells[29] and c33-A cervical cancer cells[27]. In this study, we found that silibinin alone or in combination with metformin increased PTEN expression, resulting in decreased p-Akt in COLO 205 cells, consistent with our previous reports.
Suppression of growth in human colorectal carcinoma (SW480) cells by silibinin at a concentration of 200 μmol/L has been reported[31], indicating that silibinin exerts anticancer effects at a dose of 200 μmol/L. In this study, we are the first to report that co-treatment with silibinin (100 μmol/L) and metformin (10 mmol/L) more effectively induced apoptosis than treatment with silibinin 100 μmol/L alone. Moreover, this combination treatment did not affect normal HCoEpiC. Thus, there was considerable specificity in the inhibition of cancer cells using this combination therapy.
Metformin has widely been used to treat patients with type 2 diabetes[23]. Interestingly, the concept that this compound may be a promising anti-cancer agent was first developed in the early 1970s[32]. Later on, two population-based studies provided preliminary evidence that metformin may reduce cancer risk and improve prognosis in type 2 diabetic patients[33]. Metformin inhibits cancer cells by activation of AMPK and S6 kinase and suppression of mTOR[34]. Moreover, significant down-regulation of the anti-apoptotic proteins Bcl-2 and Bcl-xL and up-regulation of the pro-apoptotic protein Bax were observed in malignant cells following metformin treatment[35]. In this study, we found that metformin alone or in combination with silibinin increased the expression of p-AMPK and inhibited the phosphorylation of mTOR, consistent with previous reports[36].
Cell apoptosis is activated in response to both intrinsic and extrinsic pathways. Drug therapies that induce apoptosis cause DNA damage-induced, p53-regulated release of cytochrome c from mitochondria. Cytochrome c then binds to apoptosis-activating factor-1 (Apaf-1), resulting in activation of caspase 9, which is followed by the activation of effector caspases including caspase 3. Following ligation, death receptors signal cell death by inducing a death-inducing signaling complex composed of the cytoplasmic adapter protein FADD (Fas-associated death domain) and caspase 8. Activated caspase 8 can activate caspase 3 both directly and indirectly by truncation of Bid[37]. Metformin[38] and silibinin[39] have been shown to induce cancer cell apoptosis through both the intrinsic and extrinsic pathways. In this study, we observed that the combination of silibinin and metformin increased caspase 3 activation or AIF expression.
Metformin at 20 mmol/L has previously been shown to be effective against breast cancer[40], melanoma[21] and gastric cancer[17]. The concentration of metformin administered to type 2 diabetic patients is approximately 30-60 μmol/L[41]. Thus, the doses of metformin that were shown to be effective against cancer cells are approximately 300-600 fold (approximately 20 mmol/L) greater than the dose routinely administered for diabetic disorders. In this study, we applied a low dose of metformin (10 mmol/L) in combination with silibinin (100 μmol/L) in order to more effectively induce apoptosis than the administration of 10 mmol/L metformin alone. Furthermore, this combination treatment did not affect normal HCoEpiC.
Similarly elevated levels of PTEN expression were observed in both the cells co-treated with metformin and silibinin and those incubated with silibinin (100 μmol/L) alone. Additionally, the increase in AMPK and decrease in mTOR phosphorylation were also the same between combination treatment and metformin (10 mmol/L) alone. In this study, we provide evidence demonstrating that combined treatment with low doses of silibinin and metformin can produce anti-cancer effects on COLO 205 cells similar to the effects of higher doses of each drug. There were no toxic effects observed on HCoEpiC with this co-treatment. We also observed a synergistic effect at the molecular level mainly for the phosphorylation of AKT. The interaction of AMPK and the PTEN/Akt pathway has yet to be elucidated. This could be a new target for our studies in the future.
Recent evidence suggests that the combination of treatment with dietary supplements and other compounds may help to alleviate toxic side effects, enhance quality of life and prolong the lifespan of patients[42]. In the present study, we are the first to examine the effects of co-treatment with low doses of metformin and silibinin on cancer cells. This combination treatment effectively induced apoptosis of COLO 205 cells, but did not affect normal HCoEpiC. Thus, this combinatory therapy may be helpful for the treatment of colorectal cancer due to not only its effectiveness, but also the reduction in toxic side effects with this treatment.
In conclusion, in the present study, the combination treatment of COLO 205 cells with silibinin and metformin synergistically enhanced the inhibition of cell survival through increased PTEN expression and AMPK phosphorylation, resulting in the induction of caspase 3 and AIF. These results suggest a novel therapeutic strategy for colorectal cancer that induces few toxic side effects on COLO 205 cells.
We thank Yan YL and Liao PR for their assistance with research.
Silibinin is the major active compound in milk thistle and is known to display high efficacy against cancer cells through increasing the expression of phosphatase and tensin homolog (PTEN) and for hepatic protection. Metformin, a well-known antidiabetic agent, has recently been reported to inhibit cancer by increasing AMP-activated protein kinase (AMPK) expression. Recent evidence suggests that combined treatment with dietary supplements and other compounds may help to alleviate toxic side effects, enhance quality of life and prolong the lifespan of patients. However, the effect from combination of silibinin with metformin is still unknown.
Silibinin is known to display high efficacy against cancer cells in addition to hepatic protection. Metformin, as the well-known antidiabetic agent, has recently been mentioned to produce cancer inhibition. The current research hotspot is to demonstrate that the combined treatment with silibinin and metformin may synergistically enhance the inhibition of COLO 205 cell survival via increased PTEN expression and AMPK phosphorylation, resulting in the induction of caspase 3 and AIF.
Metformin at 20 mmol/L has previously been found to be effective against breast cancer, melanoma and gastric cancer. In fact, the doses of metformin that were effective against cancer cells appear to be approximately 300-600 fold (about 20 mmol/L) greater than that which is administered for diabetic disorders. In this study, authors applied a low dose of metformin (10 mmol/L) in combination with silibinin (100 μmol/L) to produce the same result of apoptosis in colon cancer cells.
In the present study, combined treatment with silibinin and metformin synergistically enhanced the inhibition of COLO 205 cell survival via increased PTEN expression and AMPK phosphorylation, resulting in the induction of apoptosis. The obtained findings suggest a novel therapeutic strategy for colorectal cancer with less side toxic effects.
Colorectal cancer is one of the leading causes of global cancer associated deaths. The high mortality of patients with colorectal cancer is mainly attributed to the metastasis. Combined treatment with silibinin and metformin may induce apoptosis of human colorectal cancer cells at a dose that does not affect nonmalignant colon epithelial cells. This finding reveals a potential therapeutic strategy of colorectal cancer.
This paper assessed the efficacy of combined United Statesge of silibinin, which is a phytochemical, and metformin, which is an oral antidiabetic drug in the biguanide class. Authors successfully showed the synergistic effects of the two compounds regarding the apoptosis induction in COLO 205, a human colorectal cancer cell line. They also investigated the molecular basis for the synergism by studying activation of Akt/PTEN and AMPK. This paper is well written, showing a possibility towards a novel therapeutic regimen for the treatment of colorectal cancer.
P- Reviewer: Greenberger JS, Hsu LS, Kumiko S S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9568] [Article Influence: 869.8] [Reference Citation Analysis (0)] |
| 2. | Ottaiano A, Franco R, Aiello Talamanca A, Liguori G, Tatangelo F, Delrio P, Nasti G, Barletta E, Facchini G, Daniele B. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Naithani R, Huma LC, Moriarty RM, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Wu H, Dai Q, Shrubsole MJ, Ness RM, Schlundt D, Smalley WE, Chen H, Li M, Shyr Y, Zheng W. Fruit and vegetable intakes are associated with lower risk of colorectal adenomas. J Nutr. 2009;139:340-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, Agarwal R. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8:2366-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110-119. [PubMed] |
| 7. | Gazák R, Walterová D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem. 2007;14:315-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Hackett ES, Twedt DC, Gustafson DL. Milk thistle and its derivative compounds: a review of opportunities for treatment of liver disease. J Vet Intern Med. 2013;27:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Mohan S, Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin modulates UVB-induced apoptosis via mitochondrial proteins, caspases activation, and mitogen-activated protein kinase signaling in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2004;320:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063-3069. [PubMed] |
| 14. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3802] [Cited by in RCA: 4212] [Article Influence: 175.5] [Reference Citation Analysis (0)] |
| 15. | Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9:e87979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila). 2012;5:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 17. | Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Kovalenko IG, Poroshina TE, Semenchenko AV, Provinciali M. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 678] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 20. | Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483-491. [PubMed] |
| 21. | Janjetovic K, Harhaji-Trajkovic L, Misirkic-Marjanovic M, Vucicevic L, Stevanovic D, Zogovic N, Sumarac-Dumanovic M, Micic D, Trajkovic V. In vitro and in vivo anti-melanoma action of metformin. Eur J Pharmacol. 2011;668:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7:e33411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 445] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 23. | Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev. 2013;22:1877-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 25. | Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13:5299-5305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Lee JJ, Kong M, Ayers GD, Lotan R. Interaction index and different methods for determining drug interaction in combination therapy. J Biopharm Stat. 2007;17:461-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Yu HC, Chen LJ, Cheng KC, Li YX, Yeh CH, Cheng JT. Silymarin inhibits cervical cancer cell through an increase of phosphatase and tensin homolog. Phytother Res. 2012;26:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 684] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 29. | Su CH, Chen LJ, Liao JF, Cheng JT. Increase of phosphatase and tensin homolog by silymarin to inhibit human pharynx squamous cancer. J Med Food. 2013;16:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12:415-424. [PubMed] |
| 32. | Dilman VM. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageine, and disease. Lancet. 1971;1:1211-1219. [PubMed] |
| 33. | Berstein LM, Boyarkina MP, Teslenko SY. Familial diabetes is associated with reduced risk of cancer in diabetic patients: a possible role for metformin. Med Oncol. 2012;29:1308-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Yasmeen A, Beauchamp MC, Piura E, Segal E, Pollak M, Gotlieb WH. Induction of apoptosis by metformin in epithelial ovarian cancer: involvement of the Bcl-2 family proteins. Gynecol Oncol. 2011;121:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Ma J, Guo Y, Chen S, Zhong C, Xue Y, Zhang Y, Lai X, Wei Y, Yu S, Zhang J. Metformin enhances tamoxifen-mediated tumor growth inhibition in ER-positive breast carcinoma. BMC Cancer. 2014;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Kusmic C, L’abbate A, Sambuceti G, Drummond G, Barsanti C, Matteucci M, Cao J, Piccolomini F, Cheng J, Abraham NG. Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling. J Cell Biochem. 2010;109:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Oudejans JJ, Muris JJ, Meijer CJ. Inhibition of caspase 9 and not caspase 8 mediated apoptosis may determine clinical response to chemotherapy in primary nodal diffuse large B-cell lymphomas. Cell Cycle. 2005;4:526-528. [PubMed] |
| 38. | Wang LW, Li ZS, Zou DW, Jin ZD, Gao J, Xu GM. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol. 2008;14:7192-7198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 120] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Kauntz H, Bousserouel S, Gossé F, Raul F. Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis. 2011;16:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269-10273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 842] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 41. | Martin-Castillo B, Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Metformin and cancer: doses, mechanisms and the dandelion and hormetic phenomena. Cell Cycle. 2010;9:1057-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Yang CH, Craise LM. Development of human epithelial cell systems for radiation risk assessment. Adv Space Res. 1994;14:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |