Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18397
Revised: October 19, 2014
Accepted: November 7, 2014
Published online: December 28, 2014
Processing time: 119 Days and 10.5 Hours
AIM: To evaluate the prognostic factors and tumor stages of the 7th edition TNM classification for esophageal cancer.
METHODS: In total, 1033 patients with esophageal squamous cell carcinoma (ESCC) who underwent surgical resection with or without (neo)adjuvant therapy between January 2003 and June 2012 at the Thoracic Surgery Department II of the Beijing Cancer Hospital, Beijing, China were included in this study. The following eligibility criteria were applied: (1) squamous cell carcinoma of the esophagus or gastroesophageal junction identified by histopathological examination; (2) treatment with esophagectomy plus lymphadenectomy with curative intent; and (3) complete pathologic reports and follow-up data. Patients who underwent non-curative (R1) resection and patients who died in hospital were excluded. Patients who received (neo)adjuvant therapy were also included in this analysis. All patients were restaged using the 7th edition of the Union for International Cancer Control and the American Joint Committee on Cancer TNM staging systems. Univariate and multivariate analyses were performed to identify the prognostic factors for survival. Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used to evaluate differences between the subgroups.
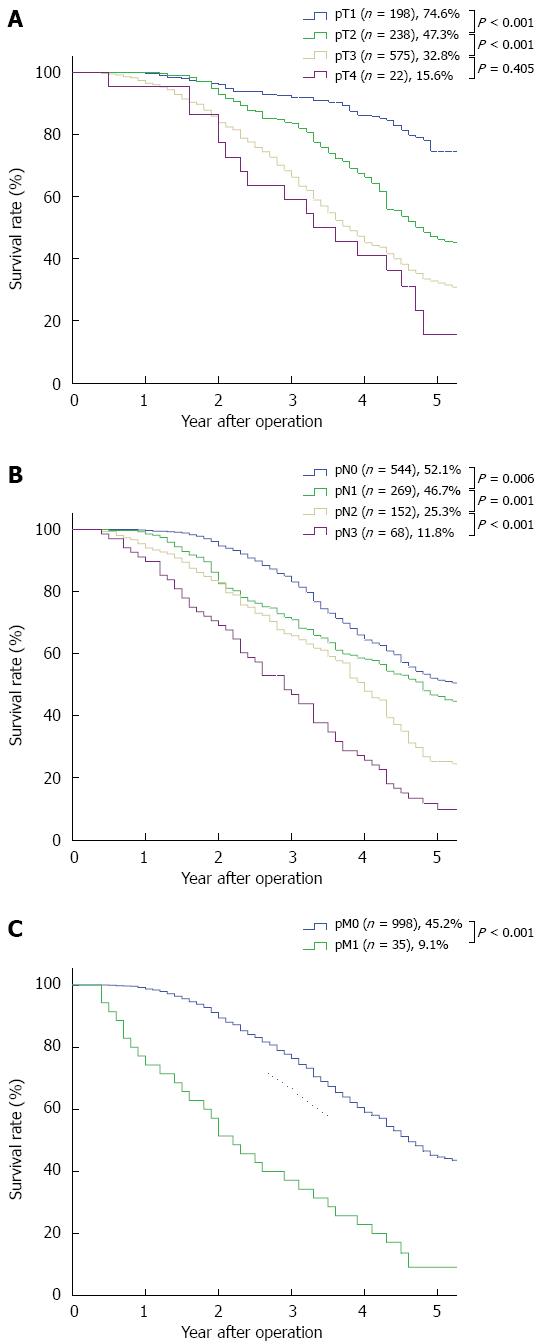
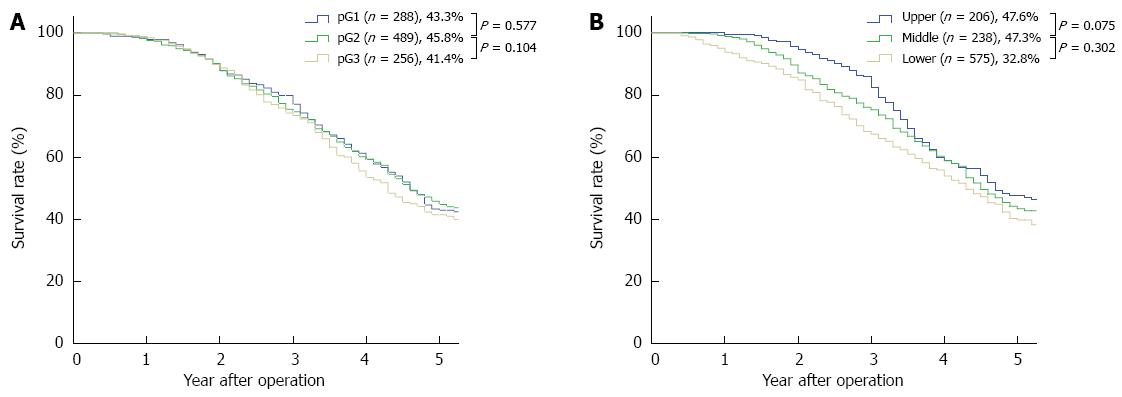
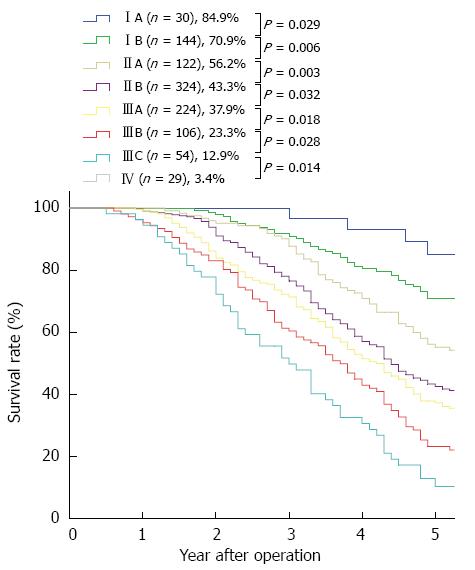
RESULTS: Of the 1033 patients, 273 patients received (neo)adjuvant therapy, and 760 patients were treated with surgery alone. The median follow-up time was 51.6 mo (range: 5-112 mo) and the overall 5-year survival rate was 36.4%. Gender, “pT” and “pN” descriptors, (neo)adjuvant therapy, and the 7th edition TNM stage grouping were independent prognostic factors in the univariate and multivariate analyses. However, neither histologic grade nor cancer location were independent prognostic factors in the univariate and multivariate analyses. The 5-year stage-based survival rates were as follows: IA, 84.9%; IB, 70.9%; IIA, 56.2%; IIB, 43.3%; IIIA, 37.9%; IIIB, 23.3%; IIIC,12.9% and IV, 3.4%. There were significant differences between each adjacent staging classification. Moreover, there were significant differences between each adjacent pN and pM subgroup. According to the pT descriptor, there were significant differences between each adjacent subgroup except between pT3 and pT4 (P = 0.405). However, there was no significant difference between each adjacent histologic grade subgroup and between each adjacent cancer location subgroup.
CONCLUSION: The 7th edition is considered to be valid for patients with resected ESCC. However, the histologic grade and cancer location were not prognostic factors for ESCC.
Core tip: The 7th edition of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging system for esophageal and gastroesophageal junction (GEJ) cancer is the first data-driven staging system for esophageal and GEJ cancers. It is based on the Worldwide Esophageal Cancer Collaboration database, which includes 4627 patients from a large multi-institutional collaboration involving 13 institutions and a data period ranging from the 1970s to the 2000s. Therefore, the surgical procedures, pathologic examinations, and patient follow-up can vary greatly between different institutions, resulting in inevitable bias. In this retrospective study, we used a large cohort of patients who had undergone potentially curative surgery for ESCC at a single institution and confirmed the predictive ability of the 7th edition of the UICC-AJCC TNM staging system.
- Citation: Wang J, Wu N, Zheng QF, Yan S, Lv C, Li SL, Yang Y. Evaluation of the 7th edition of the TNM classification in patients with resected esophageal squamous cell carcinoma. World J Gastroenterol 2014; 20(48): 18397-18403
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18397
The present (7th) edition of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging system for squamous cell carcinoma (ESCC) was released in late 2009. This edition adopted new factors associated with survival, including the number of cancer-positive lymph nodes, histopathologic cell type, histologic grade, and cancer location. The main modifications in the 7th edition are as follows: (1) T1 is subclassified into T1a (lamina propria or muscularis mucosae) and T1b (submucosa), whereas T4 is subclassified into T4a (pleura, pericardium, diaphragm, or adjacent peritoneum) and T4b (other adjacent structures, e.g., aorta, vertebral body, trachea); (2) N is subclassified according to the number of regional lymph nodes involved (N1, 1 to 2 nodes; N2, 3 to 6 nodes; and N3, ≥ 7 nodes); (3) two new prognostic factors (histologic grade and cancer location) are incorporated; and (4) the stage groups have been adjusted, and separate stage groups are used for squamous cell carcinoma and adenocarcinoma[1,2].
The large database upon which the 7th edition is based was created by 13 institutions on 3 continents, with the data period ranging from the 1970s to the 2000s; therefore, the surgical procedures, pathologic examinations, and patient follow-up can vary greatly between different institutions, resulting in inevitable bias[3]. In this retrospective study, we aimed to use a large cohort of patients who had undergone potentially curative surgery, with or without (neo)adjuvant therapy for ESCC, at a single institution to evaluate the predictive ability of the 7th edition of the UICC-AJCC TNM staging system.
The Institutional Review Board at Peking University Cancer Hospital approved this retrospective study, and the requirement for patient consent was waived. All patients who underwent esophagectomy from January 2003 to June 2012 at the Thoracic Surgery Department II of the Beijing Cancer Hospital, Beijing, China, were reviewed (n = 1086). The following eligibility criteria were applied: (1) squamous cell carcinoma of the esophagus or gastroesophageal junction identified by histopathological examination; (2) treatment with esophagectomy plus lymphadenectomy with curative intent; and (3) complete pathologic reports and follow-up data. Patients who underwent non-curative (R1) resections and patients who died in the hospital were excluded. Patients who received neo(adjuvant) therapy were also included in this analysis. Physical examinations, laboratory tests, esophagogastroduodenoscopy, barium esophagography, computed tomography scans from the neck to the upper abdomen, and abdominal and supraclavicular ultrasound scans were routinely performed to preoperatively evaluate the extent of disease. The surgical procedures used a left or right thoracic approach.
Generally, the patients were postoperatively examined at 3-mo intervals for 2 years, 6-mo intervals for an additional 3 years, and 1-year intervals thereafter to monitor disease recurrence and survival. The survival intervals (overall survival) were calculated from the date of the operation to the date of death or last follow-up.
All patients were restaged using the 7th edition of the UICC-AJCC TNM staging system. Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used to evaluate differences between the subgroups. Univariate and multivariate Cox proportional hazard models were used to evaluate the impact of each factor on overall survival. P values for differences were calculated, with a significance level of P < 0.05. SPSS software version 16.0 (SPSS Inc., Chicago, IL, United States) was used for all analyses.
Of the 1033 patients included in the study, 273 patients received (neo)adjuvant therapy, and 760 were treated with surgery alone. Forty three patients received neoadjuvant chemotherapy and/or neoadjuvant radiotherapy, 255 patients received adjuvant chemotherapy and/or adjuvant radiotherapy, and 25 patients received both neoadjuvant and adjuvant chemotherapy and/or radiotherapy. The patient characteristics and 5-year survival rates are summarized in Table 1. The median follow-up time was 51.6 mo (range: 5-112 mo). The overall 5-year survival rate was 36.4%.
| Characteristic | Value | 5-yr survival |
| Gender Male Female | 793 (76.8) 240 (23.2) | 43.1%47.2% |
| Age, yr Median (range) ≤ 60 > 60 | 63 (27-81) 688 (66.6)345 (33.4) | 44.6%42.9% |
| Cigarette smoking Yes No | 544 (52.7) 489 (47.3) | 42.7%45.5% |
| Alcohol consumption Yes No | 272 (26.3)761 (73.7) | 43.6%44.1% |
| pT 1 2 3 4 | 198 (19.2) 238 (23.0)575 (55.7)22(2.1) | 74.6%47.3%32.8%15.6% |
| pN 0 1 2 3 | 544 (52.7)269 (26.0)152 (14.7)68 (6.6) | 52.1%46.7%25.3%11.8% |
| pM 0 1 | 998 (96.6)35 (3.4) | 45.2%9.1% |
| Histologic grade Well differentiated (G1) Moderately differentiated (G2) Poorly differentiated (G3) | 288 (27.9)489 (47.3)256 (24.8) | 43.3%45.8%41.4% |
| Cancer location Upper thoracic Middle thoracic Lower thoracic + GEJ | 206 (19.3) 603 (58.6)224 (22.2) | 47.6%44.2%40.3% |
| Type of surgical approach Left thoracic Right thoracic | 126 (12.2)907 (87.8) | 40.4%44.5% |
| Length of tumorMean (range) ≤ Mean > Mean | 3.4 cm (0.4-15.0 cm)689 (66.7)344 (33.3) | 44.1%43.9% |
| Stage IA IB IIA IIB IIIA IIIB IIIC IV | 30 (2.9)144 (13.9)122 (11.8)324 (31.4)224 (21.7)106 (10.3)54 (5.2)29 (2.8) | 84.9%70.9%56.2%43.3%37.9%23.3%12.9%3.4% |
| (neo)adjuvant therapy No Yes | 760 (73.6)273 (26.4) | 49.8%30.3% |
The 5-year survival rates and the survival curves by pT, pN and pM are shown in Figure 1. With respected to the pN and pM descriptors, there were significant differences between each adjacent subgroup. According to the pT descriptor, there were significant differences between each adjacent subgroup, except between pT3 and pT4 (P = 0.405). The 5-year survival rates and the survival curves by the new incorporated descriptors in the 7th edition, pG (histologic grade) and cancer location are shown in Figure 2. According to the histologic grade and cancer location, there was no significant difference between each adjacent subgroup (with regard to histologic grade, between G1 and G2, P = 0.577, between G2 and G3, P = 0.104; with respect to cancer location, between upper and middle, P = 0.075, between middle and lower, P = 0.302). The 5-year survival rates and the survival curves by pStage grouping according to the 7th edition of the TNM classification are shown in Figure 3, and there were significant differences between each adjacent subgroup.
Univariate analysis revealed that gender (P = 0.046), age (P = 0.039), cigarette smoking (P = 0.027), (neo)adjuvant therapy (P < 0.001), the “pT” (P < 0.001), “pN” (P < 0.001) and “pM” (P < 0.001) descriptors, and the 7th edition TNM stage grouping significantly affected patient survival, but neither histologic grade nor cancer location showed any significance in terms of survival (P = 0.130 and P = 0.067, respectively). The multivariate Cox regression model was performed by incorporating gender, age, cigarette smoking, (neo)adjuvant therapy, the “pT”, “pN”, “pM” descriptors, histologic grade, cancer location, and the 7th edition TNM stage grouping. Gender, “pT” and “pN” descriptors, (neo)adjuvant therapy, and the 7th edition TNM stage grouping remained as independent prognostic factors. However, cigarette smoking and the “pM” descriptor, which were significant prognostic factors in the univariate analysis, did not significantly influence patient survival in the multivariate analysis (Table 2).
| Univariate | Multivariate | |||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Gender Male Female | 1.0000.829 | 0.690-0.996 | 0.046 | 1.0001.333 | 1.091-1.629 | 0.005 |
| Age, yr ≤ 60 > 60 | 1.0001.183 | 1.009-1.390 | 0.039 | 1.0000.999 | 0.840-1.190 | 0.995 |
| Cigarette smoking No Yes | 1.0001.190 | 1.019-1.388 | 0.027 | 1.0000.949 | 0.805-1.119 | 0.537 |
| Alcohol consumption No Yes | 1.0001.070 | 0.903-1.268 | 0.431 | |||
| (neo) adjuvant therapy No Yes | 1.0001.634 | 1.386-1.927 | < 0.001 | 1.0001.330 | 1.102-1.606 | 0.003 |
| pT 1 2 3 4 | 1.0002.4874.1865.154 | 1.856-3.3313.219-5.4443.015-8.812 | < 0.001< 0.001< 0.001 | 1.0001.9822.3031.150 | 1.254-3.1321.437-3.6930.518-2.549 | 0.0030.0010.732 |
| pN 0 1 2 3 | 1.0001.2941.9393.500 | 1.073-1.5601.567-2.3992.650-4.624 | 0.007< 0.001< 0.001 | 1.0000.3610.2900.122 | 0.242-0.5380.145-0.5800.047-0.314 | < 0.001< 0.001< 0.001 |
| pM 0 1 | 1.0003.392 | 2.359-4.875 | < 0.001 | 1.0000.789 | 0.231-2.689 | 0.704 |
| Histologic grade G1 G2 G3 | 1.0001.0541.227 | 0.875-1.2710.995-1.513 | 0.5780.056 | 1.0000.9170.874 | 0.724-1.1610.681-1.123 | 0.4720.292 |
| Cancer location Upper thoracic Middle thoracic Lower thoracic + GEJ | 1.0001.2011.326 | 0.977-1.4761.043-1.685 | 1.2011.326 | 1.0000.8081.027 | 0.634-1.0310.758-1.392 | 0.0860.862 |
| Type of surgical approach Right thoracic Left thoracic | 1.0001.149 | 0.912-1.448 | 0.238 | |||
| Length of tumor ≤ Mean > Mean | 1.0001.075 | 0.916-1.262 | 0.374 | |||
Table 3 presents the univariate and multivariate analyses for the 7th edition TNM stage grouping. The multivariate analyses revealed significant differences between each adjacent pStage, except for pStageIB and IIA (P = 0.129). The hazard ratios for stages IB and IIA disease (referred to as pStage IA disease) were 3.013 and 2.368, respectively.
| Univariate | Multivariate | |||||
| HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Stage | ||||||
| IA | 1.000 | 1.000 | ||||
| IB | 2.919 | 1.059-8.048 | 0.038 | 3.013 | 1.065-8.530 | 0.038 |
| IIA | 4.771 | 1.737-13.103 | 0.002 | 2.368 | 0.779-7.198 | 0.129 |
| IIB | 7.251 | 2.695-19.508 | < 0.001 | 6.472 | 2.083-20.108 | 0.001 |
| IIIA | 9.062 | 3.359-24.450 | < 0.001 | 15.152 | 4.282-53.616 | < 0.001 |
| IIIB | 12.314 | 4.514-33.591 | < 0.001 | 23.933 | 4.960-88.354 | < 0.001 |
| IIIC | 18.288 | 6.583-50.804 | < 0.001 | 89.566 | 18.855-425.471 | < 0.001 |
| IV | 33.720 | 11.799-96.369 | < 0.001 | 147.116 | 21.759-994.681 | < 0.001 |
In Asia, ESCC is one of the most common and aggressive diseases[4]. A robust staging system is important for ESCC patients in terms of planning treatment, estimating prognosis, evaluating the end results of therapy, and providing a standardized nomenclature to facilitate information exchange between treatment centers.
The 7th edition supports the continued use of the accepted T major stages (I, II, III, and IV) in the 6th edition[1]. In our series, which included few pT4 patients, the high prognostic value of the pT1-pT3 subclassification was confirmed. However, the survival of pT4 patients was similar to that of pT3 patients, likely because of the small number of pT4 patients in our study.
Based on the evidence of survival differences, T1 is subclassified as T1a (tumor limited in the mucosa) and T1b (tumor in the submucosa), and T4 is subclassified as T4a (tumor invading adjacent, resectable structures) and T4b (tumor invading adjacent, unresectable structures) in the 7th edition. Rice et al[5] and Wijnhoven et al[6] reported that T1a patients had a higher survival rate than T1b patients. Moreover, Sepesi et al[7] observed differences in the lymph node metastasis rate between T1a and T1b patients. Because treatment strategies for esophageal cancer are based on a precise staging system, some of these changes to the descriptors may influence therapeutic decision-making[8].
The refinement of the N classification to N0-N3, which is based on the number of positive regional lymph nodes, is one of the major modifications of the 7th edition[1]. Many investigators have reported that subdividing the “N” classification based on the absolute number of involved lymph nodes may yield better survival stratifications[9,10]. In our analyses of patient prognoses using the pN classification, there were clear differences between each adjacent pN subgroup, which significantly confirmed the high prognostic value of the pN subclassification in the 7th edition.
The M descriptor is a prognostic factor in univariate factor Cox analyses, but not in multivariate factor Cox analyses. Because few patients in our study had distant metastases, we cannot exclude the possibility that the discrepancy is caused by the small pM1 sample size.
Male gender, advanced age, cigarette smoking, and (neo)adjuvant therapy have been shown to be associated with poor survival[11-16]. In our series, gender and (neo)adjuvant therapy significantly influenced patient survival, as revealed by univariate and multivariate analyses. However, age and cigarette smoking were shown to be prognostic factors in the univariate factor Cox analyses, but not in the multivariate factor Cox analyses, which suggests that age and cigarette smoking were not strong prognostic factors in ESCC patients.
Another major modification of the 7th edition is the incorporation of 2 new descriptors: histologic grade and cancer location[1]. Along with T, N, and M descriptors, these new descriptors help to subclassify T2-3N0M0 patients into stages IB, IIA, and IIB. In the 7th edition, patient survival improves as the histologic grade changes from G3 to G1 and/or the cancer location moves from the upper esophagus to the lower esophagus[1,2]. However, according to our analysis, histologic grade and cancer location are not independent prognostic factors. In fact, the roles of histologic grade and cancer location in ESCC patient survival were inconsistent among different studies. Although the histologic grade had been shown to be a strong predictor of survival in perijunctional esophagogastric carcinoma[17], Wijnhoven et al[6] (2007) and Eloubeidi et al[13] (2002) reported that histologic grade was not significantly related to survival. Moreover, many investigators have reported that the histologic grade is not a prognostic factor in ESCC[11,14,18,19].
The results concerning cancer location are also controversial. Some investigators have found that survival improves as the tumor moves distally in the esophagus[20]; in contrast, other researchers have demonstrated that survival is worse in patients whose tumors are located in the lower thoracic portions of the esophagus[13]. Still other investigators have reported that the upper, middle, and lower locations of thoracic esophageal cancer yield similar survival rates[21]. Situ et al[14] explained that one possible reason for this discrepancy is a variation in the experience of the pathologists and endoscopic laboratory doctors from various institutes, or the different definitions of tumor segmentation in the 6th edition of the UICC-AJCC cancer staging manual, as the 7th staging system had not yet been published. In addition to the above reasons, we believe that differences in the biological behavior of ESCC and esophageal adenocarcinoma might also be responsible for the inconsistent results.
In conclusion, the survival characteristics of 1033 patients with resected ESCC in a single institution validated the 7th edition of the UICC-AJCC TNM staging system for esophageal cancer. This edition may clearly differentiate the survival rates between patients in different stages; furthermore, the revised N classification provides prognostic power, and the refinement of the T group may be referenced when determining treatment options. In addition, we demonstrated that gender and (neo)adjuvant therapy are important prognostic factors for patient survival. However, we found that histologic grade and cancer location are not important prognostic factors for ESCC patient survival.
Esophageal squamous cell carcinoma (ESCC) is one of the most common and aggressive diseases in Asia. The present (7th) edition of the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM staging system for esophageal cancer was released in late 2009. This edition adopted new factors associated with survival, including the number of cancer-positive lymph nodes, histopathologic cell type, histologic grade and cancer location.
There is controversy whether histologic grade and cancer location should be incorporated in the new staging system of esophageal cancer, and some scholars suggested the adoption of some other new descriptors into the staging system, such as (neo)adjuvant therapy.
Our research is the first retrospective study to use a large cohort of patients with ESCC at a single institution to evaluate the predictive ability of the 7th edition UICC-AJCC TNM staging system for esophageal and gastroesophageal junction cancer.
The results validated the 7th edition staging system for esophageal cancer. Moreover, the authors demonstrated that gender and (neo)adjuvant therapy are important prognostic factors for patient survival. However, the authors found that histologic grade and cancer location are not important prognostic factors for ESCC patient survival.
Squamous cell carcinoma is the main histopathologic cell type in esophageal cancer in the East, accounting for more than 90% esophageal cancers. Many reports have showed that there are marked differences between ESCC and esophageal adenocarcinoma.
The manuscript is an interesting study, which validated the 7th edition of the TNM classification in patients with this disease.
P- Reviewer: De Silva AP, Garcia-Compean D S- Editor: Qi Y L- Editor: Cant MR E- Editor: Zhang DN
| 1. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, TrottiA . American Joint Committee on Cancer (AJCC) cancerstaging manual. 7th ed. Chicago: Springer 2010; . |
| 2. | Rusch VW, Rice TW, Crowley J, Blackstone EH, Rami-Porta R, Goldstraw P. The seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals: the new era of data-driven revisions. J Thorac Cardiovasc Surg. 2010;139:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 4. | Lin CS, Chang SC, Wei YH, Chou TY, Wu YC, Lin HC, Wang LS, Hsu WH. Prognostic variables in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2009;87:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Rice TW, Blackstone EH, Rybicki LA, Adelstein DJ, Murthy SC, DeCamp MM, Goldblum JR. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg. 2007;245:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Sepesi B, Watson TJ, Zhou D, Polomsky M, Litle VR, Jones CE, Raymond DP, Hu R, Qiu X, Peters JH. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg. 2010;210:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Strong VE, D’Amico TA, Kleinberg L, Ajani J. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw. 2013;11:60-66. [PubMed] |
| 9. | Rizk N, Venkatraman E, Park B, Flores R, Bains MS, Rusch V. The prognostic importance of the number of involved lymph nodes in esophageal cancer: implications for revisions of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg. 2006;132:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Bollschweiler E, Baldus SE, Schröder W, Schneider PM, Hölscher AH. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol. 2006;94:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Hsu PK, Wu YC, Chou TY, Huang CS, Hsu WH. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Elsayed H, Whittle I, McShane J, Howes N, Hartley M, Shackcloth M, Page R. The influence of age on mortality and survival in patients undergoing oesophagogastrectomies. A seven-year experience in a tertiary centre. Interact Cardiovasc Thorac Surg. 2010;11:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Situ D, Wang J, Lin P, Long H, Zhang L, Rong T, Ma G. Do tumor location and grade affect survival in pT2N0M0 esophageal squamous cell carcinoma? J Thorac Cardiovasc Surg. 2013;146:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lee CH, Lee JM, Wu DC, Hsu HK, Kao EL, Huang HL, Wang TN, Huang MC, Wu MT. Independent and combined effects of alcohol intake, tobacco smoking and betel quid chewing on the risk of esophageal cancer in Taiwan. Int J Cancer. 2005;113:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Pandeya N, Olsen CM, Whiteman DC. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiol. 2013;37:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Dickson GH, Singh KK, Escofet X, Kelley K. Validation of a modified GTNM classification in peri-junctional oesophago-gastric carcinoma and its use as a prognostic indicator. Eur J Surg Oncol. 2001;27:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg. 1994;81:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 196] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Nomura M, Shitara K, Kodaira T, Hatooka S, Mizota A, Kondoh C, Yokota T, Takahari D, Ura T, Muro K. Prognostic impact of the 6th and 7th American Joint Committee on Cancer TNM staging systems on esophageal cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Bogoevski D, Onken F, Koenig A, Kaifi JT, Schurr P, Sauter G, Izbicki JR, Yekebas EF. Is it time for a new TNM classification in esophageal carcinoma? Ann Surg. 2008;247:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Doki Y, Ishikawa O, Takachi K, Miyashiro I, Sasaki Y, Ohigashi H, Murata K, Yamada T, Noura S, Eguchi H. Association of the primary tumor location with the site of tumor recurrence after curative resection of thoracic esophageal carcinoma. World J Surg. 2005;29:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |