Published online Apr 7, 2015. doi: 10.3748/wjg.v21.i13.4038
Peer-review started: September 15, 2014
First decision: October 14, 2014
Revised: October 17, 2014
Accepted: November 7, 2014
Article in press: November 11, 2014
Published online: April 7, 2015
Processing time: 205 Days and 1.3 Hours
AIM: To evaluate the association between alcoholic liver disease (ALD) and bone fractures or osteoporosis.
METHODS: Non-randomized studies were identified from databases (PubMed, EMBASE, and the Cochrane Library). The search was conducted using Boolean operators and keywords, which included “alcoholic liver diseases”, “osteoporosis”, or “bone fractures”. The prevalence of any fractures or osteoporosis, and bone mineral density (BMD) were extracted and analyzed using risk ratios and standardized mean difference (SMD). A random effects model was applied.
RESULTS: In total, 15 studies were identified and analyzed. Overall, ALD demonstrated a RR of 1.944 (95%CI: 1.354-2.791) for the development of bone fractures. However, ALD showed a RR of 0.849 (95%CI: 0.523-1.380) for the development of osteoporosis. BMD was not significantly different between the ALD and control groups, although there was a trend toward lower BMD in patients with ALD (SMD in femur-BMD: -0.172, 95%CI: -0.453-0.110; SMD in spine-BMD: -0.169, 95%CI: -0.476-0.138). Sensitivity analyses showed consistent results.
CONCLUSION: Current publications indicate significant associations between bone fractures and ALD, independent of BMD or the presence of osteoporosis.
Core tip: Excessive alcohol consumption is a well-established risk factor for osteoporosis and bone fractures. However, light amounts of alcohol ingestion is known to be associated with higher bone mineral density (BMD) and low fracture rates. This study evaluated the current evidence regarding osteoporosis and bone fractures in alcoholic liver disease (ALD). In this meta-analysis, there was significant associations between bone fractures and ALD, independent of BMD or the presence of osteoporosis. Although the mechanism of bone fractures in ALD is not totally understood, further research utilizing a homogenous population and controlling for confounding risk factors for fractures could elucidate the mechanism.
- Citation: Bang CS, Shin IS, Lee SW, Kim JB, Baik GH, Suk KT, Yoon JH, Kim YS, Kim DJ. Osteoporosis and bone fractures in alcoholic liver disease: A meta-analysis. World J Gastroenterol 2015; 21(13): 4038-4047
- URL: https://www.wjgnet.com/1007-9327/full/v21/i13/4038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i13.4038
Chronic excessive alcohol consumption is a well-established risk factor for low bone density and bone fractures[1]. This is included in the fracture risk assessment tool (FRAX), which estimates the 10-year probability of bone fractures combined with other clinical risk factors and the bone mineral density (BMD) of the femoral neck[2,3]. It is assumed that the decreases in bone mass and strength resulting from heavy alcohol use are due to an imbalance between bone formation and resorption[4]. However, ingestion of light or moderate amounts of alcohol is known to be associated with higher BMD and decreased fracture rates[5-15], although conflicting results exist because of inconsistent standards of classification of light, moderate, or heavy alcohol consumption[13,16].
Osteoporosis and bone fractures are frequently overlooked complications in patients with chronic liver disease that could result in serious outcomes[17]. However, the exact prevalence or mechanism of osteodystrophy in patients with alcoholic liver disease (ALD), the deleterious outcome of chronic alcohol abuse, have not been described. The aim of this study was to evaluate the association between ALD and bone fractures or osteoporosis.
MEDLINE (through PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library were searched using common keywords related to ALD, osteoporosis, and bone fractures (from inception to April 2014). Medical Subject Headings (MeSH) terminology was used because all 3 databases permit searching using MeSH terminology. The keywords used included “alcoholic liver diseases”, “osteoporosis”, or “bone fractures” using Boolean operators. Only publications on human subjects were searched, and the bibliographies of relevant articles were also reviewed to identify additional studies. The language of publication was not restricted.
Due to a lack of randomized-controlled studies relevant to this topic, we included case-control or cohort studies meeting all of the following criteria: (1) designed to evaluate ALD in the target or control group; and (2) included at least one outcome (prevalence of any bone fractures, prevalence of osteoporosis, or BMD) that enabled comparisons of osteodystrophy between patients with ALD and the control group. The exclusion criteria were as follows: (1) incomplete data; (2) review article; or (3) abstract only (study not published as full-text article).
Two of the authors (C.S.B. and G.H.B.) independently evaluated the eligibility of all studies retrieved from the databases based on the predetermined selection criteria. The abstracts of all identified studies were reviewed to exclude irrelevant articles. Full-text reviews were performed to determine whether the inclusion criteria were satisfied by the remaining studies. Disagreements between the two evaluators were resolved by discussion or by consultation with a third author (D.J.K.).
The methodological quality of the enrolled studies was assessed using the Newcastle-Ottawa Scale[18,19]. This tool is categorized into three parameters: the selection of the study population, the comparability of the groups, and the ascertainment of the exposure or outcome. Each parameter consists of subcategorized questions: selection (n = 4), comparability (n = 1), and exposure or outcome (n = 3). Stars awarded for each item serve as a quick visual assessment for the methodological quality of the studies. A study can be awarded a maximum of nine stars, indicating the highest quality[18,19]. The included studies were classified as high-quality (≥ 7 stars) or low-quality (< 7 stars). Sensitivity analyses were performed according to the criteria described above.
We investigated the relationship between ALD and bone fractures or osteoporosis using adjusted risk ratios (RRs) and standardized mean difference (SMD). The prevalence of any fractures or osteoporosis assessed by radiologic examinations and BMD assessed by dual-energy X-ray absorptiometry (DXA) or dual-photon absorptiometry (DPA) were extracted and analyzed. Osteoporosis was defined as a value for BMD that was 2.5 standard deviations or more below the reference range[20]. We also performed sensitivity analyses based on methodological quality (high or low), the area measured to determine BMD (femoral neck or spine), the type of control group (normal, healthy control or chronic liver diseases other than ALD), and the effect size, excluding outliers whenever possible. Both a cumulative analysis and a one-study-removed analysis were also performed.
For the present evaluation, the independent study was the primary unit of analysis. Thus, for the studies that had multiple control groups (reported multiple outcomes)[21-25] or multiple BMD measured at the femoral neck or spine simultaneously[23,26], an approach with a shifting unit of analysis was used for the determination of an independent estimate of the effect[27]. In these studies, the biggest effect sizes of the control groups or the BMD were used.
Comprehensive meta-analysis software (version 2.2.064, Borenstein M, Hedges L, Higgins J and Rothstein H. Englewood, NJ: Biostat; United States) was used for this meta-analysis. We calculated the RRs with 95%CIs using 2 × 2 tables from the original articles whenever possible to reveal the relationship between ALD and bone fractures or osteoporosis. To compare the BMD directly between patients with ALD and the control groups, the SMD was also used as another effect size calculation. SMD is the difference in mean value between groups divided by the SD among the populations. Therefore, even if the actual scales for BMD are different across the articles, individual data can be combined. SMD was calculated as follows: SMD = (M1 - M2) / pooled SD, where M1 is the mean BMD in the ALD group and M2 is the mean BMD in the control group[28]. A negative value of SMD means the ALD group has a lower BMD than the control group. Heterogeneity was tested using the I2 test, which measures the percentage of total variation across studies[29]. I2 was calculated as follows: I2 (%) = 100 × (Q-df)/Q, where Q is Cochrane’s heterogeneity statistic and df means the degree of freedom. Negative values for I2 were set to zero, and an I2 value over 50% was considered to be of substantial heterogeneity (range of 0-100%)[30]. Pooled RRs with 95%CIs were calculated using a random effects model and the method of DerSimonian and Laird because of methodological heterogeneity[31]. These results were confirmed again by the I2 test. A fixed effects model using the inverse variance-weighted (Woolf’s) method was used in the sensitivity analyses, including cumulative and one-study-removed analyses, based on the assumption of a common effect size shared by the subgroup studies[32,33]. Significance was set at P = 0.05 in both models. Publication bias was evaluated using Begg’s funnel plot, Egger’s test of the intercept, Duval and Tweedie’s trim and fill, and Begg and Mazumdar’s rank correlation test[34-38].
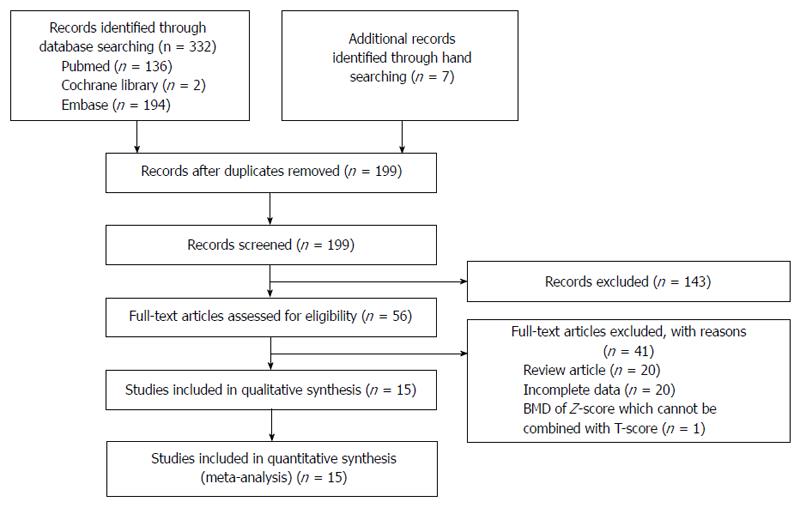
Figure 1 shows a flow diagram of how relevant studies were identified. A total of 339 articles was identified by a search of 3 core databases and a manual search of relevant bibliographies. In all, 140 duplicate studies and an additional 143 studies were excluded during the initial screening through a review of the titles and abstracts. The full texts of the remaining 56 studies were thoroughly reviewed. Among these studies, 41 were excluded from the final analysis. The reasons for study exclusion during the final review were as follows: review article (n = 20), incomplete data (n = 20), or use of Z-score for BMD, which cannot be combined with T-score (n = 1). The remaining 15 non-randomized studies were included in the final analysis.
Within the 15 studies, we identified a total of 726 participants (313 patients with ALD vs 413 controls) in the analysis of bone fractures, 470 participants (260 patients with ALD vs 210 controls) in the analysis of osteoporosis, and 769 participants (391 patients with ALD vs 378 controls) in the analysis of BMD. The clinical characteristics of the enrolled studies are shown in Table 1.
| Ref. | Population characteristics | LC in ALD | Bone fractures | Osteoporosis | ALD BMD | Control BMD | |||||||
| Fracture site | ALD fracture | Total ALD | Control fracture | Total control | ALD osteoporosis | Total ALD | Control osteoporosis | Total control | |||||
| Lindsell et al[44] | 149 CLD (72 ALD, 77 non-alcoholic CLD), 149 normal controls | 0 | Rib or clavicle | 18 | 72 | 10 | 149 (normal control) | ||||||
| Diamond et al[46] | 115 CLD (M: 72, F: 43), 113 Controls (M: 52, F: 61) | Unidentifiable | Peripheral bone | 12 | 40 | 12 | 75 (other CLD) | ||||||
| Bonkovsky et al[23] | 133 CLD (M: 70, F: 63, Mean age: 47 ± 1.1) | Unidentifiable | 5 (L), 4 (FN) | 32 | 15 (L), 5 (FN) | 48 (other LC) | |||||||
| Ninkovic et al[39] | 37 CLD (6 ALD, 31 other CLD) (M: 20, F: 17, Mean age: 51.3, range: 32-65) | Unidentifiable | Vertebra | 6 | 6 | 17 | 31 (other CLD) | ||||||
| Ninkovic et al[21] | 243 CLD (M: 128, F: 115, Mean age: 51.1 ± 10.9) | Predominantly LC | 46 | 55 (HCV) | T (L1-4): -0.94 ± 1.38, T (FN): -1.49 ± 1.15 | T (L1-4): -1.59 ± 1.6, T (FN): -1.66 ± 1.27 | |||||||
| Carey et al[45] | 207 CLD (66 ALD, 73 HCV + ALD, 68 HCV) (M: 131, F: 76, Mean age: 51, range: 32-68) | Unidentifiable | Overall fractures | 21 | 66 | 13 | 68 (HCV) | 12 | 66 | 19 | 68 (HCV) | T (L1-4): -0.87 ± 1.61 | T (L1-4): -1.43 ± 1.68 |
| Kim et al[47] | 18 ALD (Mean age: 50.2 ± 9.5), 18 normal controls (Mean age: 51.2 ± 14) | 0 | 4 | 18 | 3 | 18 (normal control) | T (L2-4): 1.04 ± 0.14, T (FN): 0.844 ± 0.12 | T (L2-4): 1.131 ± 0.22, T (FN): 0.908 ± 0.15 | |||||
| Sokhi et al[22] | 104 CLD (M: 54, F: 50, Mean age: 54.4 ± 12.9) | All (17) | 17 | 69 (LC-HCV) | T (L2-4): -1.17 ± 0.16, T (FN): 0.97 ± 0.18 | T (L2-4): 1.23 ± 0.25, T (FN): 0.98 ± 0.16 | |||||||
| Alvisa-Negrín et al[43] | 77 ALD (M: 68, F: 9, Mean age: 50.43 ± 10.81), 28 normal controls (M: 25, F: 3, Mean age: 49.83 ± 9.24) | 41 ALC, 30 Non-LC | 9 | 77 | 0 | 28 (normal control) | 1T (L): -1.2 ± 1.16, T (FN): -1.1 ± 1.28 | 1T (L): -0.49 ± 0.89, T (FN): -0.5 ± 1.23 | |||||
| González-Reimers et al[40] | 124 ALD (Mean age: 50.5 ± 11.23), 38 Controls (Mean age: 48.47 ± 11.08) | 51 ALC, 61 Non-LC | 124 | 38 (normal control) | 2T (LS): -1.17 ± 1.22, T (FN): -1.24 ± 1.38 | 2T (LS): -0.5 ± 0.86, T (FN): -0.36 ± 1.34 | |||||||
| Mitchell et al[24] | 117 CLD (M: 74, F: 43, Mean age: 50.4 ± 10.5) | Unidentifiable | 20 | 46 (viral CLD) | T (L1-4): -1.51 ± 1.35, T (FN): -1.31 ± 1.02 | T (L1-4): -1.08 ± 1.35, T (FN): -1.11 ± 1.07 | |||||||
| Mahmoudi et al[25] | 109 CLD (M: 72, mean age: 55.3 ± 11.4, F: 37, mean age: 65.2 ± 11) | All (31) | 31 | 43 (LC-HCV) | T (L1-4): -0.34 ± 1.46, T (FN): -0.07 ± 1.45 | T (L1-4): -0.83 ± 1.66, T (FN): -0.56 ± 1.49 | |||||||
| Wibaux et al[42] | 99 CLD (M: 79, F: 20, mean age: 55 ± 8) | All (39) | Vertebra | 17 | 39 | 19 | 60 (other CLD) | ||||||
| Choudhary et al[26] | 115 LC (M: 107, F: 8, mean age: 49 ± 5.5) | All (67) | 16 | 67 | 10 | 48 (viral LC) | |||||||
| González-Reimers et al[41] | 90 ALD (Mean age: 50.14 ± 10.49), 30 normal controls (Mean age: 50.11 ± 10.4) | 40 ALC, 48 Non-LC | Overall fractures | 49 | 90 | 0 | 30 (normal control) | 3T (L): -1.15 ± 1.18 | 3T (L): -0.56 ± 0.9 | ||||
The enrolled studies were published between 1982 and 2011. Eight studies were conducted in Europe[21,25,39-44], whereas the remaining studies were conducted in the United States (n = 3)[22,23,45], Canada (n = 1)[24], Australia (n = 1)[46], India (n = 1)[26], and Korea (n = 1)[47]. All 15 articles were written in English. The study format of each study was as follows; cohort study (10)[21-26,39,40,42,45] and case-control study (5)[41,43,44,46,47]. Three studies included a normal healthy population as the control group[40,41,44], although the remaining studies included patients with chronic liver diseases other than alcoholic etiologies as the control groups[21-26,39,42,43,45-47].
Among the included studies, 4 studies[22,25,26,42] had ALD groups that consisted only of cirrhotic patients, whereas 2 studies[44,47] included no alcoholic cirrhotic patients, and in 5 studies[23,24,39,45,46], the presence or absence of cirrhosis was not specified.
As for the measurement of BMD, only 1 study used DPA using Prodigy: DPX-NT /DPX-MD + (General Electric, Milwaukee, WI)[22], and the remaining studies[21,24,25,40,41,43,45,47] used DXA.
In terms of the methodological quality, the authors classified the included studies as high-quality (≥ 7 stars) or low-quality (< 7 stars). Six studies[26,39,40,45-47] were classified as high-quality, whereas the remaining 9 studies[21-25,41-44] were classified into the low-quality group (Table 2).
| Study | Selection | Comparability | Exposure or outcome | Total |
| Lindsell et al[44] | 2 | 3 | 5 | |
| Diamond et al[46] | 3 | 1 | 3 | 7 |
| Bonkovsky et al[23] | 3 | 2 | 5 | |
| Ninkovic et al[39] | 4 | 3 | 7 | |
| Ninkovic et al[21] | 3 | 3 | 6 | |
| Carey et al[45] | 3 | 1 | 3 | 7 |
| Kim et al[47] | 3 | 2 | 3 | 8 |
| Sokhi et al[22] | 3 | 3 | 6 | |
| Alvisa-Negrín et al[43] | 3 | 1 | 2 | 6 |
| González-Reimers et al[40] | 3 | 1 | 3 | 7 |
| Mitchell et al[24] | 3 | 3 | 6 | |
| Mahmoudi et al[25] | 3 | 3 | 6 | |
| Wibaux et al[42] | 3 | 3 | 6 | |
| Choudhary et al[26] | 3 | 2 | 3 | 8 |
| González-Reimers et al[41] | 3 | 1 | 2 | 6 |
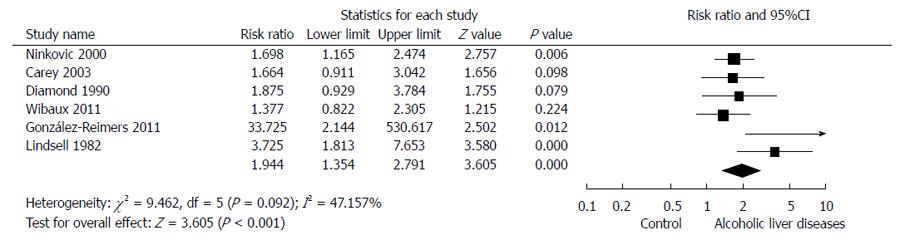
The overall association of ALD and bone fractures was evaluated by a random effects model-based meta-analysis of 6 studies[39,41,42,44-46]. Overall, ALD showed a RR of 1.944 (95%CI: 1.354-2.791, P < 0.001) for the development of bone fractures (Figure 2).
The relationship between ALD and osteoporosis was assessed by a random effects model-based meta-analysis of 5 studies[23,26,43,45,47]. ALD showed a RR of 0.849 (95%CI: 0.523-1.380, P = 0.509) for the development of osteoporosis (Figure 3).
To compare the BMD directly between patients with ALD and the control groups, the authors performed a random effects model-based meta-analysis of 7 studies[21,22,24,25,40,43,47] in which the BMD was measured at the femoral neck and 9 studies[21,22,24,25,40,41,43,45,47] in which the BMD was measured at the spine. BMD was not significantly different between the ALD and control groups, although there was a trend toward lower BMD in patients with ALD (SMD in the femoral neck BMD: -0.172, -0.453-0.110, P = 0.233; SMD in the spine BMD: -0.169, -0.476-0.138, P = 0.282).
The cumulative meta-analysis of the enrolled studies in the order of published year showed a decreasing trend of RRs, but a consistent and statistically significant increase in bone fractures. With regard to osteoporosis, the cumulative meta-analysis of the enrolled studies showed an increasing trend of RRs that was consistently non-statistically significant. For the measurement of BMD, cumulative meta-analyses of enrolled studies showed a decreasing trend in SMD, although there was still no difference in BMD vs the control group. The one-study-removed meta-analyses of the enrolled studies also showed consistent results.
In the sensitivity analyses of high-quality[39,45,46] and low-quality[41,42,44] studies for bone fractures, consistent results were noted (RR = 1.719, 95%CI: 1.285-2.299, P < 0.001; RR = 2.058, 95%CI: 1.360-3.114, P = 0.001). Consistent results were also noted in the sensitivity analysis of osteoporosis in both the high-quality[26,45,47] and low-quality[23,43] studies (RR = 0.885, 95%CI: 0.568-1.381, P = 0.592; RR = 0.642, 95%CI: 0.271-1.523, P = 0.315).
When analyzing the included studies by the characteristics of the control, the association between ALD and bone fractures was statistically significant both in studies utilizing the normal healthy population as the control arm[41,44] (RR = 7.379, 95%CI: 1.001-54.391, P < 0.05) and studies with CLD (etiology other than alcohol) serving as the control arm[39,42,45,46] (RR = 1.629, 95%CI: 1.265-2.099, P < 0.001).
In the analysis of the association between ALD and bone fractures, the outlier[41] was noted to have an effect size of 33.725 (RR) (Figure 2). After excluding this outlier[41] in the analysis of the association between ALD and bone fractures, the result was unchanged and was statistically significant (RR = 1.811, 95%CI: 1.370-2.395, P < 0.001).
For the association between ALD and osteoporosis, studies using a normal, healthy control-arm[43,47] showed a RR = 1.908 (95%CI: 0.498-7.300, P = 0.346), and studies with CLD (etiology other than alcohol) as a control arm[23,26,45] showed a RR = 0.751 (95%CI: 0.474-1.188, P = 0.221), which did not differ from the general analysis (Figure 3).
Currently, DXA is recommended for the measurement or monitoring of BMD[48]. A T-score measured by DPA, a different tool for the measurement of BMD, could result in significant bias. Thus, an analysis excluding the study[22] that utilized DPA for the measurement of BMD was performed. The sensitivity analysis for BMD excluding the study that measured BMD by DPA demonstrated results consistent with the full analysis (SMD in femoral neck BMD: -0.157, -0.357-0.043, P = 0.123; SMD in spine BMD: -0.073, -0.234-0.088, P = 0.375).
A funnel plot for the enrolled studies was asymmetrical. Egger’s regression test revealed that the intercept was 2.442 [95%CI: -0.282-5.167, t-value: 2.489, df: 4, P = 0.034 (1-tailed) and P = 0.068 (2-tailed)]. A trim and fill analysis showed that 1 study was missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.800 with a continuity correction [P = 0.012 (1-tailed) and P = 0.024 (2-tailed)].
A funnel plot for the enrolled studies of the analysis of the association between ALD and osteoporosis is also asymmetrical. However, Egger’s regression test revealed that the intercept was 1.644 [95%CI: -2.081-5.368, t-value: 1.405, df: 3, P = 0.127 (1-tailed) and P = 0.255 (2-tailed)]. A trim and fill analysis showed that 2 studies were missed or trimmed. The rank correlation test showed a Kendall’s tau of 0.300 with a continuity correction [P = 0.231 (1-tailed) and P = 0.462 (2-tailed)].
In the evaluation of the BMD measured at the femoral neck between the ALD and control groups, the resulting funnel plot was of a symmetrical shape. Egger’s regression test revealed that the intercept was -3.701 [95%CI: -12.537-5.135, t-value: 1.076, df: 5, P = 0.165 (1-tailed) and P = 0.331 (2-tailed)]. A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test showed a Kendall’s tau of -0.19 with a continuity correction [P = 0.274 (1-tailed) and P = 0.548 (2-tailed)].
In the evaluation of the BMD measured at the spine between the ALD and control groups, the funnel plot showed a symmetrical shape. Egger’s regression test revealed that the intercept was -6.231 [95%CI: -12.759-0.296, t-value: 2.257, df: 7, P = 0.029 (1-tailed) and P = 0.059 (2-tailed)]. A trim and fill analysis showed that no study was missed or trimmed. The rank correlation test showed a Kendall’s tau of -0.25 with a continuity correction [P = 0.174 (1-tailed) and P = 0.348 (2-tailed)].
Overall, publication bias was detected in the analysis of bone fractures and osteoporosis. However, there was no evidence of publication bias in the analysis of BMD.
In this meta-analysis, bone fracture was associated with ALD, whereas osteoporosis was not associated with ALD. This signifies that the fractures occurring in patients with ALD could be BMD-independent fractures. This result is consistent with the findings of a previous meta-analysis by Kanis et al[49]. This study concluded that high intake of alcohol confers a substantial risk for fractures and that this risk is largely independent of BMD[49]. However, there are several considerations when interpreting the results of these studies. The meta-analysis by John A. Kanis et al[49] aimed to quantify the fracture risk associated with alcohol consumption in the normal population. As demonstrated in previous studies, light to moderate amounts of alcohol use decrease fracture rates and increase bone density, which is contrary to the results of heavy alcohol consumption[5-15]. This is known as the threshold effect, indicating a J-shaped relationship between alcohol consumption and fracture risk, which was confirmed by another meta-analysis[15]. However, studies in the normal population have the problem of spectrum bias. The proportion of heavy alcoholics in the cohort dictates the threshold, and studies with a low proportion of heavy alcohol users lack the power required to examine this effect[49]. Moreover, these studies have the limitation of timing for alcohol consumption measurement and self-reported alcohol consumption, which could be inaccurate[15]. Contrary to the studies consisting of the normal population, our study enrolled articles utilizing the ALD population. Considering that ALD is the deleterious outcome of chronic alcohol abuse, these limitations could be minimized in our study.
In the present study, however, there was also substantial methodological heterogeneity between the enrolled studies, which had a potential effect on the risk estimates. This phenomenon was evaluated by sensitivity analyses for the confirmation of the robustness of this meta-analysis. The most noticeable modifier was the different populations used as control groups among the enrolled studies. Previous studies have suggested that metabolic bone disease is prevalent in CLD, especially in cholestatic liver disease[17,50]. Thus, studies with CLD as a control group run the potential risk of reporting an underestimated effect size (vs ALD). Moreover, CLD is a complex and vague terminology that cannot include specific populations. ALD may include alcoholic fatty liver, alcoholic hepatitis, and liver cirrhosis of alcoholic origin. Despite the anticipated problems described above, the main analysis and the sensitivity analyses divided by control-group (normal population vs CLD) showed consistent results regarding bone fractures, osteoporosis, and BMD. The proportion of liver cirrhosis in ALD was unidentifiable in most of the enrolled studies. Future studies using homogenous populations are needed to determine the applicability of these results to the subpopulations of ALD or CLD.
Another modifier was the quality of the enrolled studies. The included studies were classified as being either in the high-quality (≥ 7 stars) or the low-quality group (< 7 stars). This standard was decided by the authors. The average number of stars awarded in 15 studies was 6.4 (Table 2). Of note, 8 studies were awarded zero stars in the section of comparability. Despite the pitfalls of methodological quality, the sensitivity analyses divided by study quality showed consistent results. High-quality studies are needed for the wide application of these results.
During the main and sensitivity analyses, a significant outlier[41] was observed. In the analysis of the association between ALD and bone fractures, the effect size of the outlier (RR = 33.725) was more than 10 times the adjusted effect size (RR = 1.944) (Figure 2). The analysis excluding the outlier showed consistent results. The reason for the large effect size of the outlier is postulated to be a methodological problem. In this study, the presence of fracture was recorded by anamnesis and chest X-ray film[41]. This inaccurate methodology could overestimate the rate of bone fractures. Moreover, the quality measured by NOS was low (6 stars) in this study.
In terms of the main mechanism behind the pathophysiology of bone fractures and osteoporosis in ALD, it is inferred that alcohol causes an imbalance in bone remodeling with a predominant decrease in bone formation[4,15,51]. Alcohol is known to cause direct effects on the numbers and activity of osteoblasts and osteoclasts[4]. In addition to the direct effects of alcohol on the osteoblasts and osteoclasts, many indirect effects have also been reported. These indirect effects are mainly linked to impaired nutrition, which leads to weight loss, decreased fat and lean body mass, and hormone alterations, which may change in bone cell activity[4,15,51]. More recent studies indicated the effects that alcohol have on bone mass may be due to a suppression of the Wnt/DKK1 signaling pathway through the stimulation of oxidative stress[52,53]. With an accumulating body of evidence regarding the effects alcohol has on the skeletal system, a more detailed pathophysiology could be confirmed in the near future.
This study is the first meta-analysis examining the association between ALD and bone fractures or osteoporosis. The strength of this study is the evaluation of 3 effect sizes. The main result was confirmed, compared, and interpreted with regard to the other effect sizes. Another strength of this analysis is the selection of ALD for the study population. As described above, biases from various standards of alcohol consumption (light, moderate, heavy, or binge drinking), inaccurate amounts of alcohol consumption from self-reporting, and inconsistent timing for the measurement of alcohol consumption could be minimized. Potential modifiers were detected whenever possible from the articles and evaluated through the sensitivity analyses.
Despite its strengths, there are several limitations within the present study. First, there was no consideration given to any potential confounders, which could be influential to bone fractures and osteoporosis. These include sex, age, menopausal status, bone fracture history, family history of bone diseases or fractures, smoking, medications such as corticosteroids and body composition. However, most the important confounder is assumed to be trauma, such as frequent falls in patients with ALD. As noted in many studies, this powerful but easily overlooked confounder should be included via a thorough history in the studies relevant to this topic[4,15]. Second, the Newcastle-Ottawa scale was used to assess the methodological quality of the studies. This scale has been criticized for its low agreement between authors and reviewers[54]. Alternative tools such as the risk of bias table by the Cochrane group have been proposed as an alternative approach. However, this particular tool has strength in the evaluation of randomized studies, and poor inter-rater agreement was also noted[55]. Additional validated and commonly used tools are needed.
In conclusion, current publications indicate a significant association between bone fractures and ALD, independent of BMD or the presence of osteoporosis. Due to the qualitative and quantitative heterogeneity among studies, further studies utilizing homogenous populations and controlling for confounding risk factors for fractures are needed to elucidate the underlying mechanism of bone fractures in ALD.
Chronic excessive alcohol consumption is a well-established risk factor for low bone density and bone fractures. However, ingestion of light or moderate amounts of alcohol is known to be associated with higher bone density and decreased fracture rates. Osteoporosis and bone fractures are frequently overlooked complications in patients with chronic liver disease that could result in serious outcomes. However, the exact prevalence or mechanism of osteodystrophy in patients with alcoholic liver disease (ALD), which is the deleterious outcome of chronic alcohol abuse, have not been described
Meta-analysis by John A. Kanis concluded that high intake of alcohol confers a substantial risk for fractures and that this risk is largely independent of bone mineral density (BMD). However, this study included normal population and there has been no such analysis in the ALD population.
From the fifteen non-randomized studies, ALD demonstrated a RR =1.944 (95%CI: 1.354-2.791) for the development of bone fractures. However, ALD showed a RR = 0.849 (95%CI: 0.523-1.380) for the development of osteoporosis.
Current publications indicate significant associations between bone fractures and ALD, independent of BMD or the presence of osteoporosis. Although the mechanism of bone fractures in ALD is not totally understood, further research utilizing a homogenous population and controlling for confounding risk factors for fractures could elucidate the mechanism.
ALD: Excessive alcohol consumption is associated with a spectrum of hepatic manifestations, including alcoholic fatty liver, alcoholic hepatitis, and cirrhosis. These diseases share the core cause of hepatic injury which is an excessive alcohol consumption and generally refers to alcoholic liver disease.
The study addresses an interesting topic and merit. However, it has some intrinsic methodological limitations which decrease its potential clinical impact.
P- Reviewer: De Ponti F, Fouad YM, Han T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 772] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 2. | Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 1702] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 3. | Available from: http://www.shef.ac.uk/FRAX. |
| 4. | Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | Feskanich D, Korrick SA, Greenspan SL, Rosen HN, Colditz GA. Moderate alcohol consumption and bone density among postmenopausal women. J Womens Health. 1999;8:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Jugdaohsingh R, O’Connell MA, Sripanyakorn S, Powell JJ. Moderate alcohol consumption and increased bone mineral density: potential ethanol and non-ethanol mechanisms. Proc Nutr Soc. 2006;65:291-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Orwoll ES, Bauer DC, Vogt TM, Fox KM. Axial bone mass in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1996;124:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Pedrera-Zamorano JD, Lavado-Garcia JM, Roncero-Martin R, Calderon-Garcia JF, Rodriguez-Dominguez T, Canal-Macias ML. Effect of beer drinking on ultrasound bone mass in women. Nutrition. 2009;25:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Williams FM, Cherkas LF, Spector TD, MacGregor AJ. The effect of moderate alcohol consumption on bone mineral density: a study of female twins. Ann Rheum Dis. 2005;64:309-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Tucker KL, Jugdaohsingh R, Powell JJ, Qiao N, Hannan MT, Sripanyakorn S, Cupples LA, Kiel DP. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am J Clin Nutr. 2009;89:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Venkat KK, Arora MM, Singh P, Desai M, Khatkhatay I. Effect of alcohol consumption on bone mineral density and hormonal parameters in physically active male soldiers. Bone. 2009;45:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Ganry O, Baudoin C, Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: The EPIDOS Study. Epidémiologie de l’Ostéoporose. Am J Epidemiol. 2000;151:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Høidrup S, Grønbaek M, Gottschau A, Lauritzen JB, Schroll M. Alcohol intake, beverage preference, and risk of hip fracture in men and women. Copenhagen Centre for Prospective Population Studies. Am J Epidemiol. 1999;149:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Berg KM, Kunins HV, Jackson JL, Nahvi S, Chaudhry A, Harris KA, Malik R, Arnsten JH. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Hernández ER, Revilla M, Rico H. Total body bone mineral and pelvis bone mineral content as parameters of bone mass in men. A dual-energy X-ray absorptiometry study. Acta Anat (Basel). 1991;142:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Goel V, Kar P. Hepatic osteodystrophy. Trop Gastroenterol. 2010;31:82-86. [PubMed] |
| 18. | Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii-x, 1-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1687] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 19. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12586] [Article Influence: 839.1] [Reference Citation Analysis (0)] |
| 20. | Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 817] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 21. | Ninkovic M, Love SA, Tom B, Alexander GJ, Compston JE. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int. 2001;69:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Sokhi RP, Anantharaju A, Kondaveeti R, Creech SD, Islam KK, Van Thiel DH. Bone mineral density among cirrhotic patients awaiting liver transplantation. Liver Transpl. 2004;10:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Bonkovsky HL, Hawkins M, Steinberg K, Hersh T, Galambos JT, Henderson JM, Millikan WJ, Galloway JR. Prevalence and prediction of osteopenia in chronic liver disease. Hepatology. 1990;12:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Mitchell R, McDermid J, Ma MM, Chik CL. MELD score, insulin-like growth factor 1 and cytokines on bone density in end-stage liver disease. World J Hepatol. 2011;3:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Mahmoudi A, Sellier N, Reboul-Marty J, Chalès G, Lalatonne Y, Bourcier V, Grando V, Barget N, Beaugrand M, Trinchet JC. Bone mineral density assessed by dual-energy X-ray absorptiometry in patients with viral or alcoholic compensated cirrhosis. A prospective study. Clin Res Hepatol Gastroenterol. 2011;35:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Choudhary NS, Tomar M, Chawla YK, Bhadada SK, Khandelwal N, Dhiman RK, Duseja A, Bhansali A. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig Dis Sci. 2011;56:3323-3327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Cooper H. Synthesizing research: A guide for literature reviews. Thousand Oaks, CA: Sage 1998; . |
| 28. | Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. UK, London: The Cochrane Collaboration 2011; . |
| 29. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25721] [Article Influence: 1118.3] [Reference Citation Analysis (0)] |
| 30. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46363] [Article Influence: 2107.4] [Reference Citation Analysis (3)] |
| 31. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30361] [Article Influence: 778.5] [Reference Citation Analysis (0)] |
| 32. | Borenstein M, Hedges LV, Higgins J, Rothstein HR. Fixed - Effect Versus Random - Effects Models. Introduction to Meta-analysis. Chichester: Wiley 2009; 77-86. [DOI] [Full Text] |
| 33. | Olkin I. Statistical methods for meta-analysis. San Diego, CA: Academic 1985; . |
| 34. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9055] [Article Influence: 362.2] [Reference Citation Analysis (0)] |
| 35. | Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester: Wiley 2000; . |
| 36. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2629] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 37. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12154] [Article Influence: 405.1] [Reference Citation Analysis (0)] |
| 38. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40383] [Article Influence: 1442.3] [Reference Citation Analysis (2)] |
| 39. | Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2000;12:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | González-Reimers E, Alvisa-Negrín J, Santolaria-Fernández F, Ros-Vilamajó R, Martín-González MC, Hernández-Betancor I, García-Valdecasas-Campelo E, González-Díaz A. Prognosis of osteopenia in chronic alcoholics. Alcohol. 2011;45:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | González-Reimers E, Alvisa-Negrín J, Santolaria-Fernández F, Candelaria Martín-González M, Hernández-Betancor I, Fernández-Rodríguez CM, Viña-Rodríguez J, González-Díaz A. Vitamin D and nutritional status are related to bone fractures in alcoholics. Alcohol Alcohol. 2011;46:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Wibaux C, Legroux-Gerot I, Dharancy S, Boleslawski E, Declerck N, Canva V, Mathurin P, Pruvot FR, Cortet B. Assessing bone status in patients awaiting liver transplantation. Joint Bone Spine. 2011;78:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Alvisa-Negrín J, González-Reimers E, Santolaria-Fernández F, García-Valdecasas-Campelo E, Valls MR, Pelazas-González R, Durán-Castellón MC, de Los Angeles Gómez-Rodríguez M. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Lindsell DR, Wilson AG, Maxwell JD. Fractures on the chest radiograph in detection of alcoholic liver disease. Br Med J (Clin Res Ed). 1982;285:597-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Carey EJ, Balan V, Kremers WK, Hay JE. Osteopenia and osteoporosis in patients with end-stage liver disease caused by hepatitis C and alcoholic liver disease: not just a cholestatic problem. Liver Transpl. 2003;9:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Diamond T, Stiel D, Lunzer M, Wilkinson M, Roche J, Posen S. Osteoporosis and skeletal fractures in chronic liver disease. Gut. 1990;31:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Kim MJ, Shim MS, Kim MK, Lee Y, Shin YG, Chung CH, Kwon SO. Effect of chronic alcohol ingestion on bone mineral density in males without liver cirrhosis. Korean J Intern Med. 2003;18:174-180. [PubMed] |
| 48. | El Maghraoui A, Roux C. DXA scanning in clinical practice. QJM. 2008;101:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 369] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 50. | Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Chakkalakal DA. Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res. 2005;29:2077-2090. [PubMed] |
| 52. | Chen JR, Lazarenko OP, Shankar K, Blackburn ML, Badger TM, Ronis MJ. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25:1117-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Callaci JJ, Himes R, Lauing K, Wezeman FH, Brownson K. Binge alcohol-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral bone. Calcif Tissue Int. 2009;84:474-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1626] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 55. | Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs Seida J, Klassen TP. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ. 2009;339:b4012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |