Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.4082
Peer-review started: September 18, 2014
First decision: September 27, 2014
Revised: November 18, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: April 7, 2015
Processing time: 212 Days and 4 Hours
A 62-year-old Japanese man presented to our hospital with a history of weight loss of 6 kg in 4 mo. Imaging examinations revealed a tumor located on the third portion of the duodenum with stenosis. We suspected duodenal carcinoma and performed pancreas-preserving segmental duodenectomy. Adenocarcinoma arising from a heterotopic pancreas at the third portion of the duodenum was finally diagnosed by immunohistochemical staining. Malignant transformation in the duodenum arising from a heterotopic pancreas is extremely rare; to our knowledge, only 13 cases have been reported worldwide, including the present case. The most common location of malignancy is the proximal duodenum at the first and descending portion. Herein, we describe the first case of adenocarcinoma arising from a heterotopic pancreas, which was located in the third portion of the duodenum, with a review of the literature.
Core tip: Based on all abdominal surgeries, the incidence of heterotopic pancreas ranges from 0.25% to 1.2%. The frequency of malignant transformation ranges from 0.7% to 1.8% among all heterotopic pancreas cases. Malignant transformation arising from a heterotopic pancreas is extremely rare. The most common location of malignancy is the proximal duodenum at the first and descending portion. In this paper, we report the case of a patient with weight loss who was diagnosed as having an adenocarcinoma arising from a heterotopic pancreas, at the third portion of the duodenum, with a review of the literature.
- Citation: Fukino N, Oida T, Mimatsu K, Kuboi Y, Kida K. Adenocarcinoma arising from heterotopic pancreas at the third portion of the duodenum. World J Gastroenterol 2015; 21(13): 4082-4088
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/4082.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.4082
Heterotopic pancreas is defined as an unusual pancreatic tissue existing in other organs without any connection to the original pancreas. The most frequent locations are the duodenum (9%-36%), stomach (24%-38%), jejunum (0.5%-27%), and Meckel’s diverticulum (2%-6.5%)[1]. Heterotopic pancreas has been classified into four types by Heinrich[2,3]. The incidence of heterotopic pancreas ranges from 0.25% to 1.2% among all abdominal surgeries[4]. Malignant transformation arising from a heterotopic pancreas is extremely rare, and has been reported in several cases in the literature. The frequency of malignant transformation ranges from 0.7% to 1.8% among all cases of heterotopic pancreas[5,6]. Malignant tumors arising from a heterotopic pancreas, as well as heterotopic pancreas, commonly appear pathologically as a smooth nodule, and occasionally, as a mass with an irregular surface. These tumors are usually located in the submucosa and only occasionally expand into the muscularis[1]. Since only a small number of cases have been reported, the prognosis of patients with malignant tumors arising from a heterotopic pancreas is not clear. Herein, we describe the first case of adenocarcinoma arising from a heterotopic pancreas, which was located in the third portion of the duodenum.
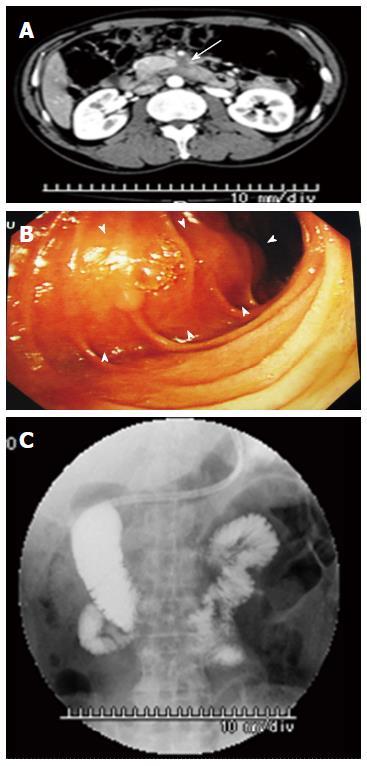
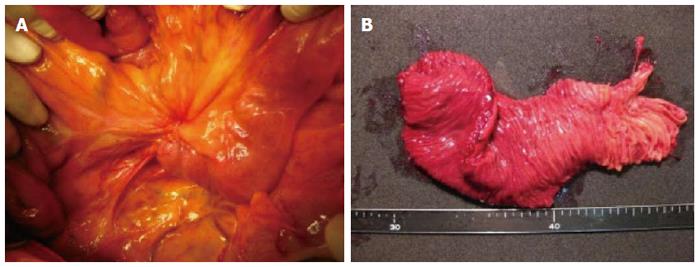
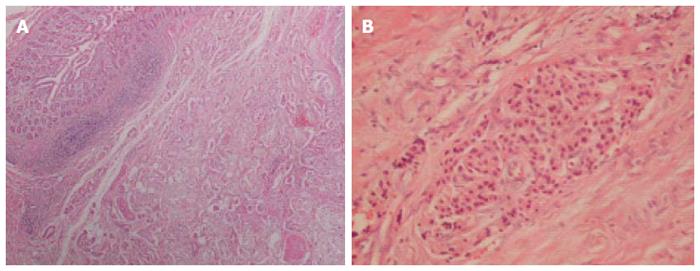
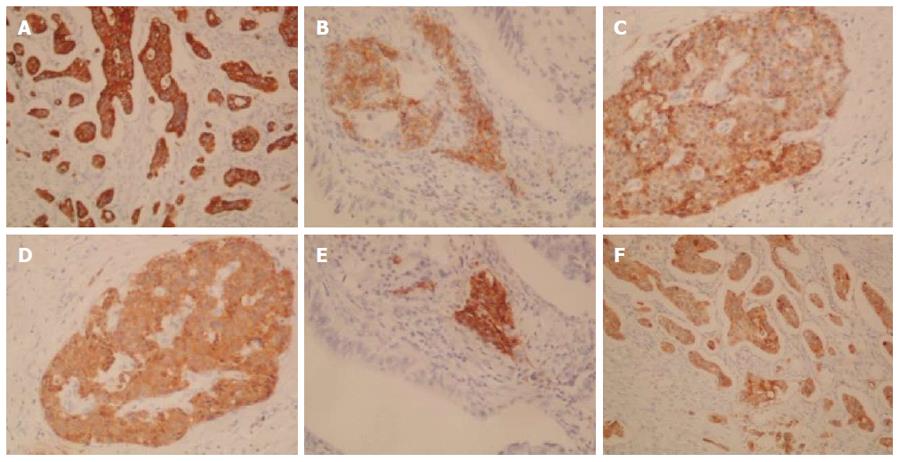
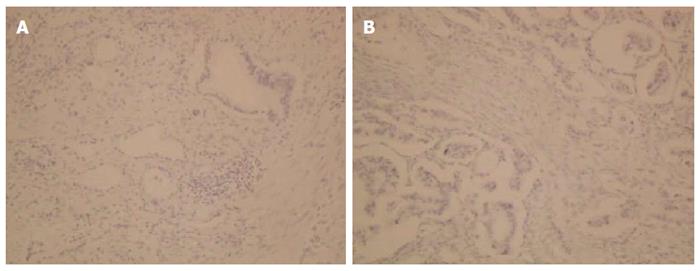
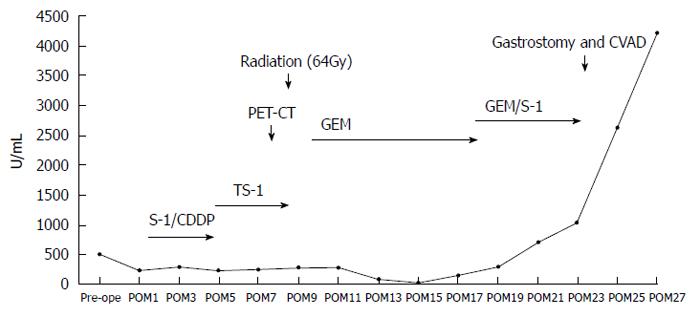
A 62-year-old Japanese man presented to our hospital with a history of weight loss of 6 kg in 4 mo. There were no remarkable findings on the patient’s medical history. The patient’s physical examination and vital signs were normal. He was admitted to our hospital for examination because of elevated serum levels of the following tumor markers (the normal range for each parameter is in parentheses): carbohydrate antigen 19-9, 500 U/mL (0-37 U/mL); DUPAN-2, 226 U/mL (less than 150 U/mL); and SPan-1, 41 U/mL (less than 30 U/mL). The level of carcinoembryonic antigen was within the normal range. The results of the complete blood count test, coagulation test, and laboratory analysis were normal. Contrast-enhanced computed tomography of the abdomen showed a poorly enhanced mass around the middle section of the third portion of the duodenum and superior mesenteric artery (Figure 1A). Duodenofiberoscopic findings (Figure 1B) and hypotonic duodenography (Figure 1C) revealed a submucosal tumor with a smooth surface and obstruction at the third portion of the duodenum. Biopsies of the submucosal tumor were taken and revealed no evidence of malignancy. We suspected a diagnosis of duodenal carcinoma from the examination results and performed open resection with intraoperative frozen section analysis. Intraoperative examination revealed that the stomach and pancreas were normal. The umbilication was located in front of the ligament of Treitz and was completely separable from the superior mesenteric artery (Figure 2A). The intraoperative frozen section analysis revealed adenocarcinoma of the duodenum. We diagnosed primary duodenal carcinoma and performed pancreas-preserving segmental duodenectomy from the third portion to the fourth portion of the duodenum. The intestinal reconstruction was carried out by side-to-side reconstruction using the GIA™ Stapling System (Covidien, Tokyo, Japan). Macroscopic findings were normal at the surface of duodenum (Figure 2B). Microscopically, the tumor was 15 mm in diameter and extended into the submucosa of the third portion of the duodenum. The major part of the tumor was moderately differentiated adenocarcinoma (Figure 3A). Moreover, only islet cells were present within and near the tumor (Figure 3B). In immunohistochemical staining, cytokeratin (CK) 7 (Figure 4A), neural cell adhesion molecule (CD56) (Figure 4B), chromogranin A (Figure 4C), synaptophysin (Figure 4D), insulin (Figure 4E), and mucin 1 (MUC1) (Figure 4F) were positive. On the other hand, CK20 (Figure 5A) and MUC2 (Figure 5B) were significantly negative. From the above results, we finally diagnosed adenocarcinoma arising from a heterotopic pancreas at the third portion of the duodenum. The postoperative course was uneventful, and our patient was discharged from our hospital 25 d after surgery. The patient was referred to the oncology department for adjuvant chemotherapy. We monitored the serum level of CA19-9 (Figure 6). On postoperative month 1, our patient underwent 3 courses of oral tegafur-gimeracil-dihydropyrimidine dehydrogenase (S-1) plus cisplatin chemotherapy and S-1 monotherapy, respectively. Unfortunately, serum levels of CA19-9 did not decrease. We diagnosed local recurrence of heterotopic pancreas carcinoma by positron emission and computed tomography (PET-CT) at 8 mo after surgery. After radiation therapy at another hospital (total 54 Gy), gemcitabine (GEM) monotherapy, 1000 mg/m2, was administered intravenously for 30 min on days 1, 8, and 15 of each 28-d cycle. The serum level of CA19-9 decreased gradually from 271 U/mL to 76 U/mL by GEM monotherapy. We changed the treatment from GEM monotherapy to GEM plus S-1 after administrating 10 courses of GEM monotherapy, because the serum level of CA19-9 increased again. At 24 mo after surgery, a gastrostomy was performed and a central venous access device was implanted for administrating total parenteral nutrition because the patient suffered from intestinal obstruction. We decided to terminate all chemotherapy and provide best supportive care. Our patient died 33 mo after the primary operation.
Heterotopic, ectopic, or aberrant pancreas, which is commonly an incidental finding at surgery or autopsy, is defined as a pancreatic tissue existing in other organs without any connection to the original pancreas and has been frequently reported in the gastrointestinal tract, especially in the stomach. The first report of heterotopic pancreas was identified by Shultz in 1727. In 1859, the histological confirmation was reported by Klob[7-9]. The frequency of heterotopic pancreas tissue has been reported to be 0.55%-13.7%, 0.25%, and approximately 1.2% in autopsy material, abdominal surgery, and gastrectomy operation, respectively[7]. In Japan, Tanaka et al[4] reported that the incidence of heterotopic pancreas was 0.25% among 6035 patients who underwent laparotomy. Clinical symptoms caused by heterotopic pancreas are not specific. Symptoms such as abdominal pain, vomiting, bleeding, and jaundice appear in conjunction with tumor growth. In the present case, the patient presented weight loss of 6 kg in 4 mo.
Heterotopic pancreas has been classified into 4 types by Heinrich in 1909[2,3]. Type I is characterized by the presence of typical pancreatic tissues with acini, ducts, and islet cells similar to those seen in a normal pancreas. Type II is characterized by the presence of pancreatic ducts and acini, while islet cells are not present. Type III is characterized by ducts with a few acini or dilated ducts only, so called adenomyoma. Type IV characterized by the presence of islet cells only. Based on Heinrich’s classification, most heterotopic pancreas cases are Type II. Although the reason is unknown, to date, Type IV heterotopic pancreas cases have not been reported. The present case was classified as Type IV according to Heinrich’s classification, since pathological findings revealed only islet cells within and near the tumor.
Guillou et al[6] indicated that the incidence of malignancy due to heterotopic pancreas was 0.7% and extremely rare. They studied the frequency of malignant transformations among 146 cases of heterotopic pancreas, including surgical and autopsy specimens, between 1975 and 1991. In 1999, Makhlouf et al[5] reported that among 109 patients diagnosed as having pancreatic heteropia in the gastrointestinal tract, the incidence of malignant transformation arising from a heterotopic pancreas was 1.8%. According to their study, there was a slight predominance of women over men; the mean age was 47-49 years. Most commonly, the tumor was located in the upper gastrointestinal tract, from the stomach to the jejunum. The most common symptoms were digestive symptoms such as nausea, vomiting, and jaundice caused by obstruction. There were no specific symptoms. The mean tumor size was 3.5-3.7 cm. Most of these tumors were ductal adenocarcinoma, similar to those seen in the pancreas[5,6]. Jaervi and Lauren[10] have proposed 3 criteria for the diagnosis of carcinoma arising from a heterotopic pancreas: (1) the tumor must be found within or close to the ectopic pancreatic tissue; (2) direct transition must be observed between pancreatic structures and the carcinoma (malignant transformation of an ectopic pancreas must be differentiated from a metastatic deposit or a neoplastic invasion from a neighboring digestive cancer, especially from the stomach, the biliary tract, and the eutopic pancreas); and (3) the non-neoplastic pancreatic tissue must comprise at least fully developed acini and ductal structures. According to those criteria, Guillou et al[6] suggested that it was impossible to ascertain the pancreatic origin of the tumor in the absence of acini and/or islets of Langerhans, since ductal structures might have a local origin by means of a metaplastic phenomenon. Therefore, Heinrich’s type III, so called adenomyoma, should not be classified as an aberrant pancreas. Carcinomas arising from a heterotopic pancreas have been classified by most authors according to the 3 criteria and the opinion of Guillou et al[6]. However, as mentioned above, Heinrich classified the histologic pattern of heterotopic pancreas into 4 types. In the present case, the tumor extended into the submucosa microscopically. The major part of the tumor changed into moderately differentiated adenocarcinoma. Moreover, only the islet cells were present within and near the adenocarcinoma. For all these reasons, we diagnosed the patient as having type IV heterotopic pancreas according to Heinrich’s classification.
To our knowledge, 36 cases of malignant transformation arising from a heterotopic pancreas, including the present case, have been reported in PubMed (key words: heterotopic (ectopic, aberrant) pancreas, carcinoma). The incidence according to location is as follows: duodenum, 36%; stomach, 36%; jejunum, 8%, and other (including the mediastinum, liver, esophagus, hiatal hernia, periaorta, and rectum), 19%. We summarized 13 cases of malignant transformation arising from a heterotopic pancreas, in the duodenum (Table 1)[4,9,11-18]. The mean age was 70.9 years (range: 56-86 years). There was no sex predilection. Most commonly, the tumor was located from the bulb to the descending portion in the duodenum; only in the present case, the tumor was located in the third portion of the duodenum. The mean tumor size was 25 mm (range: 12-50 mm). Approximately 80% and 50% of the tumors were pathologically diagnosed as adenocarcinomas (tubular adenocarcinoma, poorly differentiated adenocarcinoma, papillary adenocarcinoma, and mucinous adenocarcinoma) and classified as Heinrich’s type I. The mean tumor size of the malignant transformation is unknown; however, most tumors were bigger than the previously reported mean tumor size of heterotopic pancreas (mean 18 mm; range: 4-5 mm)[4]. In our study, the mean size varied from 12 to 50 mm in maximum dimension with a mean of 25 mm.
| Case | Year | Author | Age(yr) | Sex | Clinial symptoms | Location | Preoperative diagnosis | Diagnostic approach | Size (mm) | Pathology | Heinrich | Outcome |
| 1 | 1993 | Tanaka | 72 | M | Abdominal pain | D (ND) | ND | Operation | 12 | Cancer | ND | ND |
| 2 | 1996 | Inoue | 81 | F | Anorexia | D (ND) | Duodenal cancer | Biopsy | 25 | pap + muc | I | ND |
| 3 | 2006 | Inoue | 75 | M | Melena | D (ND) | No evidence of malignancy | Operation | 20 | tub | III | ND |
| 4 | 2007 | Tison | 72 | M | Abdominal pain, jaundice | D (2nd; Vater) | Tumor of the ampulla of Vater | Biopsy | ND | Adenocarcinoma, CDHP | ND | Death (16 mo) |
| 5 | 2007 | Kawakami | 68 | F | Jaundice | D (2nd; Vater) | Carcinoma of the ampulla of Vater | Biopsy | 12 | Acinar cell carcinoma | ND | Not death (19 mo) |
| 6 | 2008 | Rosok | 59 | F | No symptom | D (proximal) | No evidence of malignancy | Operation | 50 | IPMC | ND | Not death (36 mo) |
| 7 | 2010 | Inoue | 75 | M | Nausea, vomiting | D (2nd) | Cancer, GIST, MTL | Operation | ND | tub (well) | III | Not death (72 mo) |
| 8 | 2010 | Bini | 56 | M | Vomiting | D (1st) | Adenocarcinoma of uncertain origin | Biopsy | ND | Adenocarcinoma | I | ND |
| 9 | 2011 | Stock | 79 | F | Abdominal fullness, | D (4th) | No evidence of malignancy | Operation | 30 | Adenocarcinoma | I | ND |
| intermittent abdominal pain | ||||||||||||
| 10 | 2012 | Kinoshita | 62 | F | Vomiting, epigastralgia | D (1st) | Gastric cancer | Operation | 34 | tub (mode) | I | Not death (12 mo) |
| 11 | 2013 | Ginori | 86 | F | Abdominal pain, nausea, | D (1st) | Acute cholecystitis | Operation | 30 | well + muc + por | I | ND |
| vomiting | ||||||||||||
| 12 | 2014 | Endo | 75 | M | Epigastric pain, tarry stool | D (2nd) | Adenocarcinoma of uncertain origin | EUS-FNA | 22 | por | I | Not death (60 mo) |
| 13 | Present case | 62 | M | Weihgt loss | D (3rd) | Suspected duodenal cancer | Operation | 15 | tub (mode) | IV | Death (33 mo) | |
Most of these tumors were pathologically diagnosed as ductal adenocarcinomas. In addition, there are noninvasive carcinomas within intraductal papillary mucinous neoplasia and acinar cell carcinoma. It is impossible to diagnose heterotopic pancreas and malignant transformation preoperatively and precisely, because these tumors are located within the submucosal and subserosal layers. Endo et al[11] diagnosed ectopic pancreas adenocarcinoma preoperatively by endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) in 2 cases. They suggest that EUS-FNA is particularly helpful for the preoperative diagnosis. However, cytological examinations are inconclusive in about 50% of cases[16]. In our study, the accurate preoperative diagnosis rate by EUS-FNA and biopsy was about 40%. We diagnosed adenocarcinoma of the duodenum by pathological diagnosis during surgery, and malignant transformation arising from a heterotopic pancreas of the duodenum by the immunohistochemical staining, similar to previous articles.
The prognosis of patients with adenocarcinoma arising from an ectopic pancreas seems to be somewhat better than that of patients with tumors arising from the pancreas itself, probably because of earlier presentation[19,20]. To our knowledge, long-term survival was reported in a case of invasive ductal carcinoma arising from an ectopic pancreas within the gastric wall, with no recurrence for 11 years[20]. In the present case, our patient died 33 mo after surgery because of local recurrence of the adenocarcinoma.
There is no evidence of the efficacy of chemotherapy for adenocarcinoma arising from a heterotopic pancreas. In the present case, it took time to reach a definite diagnosis. First, we diagnosed primary adenocarcinoma of the duodenum only when the immunohistochemical staining results were available. We selected a regimen of S-1 plus cisplatin as first-line therapy for duodenal cancer, based on the guidelines for the treatment of gastric cancer in Japan. Our case was referred for 3 courses of adjuvant chemotherapy with S-1 plus cisplatin and S-1 monotherapy, respectively. Unfortunately, the serum level of CA19-9 remained high. On postoperative month 8, PET-CT revealed local recurrence. After radiation in another hospital (total 54 Gy), the patient was administered GEM. The serum level of CA19-9 decreased gradually to 76 U/mL. Our patient eventually developed tolerance to chemotherapy and died 33 mo after the primary operation. To the best of our knowledge, there is no literature in PubMed on the efficacy of chemotherapy (including adjuvant and systemic chemotherapy) for adenocarcinoma arising from a heterotopic pancreas. However, our experience suggests that GEM is effective for these tumors, as in cases of primary pancreatic cancer. The study of further cases of adenocarcinoma due to a heterotopic pancreas, including the efficacy of chemotherapy treatment, is necessary.
In conclusion, we herein present the first case of malignant transformation arising from a heterotopic pancreas at the third portion of the duodenum.
A 62-year-old Japanese man with a weight loss of 6 kg in 4 mo presented to our hospital.
The physical examination was normal.
Malignant tumors (primary duodenal adenocarcinoma and metastatic tumors of uncertain origin) and benign neoplasms (submucosal tumors, heterotopic pancreas, and adenoma).
The patient had elevated serum levels of carbohydrate antigen 19-9 (500 U/mL) DUPAN-2 (226 U/mL), and SPan-1 (41 U/mL), while there were no remarkable findings on other laboratory analyses.
Abdominal enhanced computed tomography, duodenofiberoscopy, and hypotonic duodenography revealed a mass and submucosal tumor located in the third portion of the duodenum.
Histological examination revealed moderately differentiated adenocarcinoma with islet cells only; therefore, the immunohistochemical staining revealed positive cytokeratin 7, neural cell adhesion molecule (CD56), chromogranin A, synaptophysin, insulin, and mucin 1.
The patient underwent pancreas-preserving segmental duodenectomy; therefore, chemotherapy and radiation was administered after surgery.
A total of 13 cases of malignant transformation arising from a heterotopic pancreas in the duodenum have been reported in the literature. This is the first report of adenocarcinoma arising from a heterotopic pancreas at the third portion of the duodenum, worldwide, and the chemotherapeutic method is controversial.
Heterotopic, ectopic, or aberrant pancreas, which is commonly an incidental finding at surgery or autopsy, is defined as pancreatic tissue existing in other organs without any connection to the original pancreas and has been frequently reported in the gastrointestinal tract, especially in the stomach.
Commonly, the tumor, which is an adenocarcinoma arising from a heterotopic pancreas, is located from the bulb to the descending portion in the duodenum. This article presents the first case of a patient with an adenocarcinoma arising from a heterotopic pancreas, located at the third portion of the duodenum.
This is a well written and interesting study that addresses an unusual case in pancreatic adenocarcinoma.
P- Reviewer: Cosen-Binker L, Liu XF, Olah A, Sakata N S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Thoeni RF, Gedgaudas RK. Ectopic pancreas: usual and unusual features. Gastrointest Radiol. 1980;5:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Von Heinrich H. Ein Beitrag zur Histologie des sogen. Akzessorischen Pankreas. Virchows Arch A Pathol Anat Histopathol. 1909;198:392-401. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 84] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Gaspar Fuentes A, Campos Tarrech JM, Fernández Burgui JL, Castells Tejón E, Ruíz Rossello J, Gómez Pérez J, Armengol Miró J. [Pancreatic ectopias]. Rev Esp Enferm Apar Dig. 1973;39:255-268. [PubMed] |
| 4. | Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32-35. [PubMed] |
| 5. | Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med. 1999;123:707-711. [PubMed] |
| 6. | Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568-571. [PubMed] |
| 7. | Dolan RV, ReMine WH, Dockerty MB. The fate of heterotopic pancreatic tissue. A study of 212 cases. Arch Surg. 1974;109:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 213] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Papaziogas B, Koutelidakis I, Tsiaousis P, Panagiotopoulou K, Paraskevas G, Argiriadou H, Atmatzidis S, Atmatzidis K. Carcinoma developing in ectopic pancreatic tissue in the stomach: a case report. Cases J. 2008;1:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Stock C, Keutgen XM, Pisapia D, Crawford C, Zarnegar R. Heterotopic pancreatic neoplasm presenting as an obstructing mass at the fourth portion of the duodenum. JOP. 2011;12:241-243. [PubMed] |
| 10. | Jaervi O, Lauren P. Gastric glandular tumors provided with excretory ducts, and criticism of the theory of the tumors arising in heterotopic pancreas; observations on the occurrence of atypical glands in the stomach. Acta Pathol Microbiol Scand. 1964;62:1-23. [PubMed] |
| 11. | Endo S, Saito R, Ochi D, Yamada T, Hirose M, Hiroshima Y, Yamamoto Y, Ueno T, Hasegawa N, Moriwaki T. Effectiveness of an endoscopic biopsy procedure using EUS-FNA and EMR-C for diagnosing adenocarcinoma arising from ectopic pancreas: two case reports and a literature review. Intern Med. 2014;53:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Tison C, Regenet N, Meurette G, Mirallié E, Cassagnau E, Frampas E, Le Borgne J. Cystic dystrophy of the duodenal wall developing in heterotopic pancreas: report of 9 cases. Pancreas. 2007;34:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kawakami H, Kuwatani M, Onodera M, Hirano S, Kondo S, Nakanishi Y, Itoh T, Asaka M. Primary acinar cell carcinoma of the ampulla of Vater. J Gastroenterol. 2007;42:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Røsok BI, Rosseland AR, Grzyb K, Mathisen O, Edwin B. Laparoscopic resection of an intraductal papillary mucinous carcinoma in ectopic pancreatic tissue. J Laparoendosc Adv Surg Tech A. 2008;18:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Inoue Y, Hayashi M, Arisaka Y, Higuchi K, Egashira Y, Tanigawa N. Adenocarcinoma arising in a heterotopic pancreas (Heinrich type III): a case report. J Med Case Rep. 2010;4:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bini R, Voghera P, Tapparo A, Nunziata R, Demarchi A, Capocefalo M, Leli R. Malignant transformation of ectopic pancreatic cells in the duodenal wall. World J Gastroenterol. 2010;16:1293-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kinoshita H, Yamaguchi S, Shimizu A, Sakata Y, Arii K, Mori K, Nasu T. Adenocarcinoma arising from heterotopic pancreas in the duodenum. Int Surg. 2012;97:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Ginori A, Vassallo L, Butorano MA, Bettarini F, Di Mare G, Marrelli D. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56-58. [PubMed] |
| 19. | Eisenberger CF, Gocht A, Knoefel WT, Busch CB, Peiper M, Kutup A, Yekebas EF, Hosch SB, Lambrecht W, Izbicki JR. Heterotopic pancreas--clinical presentation and pathology with review of the literature. Hepatogastroenterology. 2004;51:854-858. [PubMed] |
| 20. | Okamoto H, Kawaoi A, Ogawara T, Fujii H. Invasive ductal carcinoma arising from an ectopic pancreas in the gastric wall: a long-term survival case. Case Rep Oncol. 2012;5:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |