Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3527
Peer-review started: July 21, 2014
First decision: August 15, 2014
Revised: September 15, 2014
Accepted: October 14, 2014
Article in press: October 15, 2014
Published online: March 28, 2015
Processing time: 251 Days and 23.2 Hours
AIM: To optimize the viral persistence rate in a hydrodynamic injection (HI) based hepatitis B virus (HBV) transfection mouse model.
METHODS: (1) 5-6-wk-old male C3H/HeN and C57BL/6 mice were hydrodynamically injected with 10 μg endotoxin-free pAAV/HBV1.2 plasmid DNA via the tail vein. Hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg) and HBV DNA, both in the serum and liver, were detected at different time points post HI by ELISA, immunohistochemical staining or quantitative polymerase chain reaction (PCR); (2) male C3H/HeN and C57BL/6 mice, either hydrodynamically injected mice at 10 wk post HI or naïve mice, were all immunized subcutaneously with 5 μg HBsAg formulated in complete Freund’s adjuvant three times at a 2-wk interval. Two weeks after the final immunization, splenocytes were isolated for T cell function analysis by ELISPOT assay; and (3) five weeks post HI, C3H/HeN mice were intragastrically administered 0.1 mg/kg entecavir once a day for 14 d, or were intraperitoneally injected with 1 mg/kg interferon (IFN)-α twice a week for 2 wk, or were treated with PBS as controls. The sera were collected and assayed for HBV DNA on days 0, 7 and 14 after drug treatment.
RESULTS: (1) Approximately 90% (22/25) of the injected C3H/HeN mice were still HBsAg-positive at 46 wk post HI, whereas HBsAg in C57BL/6 mice were completely cleared at 24 wk. Serum levels of HBeAg in C3H/HeN mice were higher than those in C57BL/6 mice from 4 wk to 46 wk. HBV DNA levels in the hydrodynamically injected C3H/HeN mice were higher than those in the C57BL/6 mice, both in the serum (from 4 wk to 46 wk) and in the liver (detected at 8 wk and 46 wk post HI). Histology showed that hepatitis B core antigen and HBsAg were expressed longer in the liver of C3H/HeN mice than in C57BL/6; (2) HBsAg specific T cell responses after HBsAg vaccination in hydrodynamically injected C3H/HeN and C57BL/6 mice, or naive control mice were detected by ELISPOT assay. After stimulation with HBsAg, the frequencies of IFN-γ producing splenocytes in the hydrodynamically injected C3H/HeN mice were significantly lower than those in hydrodynamically injected C57BL/6 mice, control C3H/HeN and control C57BL/6 mice, which were 0, 17 ± 7, 18 ± 10, and 41 ± 10 SFCs/106 splenocytes, respectively, and the mean spot sizes showed the same pattern. Even just stimulated with PMA and ionomysin, T-cell responses elicited in the vaccinated control C3H/HeN were much higher than those in hydrodynamically injected C3H/HeN mice; and (3) For drug treatment experiments on the hydrodynamically injected C3H/HeN mice, serum HBV DNA levels in the entecavir treatment group declined (131.2 folds, P < 0.01) on day 7 after treatment and kept going down. In the group of IFN-α treatment, serum HBV DNA levels declined to a lowest point (6.42 folds, P < 0.05) on 7 d after treatment and then rebounded.
CONCLUSION: We have developed a novel HI-based HBV transfection model using C3H/HeN mice, which had a higher HBV persistence rate than the classic C57BL/6 mouse model.
Core tip: In the classic hepatitis B virus (HBV) hydrodynamic injection (HI) model using C57BL/6 mice, only about 30% of the injected mice carried HBV for more than 12 wk. Here we injected the pAAV-HBV1.2 plasmid into C3H/HeN mice and observed that the hepatitis B surface antigen, hepatitis B e antigen and viral DNA persisted even up to 46 wk in about 90% of the hydrodynamically injected mice. Applying interferon-α or entecavir in this HI model decreased HBV DNA in vivo. Hence, C3H/HeN is a suitable mouse strain for the persistent HBV HI model, which might be useful for chronic hepatitis B research and therapeutic drug development.
- Citation: Peng XH, Ren XN, Chen LX, Shi BS, Xu CH, Fang Z, Liu X, Chen JL, Zhang XN, Hu YW, Zhou XH. High persistence rate of hepatitis B virus in a hydrodynamic injection-based transfection model in C3H/HeN mice. World J Gastroenterol 2015; 21(12): 3527-3536
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3527.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3527
Chronic infection with hepatitis B virus (HBV) in the human liver remains a major health problem globally[1]. More than 400 million people have been infected and about one million patients die annually from HBV infection[2]. Currently, interferon (IFN)-α and nucleoside/nucleotide analogs have been mainly used for clinical treatment of chronic hepatitis B (CHB). Although nucleoside/nucleotide analogs inhibit HBV replication, drug resistance remains an unsettled tough issue in clinical practice[3]. On the other hand, IFN-α enhances host immune responses and promotes HBV clearance. However, only 30% of CHB patients showed a sustained response to IFN-α treatment, which limits the clinical effect and application of IFN-α[4]. New therapeutic strategies are needed to be developed to improve the treatment of CHB.
It is believed that the balance between viral replication and the host immune response during chronic HBV infection determines the pathogenesis and outcomes of CHB. Nevertheless, the etiological mechanisms of the host immune responses that lead to HBV persistence are still to be elucidated completely yet, though some components of the immune system, particularly cellular immune responses, have shown to be involved in the clearance of HBV[5].
However, the research of HBV infection, either studies on immunological mechanisms or therapeutic drug development, has been hampered by the shortage of suitable animal models[6]. Compared with chimpanzees, woodchuck and duck, mouse is the ideal laboratory animal for its convenient availability, easy husbandry and low cost, and most importantly, their well characterized genetic background, techniques for genetic modification, and abundance of immunological reagents. Despite the inability of HBV to propagate in mouse, several mouse models of HBV have been developed, including HBV transgenic mice, adenovirus or adenovirus-associated-virus (AAV) based HBV transduction models, and hydrodynamic injection (HI) based HBV transfection models[6].
Introducing the HBV genome into the mouse liver by HI via the tail vein represents a model that mimics the natural course of chronic HBV infection in human without side effects from the viral vectors, such as immune responses against adenovirus[7]. Nonetheless, in the classic model developed by HI of an HBV plasmid into C57BL/6 mice, only less than 20%-30% injected mice carried HBV for 24 wk[8]. Recently we hydrodynamically injected the HBV plasmid into C3H/HeN mice and succeeded in delaying the mouse immune clearance of HBV. About 90% the injected C3H/HeN mice maintained HBV persistence even up to 46 wk post HI. Applying IFN-α and entecavir in this model led to HBV DNA decrease in vivo. Thus, this novel HI based model of HBV might provide a more stable platform for the research of HBV persistence infection.
All procedures on mouse were reviewed by the Institutional Animal Care and Use Committee of Shanghai Public Health Clinical Center and were performed in strict accordance with the approved protocol.
The replication-competent recombinant HBV plasmid pAAV/HBV1.2 was kindly provided by Prof. Peijer Chen, National Taiwan University College of Medicine. Specific pathogen free (SPF) C3H/HeN and C57BL/6 mice were purchased from the animal facilities of Shanghai Public Health Clinical Center. Male C3H/HeN and C57BL/6 mice (5-6-wk-old) were injected with 10 μg endotoxin-free pAAV/HBV1.2 plasmid DNA into the tail vein in a volume of PBS equivalent to 10% of the mouse body weight and the total volume was delivered within 5 s, which was so-called HI as previously described[9,10]. The serum specimens were assayed for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg) or HBV DNA at the indicated time points after injection. When the mice were sacrificed, the spleens were collected for T cell function analysis and the livers were collected and preserved in 4% PFA (paraformaldehyde) for immunohistochemical analysis.
Serum specimens were collected and assayed for HBsAg and HBeAg at 0, 1, 2, 3, 4, 5, 8, 10, 16, 24 and 46 wk after HI of pAAV/HBV1.2. Serum levels of HBsAg were determined with an ELISA kit (Kehua, Shanghai, China). The levels of HBeAg were determined by ELISA (Kehua, Shanghai, China).
Serum samples were collected at 0, 1, 2, 3, 4, 5, 8, 12, 16, 24 and 46 wk after HI of pAAV/HBV1.2. For HBV DNA extraction, 10 μL mouse serum was added into 40 μL PBS, and digested with 10 μg DNaseI for 1 h at 37 °C. Then, 100 μL lysis buffer (20 mmol/L Tris-HCl, 20 mmol/L EDTA, 50 mmol/L NaCl, and 0.5% SDS) containing 50 mg proteinase K was added. After incubation at 65 °C overnight, viral DNA was isolated by phenol/chloroform extraction and ethanol precipitation. The DNA pellet was rinsed with 70% ethanol and resuspended in 10 μL ddH2O. The quantification of HBV DNA was performed using a routine real-time PCR procedure described previously, with a SYBR Green Real-time PCR Master Mix kit (TOYOBO, Osaka, Japan)[11].
Liver tissues were collected from mice receiving HI killed at 46 wk. The total DNA of the liver was extracted as described above and detected for HBV DNA by real-time PCR or DpnI enzyme before real-time PCR.
Liver samples were collected 46 wk post HI. Intrahepatic HBsAg was visualized by immunohistochemical staining of tissues incubated with mouse anti-HBs antibody (Maixin.Biotech, Fuzhou, China), rabbit anti-HBc antibody (Maixin.Bio city, Fuzhou, China), and HRP (Maixin.Bio city, Fuzhou, China).
Male C3H/HeN and C57BL/6 mice at 10 wk after HI with 10 μg pAAV/HBV1.2 plasmid or naïve control male C3H/HeN and C57BL/6 mice were all immunized subcutaneously with 5 μg HBsAg formulated in complete Freund’s adjuvant (CFA) three times at a 2-wk interval. Mice were euthanized 2 wk after the final immunization and fresh splenocytes were collected.
Freshly isolated mouse splenocytes were adjusted to a density of 4 × 106 cells/mL and plated into 96-well ELISPOT plates (BD Bioscience, Franklin Lakes, New Jersey, United States) coated with an anti-mouse IFN-γ antibody at 50 μL/well (2 × 105 cells per well). The splenocytes were stimulated with 10 μg/mL HBsAg protein. After incubation at 37 °C with 5% CO2 for 20 h, the ELISPOT plates were developed according to the manufacturer’s manual and read with Immunospot Reader (ChampspotIII, Beijing Sage Creation Science, China).
An HI based mouse model of HBV was established as described above in fifteen 4-6-wk-old male C3H/HeN mice. Five weeks post HI, the mice were randomly divided into three groups (five mice each group). The first group was intragastrically administered 0.1 mg/kg entecavir (Baraclude, Bristol-Myers Squibb, New York, United States) once a day for 14 d; the second group was intraperitoneally injected with 1 mg/kg interferon-α (IFN-α, R and D, R and D Systems, Minneapolis, United States) twice a week for 2 wk; the third group was treated with PBS as a control. Serum specimens were collected and assayed for HBV DNA on days 0, 7 and 14 after treatment.
Comparisons between two groups were performed by unpaired t-test and comparisons among three or more groups were performed using one-way analysis of variance (GraphPad Software, Inc.). Significant differences were defined as P≤ 0.05.
In the hydrodynamically injected C57BL/6 mice, the HBsAg level increased promptly within 1 wk after pAAV/HBV1.2 injection but dropped quickly thereafter. In C3H/HeN mice, the HBsAg level declined much more slowly after injection of the same plasmid. Even at 46 wk post HI, it was still detectable (Figure 1A). Approximately 88% (22/25) of the injected C3H/HeN mice were still HBsAg-positive at 46 wk post HI whereas HBsAg in C57BL/6 mice was completely cleared at 24 wk (Figure 1B). Serum levels of HBeAg were increased to a peak quickly within a week, then decreased at 2 wk post HI, and increased again. Serum levels of HBeAg in C3H/HeN mice were higher than those in C57BL/6 mice from 4 wk to 46 wk (Figure 1C). Serum samples from hydrodynamically injected C3H/HeN and C57BL/6 mice were also assayed for the presence of HBV DNA. In the hydrodynamically injected C3H/HeN mice, HBV DNA levels were higher than those in hydrodynamically injected C57BL/6 mice. At 46 wk post HI, HBV DNA in the C3H/HeN mice could still be detected, but it was undetectable in the C57BL/6 mice (Figure 1D).
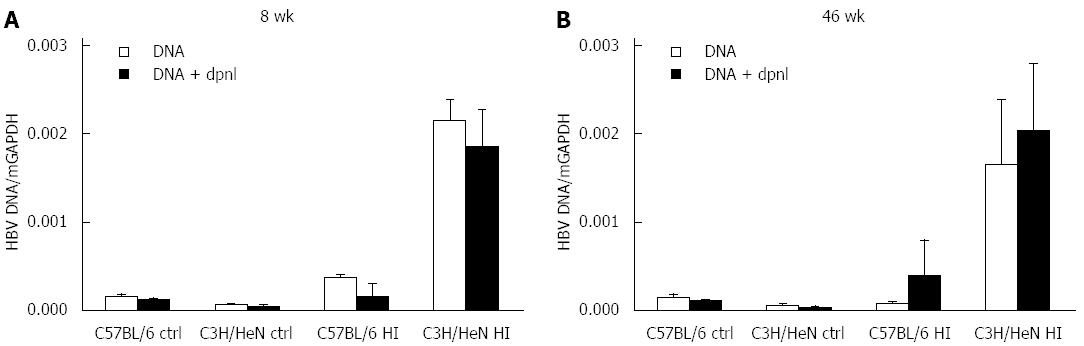
We further examined the existing way of the liver HBV DNA. The liver tissues were collected from hydrodynamically injected C57BL/6 mice, C3H/HeN mice at 46 wk post injection, and naive mice as controls. The liver total DNA was assayed for the presence of encapsidated HBV DNA by real-time PCR. We used 0.5 μL DpnI to digest the potential free plasmids and then also assayed the HBV DNA level by real-time PCR. Interestingly, we found that the liver HBV DNA level was not significantly changed between before and after DpnI digestion (Figure 2). We surmise that the DNA in serum HBsAg-positive C3H/HeN mice at 46 wk was not from free plasmid of pAAV/HBV1.2, but could be from actively replicating cytoplasmic nucleocapsid.
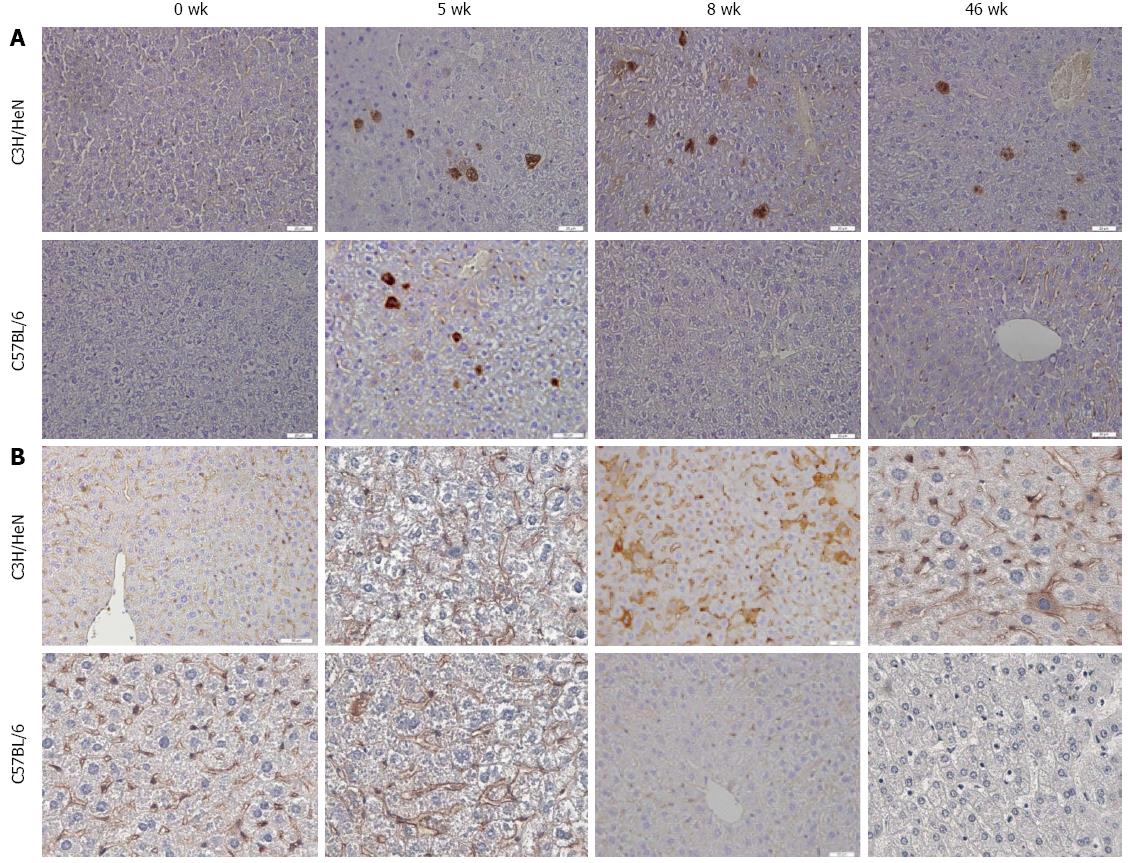
Liver tissues were collected from hydrodynamically injected C3H/HeN and C57BL/6 mice. Immunohistochemical staining was performed to determine the expression of hepatitis B core antigen (HBcAg) and HBsAg in the liver of the mice at 0, 5, 8, 46 wk post HI. As shown in Figure 3A, the number of HBcAg positive cells in C3H/HeN mice were not significantly different from that in C57BL/6 at 5 wk post HI. At 8 and 46 wk post HI, both cytoplasmic and nuclear HBcAg were detected in the liver of C3H/HeN mice but not in C57BL/6 mice. The HBsAg expression in the liver of C3H/HeN and C57BL/6 mice was not significantly different at 5 wk post HI, but was obviously higher in C3H/HeN than in C57BL/6 mice at 8 wk and 46 wk post HI, as shown in Figure 3B.
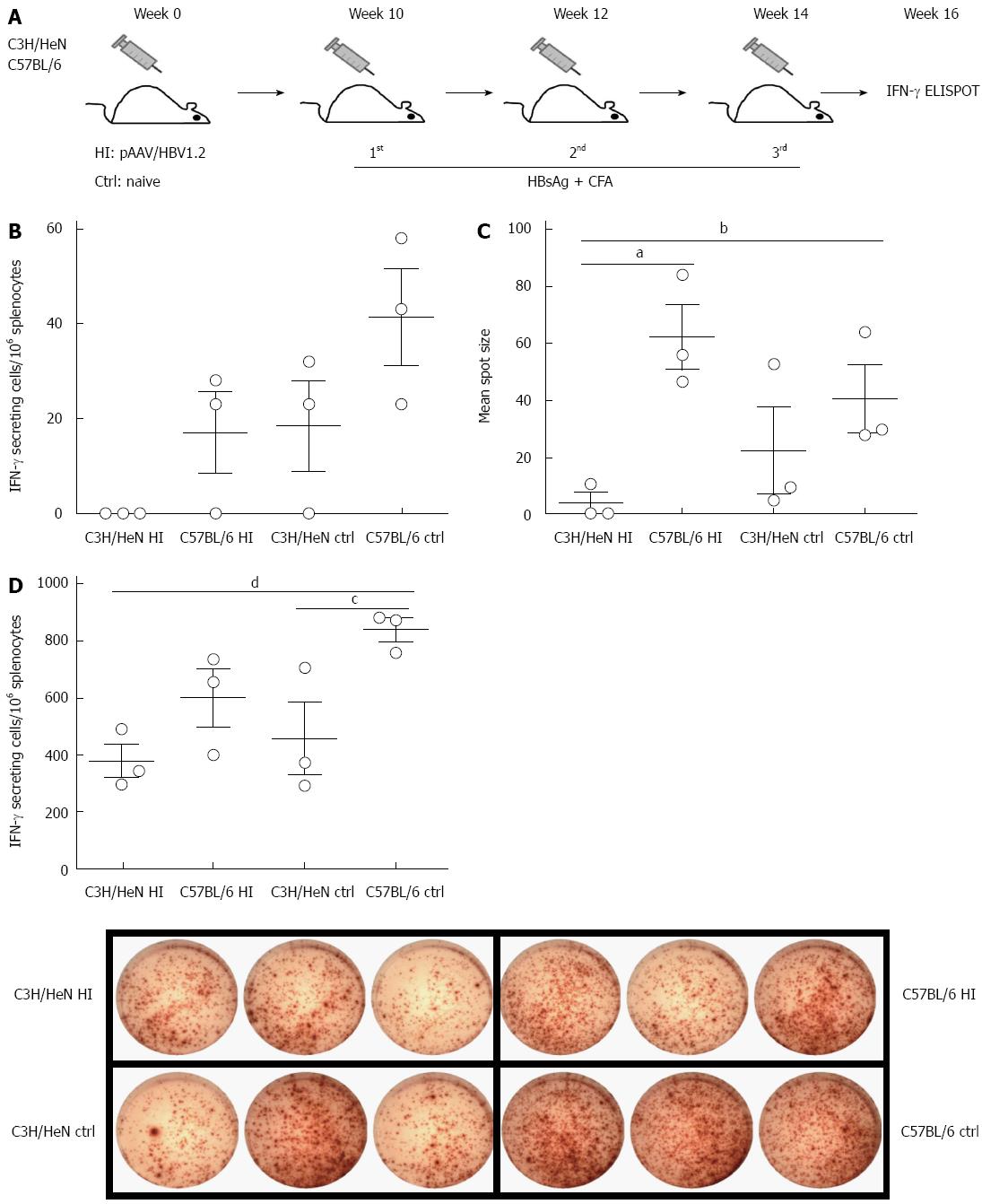
Specific T cell responses against HBV antigens (such as HBsAg) have been suggested to play critical roles in viral clearance[12]. Here we addressed whether HBsAg-specific immunity is associated with the HBV persistence/clearance in C3H/HeN and C57BL/6 mice at 10 wk post HI of pAAV/HBV1.2 or untreated controls (Figure 4A). We examined the T cell responses against HBsAg two weeks after the final vaccination with HBsAg by IFN-γ-ELISPOT in hydrodynamically injected C3H/HeN mice, hydrodynamically injected C57BL/6 mice, control C3H/HeN and control C57BL/6 mice, and the average frequency was 0, 17 ± 7, 18 ± 10, and 41 ± 10 SFCs/106 splenocytes, respectively. The frequency was significant lower in hydrodynamically injected C3H/HeN mice than in control C3H/HeN and C57BL/6 mice (Figure 4B), and the mean spot sizes showed the same pattern (Figure 4C). In contrast to hydrodynamically injected C57BL/6 capable of responding to HBsAg vaccination, the hydrodynamically injected C3H/HeN mice showed a totally tolerant phenotype to HBV with no responses to HBsAg vaccination; whereas naive control C3H/HeN mice responded to HBsAg vaccination, though it was lower than that in control C57BL/6 mice. Even just stimulated with PMA and ionomysin, frequency of IFN-γ positive T cells in the hydrodynamically injected C3H/HeN mice was much lower than that in control C57BL/6 mice (P < 0.05), and a little bit lower than those in hydrodynamically injected C57BL/6 and control C3H/HeN mice (Figure 4D). These data indicated that the HI of HBV genome into C3H/HeN mice could impair the T cell function in these mice, both specifically (i.e., HBsAg-specific T-cell immunity) and globally.
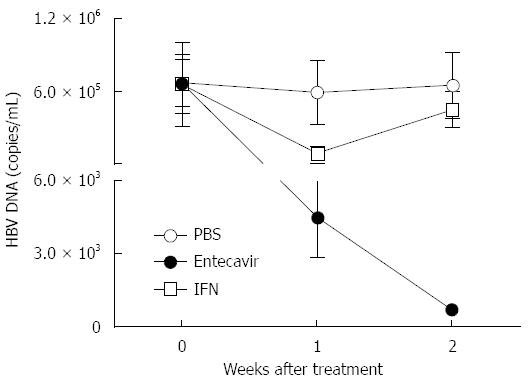
Next, we tested whether this novel HI based C3H/HeN mouse model could be applied to the drug evaluation for HBV. IFN-α and entecavir treatment was performed in those hydrodynamically injected C3H/HeN mice. In the group of intragastric administration with entecavir (0.1 mg/kg, daily), real-time PCR analysis showed that serum HBV DNA levels declined (131.2-fold, P < 0.01) and kept going down 7 d post HI (Figure 5). In the group of IFN-α treatment (1 mg/kg, twice a week), serum HBV DNA levels declined to a lowest point (6.42 folds, P < 0.05) 7 d post HI and rebounded from then on (Figure 5). These data indicated that this HI based C3H/HeN mouse model can be used for antiviral drug evaluation for HBV.
Due to the narrow host restriction of HBV, the ideal experimental animal model of HBV should be transgenic mice expressing human receptors for HBV. However, it was elusive for a long time about the receptors mediating HBV entry, though Li’s group recently identified that NTCP (sodium taurocholate cotransporting polypeptide) was a functional receptor for human HBV[13]. Whether there are coreceptors is still under investigation. Therefore, the success of HBV receptor-transgenic mice could be a long way to reach yet, considering the fact that the HCV receptors (CD81, SCARB1, CLDN1, OCLN) have been identified for a long time, but the HCV receptor-transgenic mouse has not succeeded until now[14].
Current available animal models for HBV studies include duck HBV (DHBV)[15] and woodchuck HBV (WHV)[16] infection in their natural hosts, HBV-infected chimpanzees[17], and HBV transgenic mice[18]. DHBV and WHV are genetically different from HBV, and it is difficult to perform immunological studies in those animals due to their uncharacterized background. Chimpanzees are not available easily, and the high cost as well as ethical considerations limit their applications to HBV study. HBV transgenic mice were widely used, but the drawback of immunological tolerance to the virus limits its applications in HBV immunological studies.
The HBV genome can be introduced into the mouse liver by transduction based on viral vectors, for example, adenovirus or adeno-associated viral vectors (AAVs) containing HBV DNA[19,20]. Transduction of the HBV genome into mice using viral vectors leads to efficient viral gene expression in the liver and host immune response against HBV. However, the viral vector-induced immune responses (such as induction of type I IFN and other innate immune responses) may interfere with the host immune responses against HBV.
HI can efficiently deliver DNA into the liver in vivo. The DNA internalization by this hydraulic pressure-based physical transfection is receptor-independent and can achieve delivery to approximately 10%-40% of hepatocytes[5,21]. Yang et al[22] first reported an acute HBV infection model by this method in B10.D2 mice and persistent expression of HBV antigens was observed in hepatocytes of immunocompromised CB17 NOD/SCID mice. Huang et al[8] further reported that delivery of the HBV genome into immunocompetent mouse liver by HI could induce HBV hepatitis with very different rates of viral clearance. Both plasmid backbone and genetic background of recipient mice contributed to the long-term maintenance of HBV in the mouse liver. The plasmid pAAV/HBV1.2 is better than the plasmid pGEM4A/HBV1.2, though both of them harboured replication-competent HBV DNA[8]. By using the same pAAV/HBV1.2, all BALB/c showed a rapid clearance of viral DNA template, whereas about 30% of the C57BL/6 mice showed the persistence of HBV[8]. However, the bottleneck of a relative low rate of persistence of HBV in C57BL/6 mice compromised its potential strength in chronic HBV infection studies.
The different persistence rates of HBV in hydrodynamically injected BALB/c (H-2d) and C57BL/6 (H-2b) mice encourage efforts to find more suitable mouse strains for optimisation of the HBV persistent rate. Indeed, Chen et al[23] reported that long-term maintaining of HBV antigenemia can be detected in FVB/N (H-2q) mice receiving HI of pGEM4Z/HBV1.3, and around 85% (6/7) of the mice were positive at 50 wk, compared with rapid clearance of HBV antigenemia in BALB/c mice within 4 wk and in C57BL/6 within 8 wk.
Our study successfully established HBV persistence in another inbred strain, C3H/HeN mice (H-2k), through HI of pAAV/HBV1.2 plasmid. Around 90% (22/25) of the injected C3H/HeN mice were HBsAg-positive and the HBcAg positive cells in the liver were detected at 46 wk, though the detailed mechanisms are not clear yet. Chang et al[24] reported an acute HBV hepatitis model by hydrodynamically injecting pHBV3.6 into 8-12-wk-old C3H/HeN mice. The age of C3H/HeN mice might be critical for the different persistence rates between their model and ours. Actually, we found 5-6-wk-old C3H/HeN mice are best for persistence of HBV after HI, and the mice older than 8 wk showed a similar acute hepatitis to Chang Wang’s model (data not shown).
It was well established that host immune responses contributed to HBV clearance[5]. Chang et al[24] reported that C3H/HeJ mice, which had a defect in TLR4 signaling, showed higher HBV antigenemia and viral replication than C3H/HeN in the acute model (viraemia within 2 wk), indicating that TLR4 mediated innate immune response played a role in the HBV clearance. Adaptive immune responses, especially HBV-specific T cell response, are most critical for HBV clearance[12]. Indeed, we found the HI of the HBV genome into C3H/HeN mice could impair the T cell function, both globally and specifically (i.e., HBsAg-specific T-cell immunity), in contrast to hydrodynamically injected C57BL/6 mice capable of responding to HBsAg vaccination. These results were consistent with the previous reports on correlations between persistence of HBV in hydrodynamically injected mice and few activated specific cytotoxic T cells. Hence, C3H/HeN (H-2K) mice, which were like FVB/N (H-2q) mice[23], were weaker in induction of HBV specific T cell responses than C57BL/6 (H-2b) mice; and the latter might be weaker than BALB/c (H-2d) mice[8]. These findings suggested that HI of the HBV genome could cause tolerance to HBV in the hydrodynamically injected C3H/HeN mice and this tolerance might be largely related to the HBV persistence phenotypes in those mice.
Taken together, though the route of viral genome delivery by hydrodynamic-based transfection is different from that of natural infection via receptors, this immunocompetent non-transgenic mouse model can mimic the nature course of chronic HBV infection in human to a great extent. Our novel hydrodynamically injected C3H/HeN mice with a high persistence rate of HBV described in this study could provide a new approach to dissect the immunomechanism of HBV clearance or persistence, a new platform to evaluate the antiviral drugs against HBV, and a new model to analyse the different pathogenecities of clinical HBV isolates.
We appreciate Professor Pei-Jer Chen from National Taiwan University College of Medicine, Taiwan, for kindly providing the pAAV/HBV1.2 plasmid; Professor Zheng-Hong Yuan from Fudan University, Shanghai, China for his intensive discussion. We thank the animal facilities in Shanghai Public Clinical Center, Fudan University, China for providing the platform and help for animal experiments.
The research of hepatitis B virus (HBV) infection, either studies on immunological mechanisms or therapeutic drug development, has been hampered by the shortage of suitable small animal models, albeit hydrodynamic injection (HI) of plasmids containing replication-competent HBV genome via the tail vein into immune-competent mice has been a model for HBV studies. However, in the case of classic HI model using C57BL/6 mice, only about 30% of the injected mice carried HBV for more than 12 wk, which limits its applications especially in the studies of chronic hepatitis B (CHB).
In this manuscript the authors injected the pAAV-HBV1.2 plasmid into a different inbred mouse strain, C3H/HeN, and observed that the serum hepatitis B surface and hepatitis B e antigens and viral DNA persisted even up to 46 wk in about 90% of the injected mice, while almost all of the injected C57BL/6 mice, as controls, cleared HBV at 24 wk. The authors also detected HBsAg, HBeAg and HBV DNA expression in the liver tissues of the C3H/HeN mice at 46 wk post HI. Moreover, those mice showed impaired ability to induce HBsAg specific T cells responses, which is an important phenotype of immune tolerance to HBV. Applying IFN-α or entecavir (an analog of guanosine) in this HI model decreased HBV DNA in vivo.
The mouse background can affect the result of the long-term maintenance of HBV in the mouse liver. After HI of the same pAAV/HBV1.2, BALB/c mice showed a rapid clearance of viral DNA template, whereas about 30% of the C57BL/6 mice showed the persistence of HBV (Huang et al). However, the bottleneck of a relative low rate of the persistence of HBV in the C57BL/6 mice compromised its potential strength in chronic HBV infection studies. Thus, efforts were encouraged to find more suitable mouse strains for optimisation of the HBV persistent rate. Indeed, Chen et al reported that a long term maintaining of HBV antigenemia can be detected in FVB/N (H-2q) mice receiving HI of pGEM4Z/HBV1.3, and around 85% (6 in 7) of the mice were positive at 50 wk, compared with rapid clearance of HBV antigenemia in BALB/c mice within 4 wk and in C57BL/6 within 8 wk. This study successfully established HBV persistence in another inbred strain, C3H/HeN mice (H-2k), through HI of pAAV/HBV1.2 plasmid. Around 90% of the injected C3H/HeN mice were HBsAg-positive and the hepatitis B core antigen positive cells in the liver were detected at 46 wk. The data came from an observation in 25 mice/group and could be more solid. In addition, more inbred strains (including H-2k background) available for HI HBV models could expand the possibilities to study the genetic factors on HBV persistence. Chang et al reported an acute HBV hepatitis model by hydrodynamically injecting pHBV3.6 into 8-12-wk-old C3H/HeN mice. The age of C3H/HeN mice might be critical for the different persistence rates between their model and ours. Actually, this study showed 5-6-wk-old C3H/HeN mice are best for persistence of HBV after HI.
The results in this paper suggested that the HI-based HBV infection model using the C3H/HeN mice might provide a more stable platform for mechanistic research of CHB and therapeutic development.
HI means injection with 10 μg endotoxin-free plasmid DNA into the tail vein of mouse, in a volume of PBS equivalent to 10% of the mouse body weight, and the total volume was delivered within 5 s. HI can efficiently deliver DNA into the liver in vivo. The DNA internalization by this hydraulic pressure-based physical transfection is receptor-independent and can achieve delivery to approximately 10%-40% of hepatocytes. This method has been used to establish HBV transfection mouse models, classically in C57BL/6 mice.
The paper showed that HBV persisted longer in C3H/HeN (H-2k) mice after the HI compared with C57BL/6 (H-2b) mice, suggesting that host genetic background determines the rate of HBV clearance. The authors suggested that this could be a novel animal model for CHB infection to elucidate the disease pathogenesis and develop new antiviral treatments. Overall, the study showed a clear association between mouse genetic background and the rate of persistence. However, the authors should elucidate the mechanistic basis for the frequent persistence in the C3H/HeN (H-2k) mice.
P- Reviewer: Li ZF, Osna NA, Watanabe T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 610] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 2. | McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Li X, Liu Y, Zhao P, Wang Y, Chen L, Xin S, Zhang XX, Xu D. Investigation into drug-resistant mutations of HBV from 845 nucleoside/nucleotide analogue-naive Chinese patients with chronic HBV infection. Antivir Ther. 2014;Jul 3; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Férir G, Kaptein S, Neyts J, De Clercq E. Antiviral treatment of chronic hepatitis B virus infections: the past, the present and the future. Rev Med Virol. 2008;18:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, Chisari FV. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci USA. 2010;107:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Tzeng HT, Hsu PN, Chen PJ. Immunocompetent nontransgenic mouse models for studying hepatitis B virus immune responses. J Gastroenterol Hepatol. 2013;28 Suppl 1:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, Amalfitano A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol. 2007;81:1796-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103:17862-17867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1387] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 10. | Zhang G, Ludtke JJ, Thioudellet C, Kleinpeter P, Antoniou M, Herweijer H, Braun S, Wolff JA. Intraarterial delivery of naked plasmid DNA expressing full-length mouse dystrophin in the mdx mouse model of duchenne muscular dystrophy. Hum Gene Ther. 2004;15:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | von Freyend MJ, Untergasser A, Arzberger S, Oberwinkler H, Drebber U, Schirmacher P, Protzer U. Sequential control of hepatitis B virus in a mouse model of acute, self-resolving hepatitis B. J Viral Hepat. 2011;18:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Loggi E, Gamal N, Bihl F, Bernardi M, Andreone P. Adaptive response in hepatitis B virus infection. J Viral Hepat. 2014;21:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1598] [Article Influence: 122.9] [Reference Citation Analysis (1)] |
| 14. | Bukh J. Animal models for the study of hepatitis C virus infection and related liver disease. Gastroenterology. 2012;142:1279-1287.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Mason WS, Seal G, Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980;36:829-836. [PubMed] |
| 16. | Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533-4537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 424] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 913] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 18. | Chisari FV. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr Top Microbiol Immunol. 1996;206:149-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Huang LR, Gäbel YA, Graf S, Arzberger S, Kurts C, Heikenwalder M, Knolle PA, Protzer U. Transfer of HBV genomes using low doses of adenovirus vectors leads to persistent infection in immune competent mice. Gastroenterology. 2012;142:1447-50.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Huang YH, Fang CC, Tsuneyama K, Chou HY, Pan WY, Shih YM, Wu PY, Chen Y, Leung PS, Gershwin ME. A murine model of hepatitis B-associated hepatocellular carcinoma generated by adeno-associated virus-mediated gene delivery. Int J Oncol. 2011;39:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Herweijer H, Wolff JA. Gene therapy progress and prospects: hydrodynamic gene delivery. Gene Ther. 2007;14:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci USA. 2002;99:13825-13830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 324] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Chen SH, Wu HL, Kao JH, Hwang LH. Persistent hepatitis B viral replication in a FVB/N mouse model: impact of host and viral factors. PLoS One. 2012;7:e36984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Chang WW, Su IJ, Lai MD, Chang WT, Huang W, Lei HY. Toll-like receptor 4 plays an anti-HBV role in a murine model of acute hepatitis B virus expression. World J Gastroenterol. 2005;11:6631-6637. [PubMed] |