Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17699
Revised: August 27, 2014
Accepted: November 18, 2014
Published online: December 21, 2014
Processing time: 206 Days and 9.2 Hours
Toll-like receptors (TLRs) are germ line encoded innate immune sensors that recognize conserved microbial structures and host alarmins, and signal expression of major histocompatibility complex proteins, costimulatory molecules, and inflammatory mediators by macrophages, neutrophils, dendritic cells, and other cell types. These protein receptors are characterized by their ability to respond to invading pathogens promptly by recognizing particular TLR ligands, including flagellin and lipopolysaccharide of bacteria, nucleic acids derived from viruses, and zymosan of fungi. There are 2 major TLR pathways; one is mediated by myeloid differentiation factor 88 (MYD88) adaptor proteins, and the other is independent of MYD88. The MYD88-dependent pathway involves early-phase activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 (NF-κB1) and all the TLRs, except TLR3, have been shown to activate this pathway. TLR3 and TLR4 act via MYD88-independent pathways with delayed activation of NF-κB signaling. TLRs play a vital role in activating immune responses. TLRs have been shown to mediate inflammatory responses and maintain epithelial barrier homeostasis, and are highly likely to be involved in the activation of a number of pathways following cancer therapy. Colorectal cancer (CRC) is one of the most common cancers, and accounts for almost half a million deaths annually worldwide. Inflammation is considered a risk factor for many common malignancies including cancers of the colorectum. The key molecules involved in inflammation-driven carcinogenesis include TLRs. As sensors of cell death and tissue remodeling, TLRs may have a universal role in cancer; stimulation of TLRs to activate the innate immune system has been a legitimate therapeutic strategy for some years. TLRs 3/4/7/8/9 are all validated targets for cancer therapy, and a number of companies are developing agonists and vaccine adjuvants. On the other hand, antagonists may favor inhibition of signaling responsible for autoimmune responses. In this paper, we review TLR signaling in CRC from carcinogenesis to cancer therapy.
Core tip: Toll-like receptors (TLRs) are innate immune sensors which can recognize inflammatory mediators. TLRs have been shown to mediate inflammatory response and maintain epithelial barrier homeostasis. Inflammation is a risk factor for many cancers including colorectal cancer (CRC). The key molecules involved in inflammation-driven carcinogenesis include TLRs. In this paper, we reviewed TLR signaling in CRC from carcinogenesis to cancer therapy.
- Citation: Li TT, Ogino S, Qian ZR. Toll-like receptor signaling in colorectal cancer: Carcinogenesis to cancer therapy. World J Gastroenterol 2014; 20(47): 17699-17708
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17699
Toll-like receptors (TLRs) are a family of evolutionally conserved pattern recognition receptors (PRRs)[1-3]. TLRs are included in the type I transmembrane glycoprotein receptor family with N-terminal ligand-recognition, transmembrane, and intracellular C-terminal signaling domains[4]. Currently, 13 TLRs have been identified in humans and mice, and equivalent forms of many of these have been found in other mammalian species[5]. TLRs recognize a wide range of microbial moieties, and engagement by their respective ligand(s) triggers activation of intracellular signaling cascades leading to the induction of genes involved in antimicrobial host defence, such as those encoding proinflammatory cytokines and chemokines[6,7].
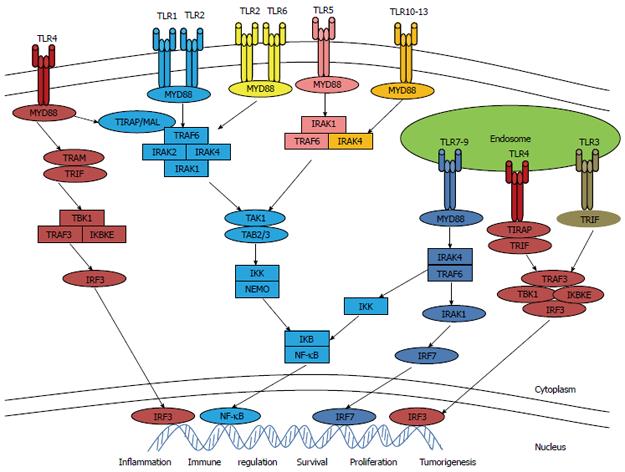
TLR signaling has been investigated extensively in recent years. There are two important TLR pathways: one is dependent on myeloid differentiation factor 88 (MYD88) adaptor proteins and the other is independent of MYD88. All TLRs commonly use MYD88 as the downstream adapter protein except TLR3. After activation with their individual ligands, TLRs recruit MYD88, leading to subsequent activation of downstream factors, including nuclear factor κB (NF-κB), mitogen-associated protein kinase (MAPK), and interferon (IFN) regulatory factors[8,9]. TLR signaling activates transcription factors, and generates cytokines as well as chemokines via intracellular pathways (Figure 1). TLR2 and TLR4 combine with their respective ligands to form dimeric complexes. The configuration is then changed and 5 specific adapters within cells are recruited, including MYD88, TIR domain-containing adaptor protein (TIRAP)/MYD88 adaptor-like (Mal), TIR domain-containing adaptor-inducing IFNβ (TRIF), TRIF-related adaptor molecule (TRAM), as well as sterile α and armadillo motif-containing protein (SARM)[4]. Immune cell expressing TLRs play important roles in immune responses against invading pathogens. TLRs recognize conserved pathogen-associated molecular patterns (PAMPs) expressed on a wide array of microbes, as well as danger-associated molecular patterns (DAMPs) released from stressed or dying cells[10].
TLRs play a major role in microbe-host interactions and innate immunity[11]. TLRs are very important in early innate immune defense mechanisms by activating canonical and non-canonical pathways of inflammation. Because TLRs are primary sensors of PAMPs, DAMPs, and stress signals associated with allergen exposure, genetic variations in the TLR genes may influence the incidence, severity, and outcome of allergic diseases[2]. Several single-nucleotide polymorphisms within the TLR genes are associated with altered susceptibility to infectious, allergic, and inflammatory diseases as well as cancers[2].
More and more evidence suggests that malfunction of TLR signaling contributes significantly to the development of autoimmune connective tissue diseases[3], tuberculosis[9], severe acute pancreatitis[12], necrotizing enterocolitis[13], atherosclerosis[14], alcohol-induced liver disease and non-alcoholic steatohepatitis[15]. TLR signaling plays a role in regulating injury responses of chronically injured precancerous organs and promoting malignant cell survival[16]. The TLR/MYD88 pathway is essential for microbiota-induced development of colitis-associated cancer, and it was demonstrated that the severity of chronic colitis directly correlates with colorectal tumor development and that bacterial-induced inflammation drives progression from adenoma to invasive carcinoma[17]. TLRs and MYD88 signaling have been shown to be associated with hepatic inflammation and hepatomitogen expression which is important for hepatocarcinogenesis, suggesting that a better understanding of TLR signaling pathways may help to clarify the mechanisms of tumorigenesis, and provide new therapeutic targets for hepatocellular carcinoma[15]. Researchers have found that TLR9 initiates a cascade of immune responses: expression of TLR9 promotes angiogenesis and cancer progression, and reduces survival, so an understanding of how TLR9 boosts angiogenesis may help refine the development of anti-cancer agent[18,19]. Table 1 showed TLR functions in disease and cancer.
| TLR | Disease | Function |
| TLRs | Autoimmune disease | Microbe-host interactions and innate immunity |
| TLRs | Infectious disease | Activating canonical and non-canonical pathways of inflammation |
| TLRs | Cancer (CRC, HCC, etc) | Carcinogenesis |
| TLRs | Allergic diseases | Primary sensors of PAMPs, DAMPs and stress signals associated with allergen exposure |
| TLRs | Tuberculosis | Recognition of Mycobacterium tuberculosis |
| TLRs | Systemic inflammatory response syndrome | Development of syndrome |
| TLR2/TLR4 | Atherosclerosis | Development of disease |
| TLR4 | NEC | Development of intestinal barrier failure |
| TLR4 | Alcohol-induced liver injury | Activation of Kupffer cells |
| TLR9 | NASH | Development of disease |
| TLR9 | Cancer | Angiogenesis |
As the evidence for the involvement of TLRs in multiple immune diseases has increased, more and more research has shown that TLRs could be a therapeutic target for inflammatory diseases. TLR2 could be a useful therapeutic target for the development of antagonists given the range of diseases that are associated with this receptor[20]. The humanized version of OPN-305 entered phase I clinical trials for the treatment of inflammatory autoimmune diseases[20-22]. Small synthetic compounds, acting as TLR3 agonists and/or TLR2/TLR4, TLR7/9 and MYD88 antagonists may favor the inhibition of signaling responsible for autoimmune responses in multiple sclerosis and experimental autoimmune encephalitis[20].
Various TLR agonists have been considered for multiple clinical applications, including cancer immunotherapy, and the TLR7 agonist imiquimod is approved for topical therapy of basal cell carcinoma[23]. Most TLR-targeted therapeutics are intercellular nucleic acid-derived immunoregulatory sequences. such as TLR3, TLR7, TLR8, and TLR9. Agents can also target cell surface TLRs, including TLR2 and TLR4. These therapeutics may be used in oncology, immune disease, and infectious disease[1].
Activation of the TLR4 pathway may cause chronic inflammation and increase production of reactive oxygen and nitrogen species (ROS/RNS), leading to oxidative and nitrosative stress and TLR-related diseases. This implies that drugs or substances that modify these pathways may prevent or improve TLR-related diseases, for example, anti-lipopolysaccharide (LPS) strategies, aim to neutralize LPS and TLR4/MYD88 antagonists, including eritoran, CyP, EM-163, epigallocatechin-3-gallate, 6-shogaol, cinnamon extract, N-acetylcysteine, melatonin, and molecular hydrogen[24]. Rajput et al[25] correlated TLR4 expression with resistance to paclitaxel in either depleted or overexpressed TLR4 protein breast cancer cell lines and found that paclitaxel not only killed tumor cells but also enhanced their survival by activating the TLR4 pathway, suggesting that blocking TLR4 could significantly improve the response to paclitaxel therapy. TLR4 is critical for the airway inflammatory response, and agents targeting TLRs are being actively pursued as novel therapies for the treatment of airway diseases such as asthma[26]. Synthetic oligodeoxynucleotide-expressing CpG motifs (CpG-ODN) are TLR9 agonists that can enhance the antitumor activity of DNA-damaging chemotherapy and radiation therapy in preclinical mouse models, and findings provide evidence that the tumor microenvironment can sensitize cancer cells to DNA-damaging chemotherapy, thereby expanding the benefits of CpG-ODN therapy beyond induction of a strong immune response[27]. TLR9 agonists can exert antitumor effects by blocking angiogenesis; it is likely that TLR-induced IFNs play an important role as IFNα is well known to suppress tumor angiogenesis[16].
Colorectal cancer (CRC) is the fourth leading cause of cancer-related death in the world and the third leading cause in the United States[28]. Initiation and progression of malignancies is the result of a series of complex processes that depend upon multiple interactive factors[29]. There are 3 distinct molecular mutagenic pathways, including chromosomal, microsatellite instability, and epigenetic pathway in colon carcinogenesis[11,22].
Inflammation is considered a risk factor for many common malignancies including CRC[29,30]. The key molecules involved in inflammation-driven carcinogenesis include TLRs, NF-κB signaling, pro- and anti-inflammatory cytokines, growth factors, kinase tumor suppressor proteins, cyclooxygenases, and nitric oxide synthases[31]. Pimentel-Nunes et al[32] found persistently positive TLR expression and lower expression of TLR inhibitors associated with higher TLR protein levels throughout the spectrum of lesions of colon carcinogenesis[22]. TLR3 may indicate the tendency of normal tissue to form adenoma or CRC[17].
TLR2: TLR2 is encoded by a DNA sequence that codes 784 amino acids[9]. This type I transmembrane receptor is composed of an extracellular leucine-rich domain, a single transmembrane domain, and a cytoplasmic domain[9]. Colon carcinogenesis is associated with increased expression levels of TLR2 and TLR4. Functional TLR2 and TLR4 polymorphisms significantly alter the risk of CRC. Smoking and obesity may influence the risk of CRC along with these genetic profiles[33]. TLR2 is unique in its requirement to form heterodimers with TLR1 or TLR6 for the initiation of signaling and cellular activation[11]. Tumor cells from TLR2 knockout mice showed less cell death and suppressed senescence[16]. Nihon-Yanagi et al[34] suggested that TLR2 activation may also be involved in sporadic colon carcinogenesis in humans.
In CRC , the role of TLR2 is still controversial. One study showed that there were no differences between wild-type and TLR2-deficient mice in CRC[16,35]. However, another study showed increased tumor development and higher interleukin (IL) 6, IL17A and phospho-signal transducer and activator of transcription 3 (STAT3) levels in CRC in TLR2-deficient mice[16,36]. In colitis, TLR2 plays a protective role against the development of colitis-associated cancer[36]. TLR2 plays a key role in Gram-positive bacterial, mycobacterial, fungal, and spirochetal cell wall component recognition, while TLR4 seems to be a key receptor of the Gram-negative component LPS; both TLR2 and TLR4 in cancer patients are implicated in carcinogenesis and antitumor treatment; the lower stress response in laparoscopic colectomy vs open colectomy provides an impetus to investigate the long-term results of laparoscopic colectomy vs open colectomy for CRC[37]. Some papers also showed that TLR2 and TLR4 were both associated with survival after diagnosis of colon cancer, but not rectal cancer[38].
TLR4: TLR4 is composed of 839 amino acids. It is activated by bacterial LPS as well as lipotechoic acid[9]. TLR4 is expressed on human colon cancer cells and is functionally active. It is important in promoting immune escape of human colon cancer cells by inducing immunosuppressive factors as well as apoptosis resistance[31]. The TLR4 signaling pathway has oncogenic effects both in vitro and in vivo. The increased individual expression of TLR4 and IL6 is a common feature of CRCs and is associated with poor prognosis[39-41]. To demonstrate the role of TLR4 signaling in colon tumorigenesis, Wang et al[39] examined the expression of TLR4 and MYD88 in CRC, and suggested that high expression of TLR4 and MYD88 is associated with liver metastasis, and is an independent predictor of poor prognosis in patients with CRC. Their findings also suggest that TLR4/MYD88 signaling contributes to CRC tumorigenesis not only in colitis-associated cancer but also in sporadic CRC[39]. Other studies also showed that TLR4 signaling activates NF-κB through the MYD88 pathway, leading to transcription of pro-inflammatory cytokines as well as many important components of the inflammatory response[42].
TLR4 is overexpressed in mouse and human inflammation-associated CRC, and TLR4-deficient mice are strongly protected against colon carcinogenesis, suggesting that TLR expression on tumor cells promotes tumor progression directly or indirectly[8]. TLR4 expression by stromal fibroblasts is associated with poor prognosis in CRC[43]. The TLR4 variant D299G induces neoplastic progression in Caco-2 intestinal cells and is associated with advanced human colon cancer, implying a novel link between colonic carcinogenesis and aberrant innate immunity[44]. Single TLR4, LY96 (MD-2), and CXC chemokine receptor 7 (CXCR7) expression levels are significantly correlated with human CRC TNM stage, advanced histological grade, tumor size, and lymph node metastasis; furthermore, concomitant expression of TLR4, LY96 and CXCR7 has been shown to be associated with increased potential for carcinoma growth and metastasis in human CRC[29]. Cammarota et al[45] found that adenocarcinoma patients (pT1-4) with higher TLR4 expression in the stromal compartment had a significantly increased risk of disease progression, and high TLR4 expression in the tumor microenvironment represents a possible marker of disease progression in colon cancer. Nox enzymes are major sources of endogenous ROS generation in response to inflammatory mediators, including cytokines, growth factors, and hypoxic conditions, all of which are elevated in response to surgical trauma[42,46,47]. It was shown that the LPS-Nox1 redox signaling axis plays a crucial role in facilitation of colon cancer cell adhesion, thus increasing the potential for colon cancer cell metastasis. Nox1 may represent a valuable target to prevent colon cancer metastasis[42].
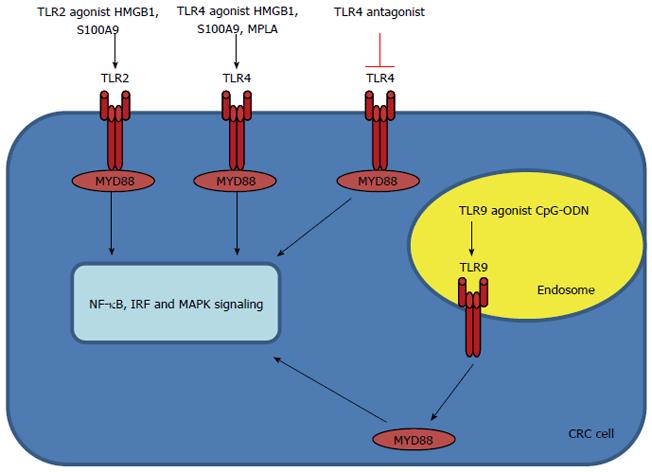
TLR9: TLR9 recognizes unmethylated CpG motifs in bacterial DNA[9]. TLR9 is expressed mainly in intracellular vesicles such as the endoplasmic reticulum, lysosomes, endosomes and endolysosomes, where they recognize microbial nucleic acids[1]. TLR9 recognizes DNA derived from both DNA bacteria and viruses[1,48]. Several studies have shown that TLR9 engagement on CD4 T cells can enhance their survival and therefore, could potentiate antitumor responses by prolonging T cell survival[10,49]. The role of TLR9 signaling in colonic carcinogenesis remains unclear. It was recently reported that oligodeoxynucleotides targeting TLR9 have opposite effects in modulating DNA repair genes in tumor cells vs immune cells, and enhance the biologic effects of chemotherapy. TLR9 expression was decreased in hyperplastic and villous polyps from patients who developed CRC, suggesting a possible protective role of TLR9 expression against malignant transformation in the colorectal mucosa[50]. Table 2 and Figure 2 show the TLRs involved in CRC.
| TLR | Carcinogenesis | Prognosis | Treatment |
| TLR2 | Controversial role in mouse model; protective against development of CRC in colitis | Associated with survival after diagnosis of colon cancer | HMGB1, S100A9 |
| TLR4 | Oncogenic effects in vitro and in vivo | Poor progression | HMGB1, S100A9, MPLA |
| TLR9 | Remain unclear; possible protection against malignant transformation in colorectal mucosa | CpG-ODN |
It was reported that high expression of the TLR4/ MYD88 signal was correlated with poor prognosis of CRC[51]. In the tumor microenvironment, high TLR4 expression represents a possible marker of disease progression in colon cancer[45]. TLR4 expression in stromal fibroblasts is associated with poor prognosis in CRC, therefore, TLR4 expression in fibroblasts could be a useful prognostic marker in CRC[43]. It has been documented that the deregulated activation of STAT3 and NF-κB is a common feature of gastrointestinal cancers and invariably correlates with poor prognosis; NF-κB and STAT3 are key downstream signal transducers of the TLR families and IL-6 cytokine, respectively; the molecular mechanisms are associated with cross-talk between the IL-6 cytokine family/STAT3 signaling network and the TLR family/NF-κB signaling network, and there is potential benefit in their therapeutic targeting in colorectal and gastric cancers[7]. Genetic variations in TLR2, TLR3 and TLR4 may influence colon cancer development as well as survival after diagnosis with colon cancer[38]. Persistent TLR-specific activation of NF-κB in CRC and particularly in tumor-initiating cells may sustain further tumor growth and progression through perpetuation of signaling from inflammatory and tissue repair mechanisms, with consequent self-renewal of pluripotent tumor cells. TLR7 and TLR8 expression on PROM1 (CD133)+ cells in CRC may play a specific role in tumorigenesis and tumor progression[52].
TLR agonists play a fundamental role in activating innate and adaptive immune responses[10]. TLR agonists are currently under investigation as vaccine adjuvants in anticancer therapies for their ability to activate immune cells and promote inflammation[10]. A growing body of evidence indicates that TLRs are expressed or can be induced on various cell types, including T cells and tumor cells[8,10].
Current available synthetic TLR2 ligands are based on cell wall constituents of (potential) pathogens, and adjuvant research could possibly benefit from elucidating the variations in the LPS make-up of probiotic strains. With regard to the indispensable role of pattern recognition receptors (PRRs) in facilitating microbe-induced TLR2 function, determination of specific PRRs involved in the recognition of probiotic strains would aid research on the mechanism of action of probiotics. In addition, because microbial manipulation of PRR-TLR crosstalk is used by pathogens to subvert appropriate immune responses, determination of the specific PRRs involved could lead to new therapeutic approaches[11]. The TLR2⁄4 agonists S100A9 and HMGB1 have been touted as potential biomarkers for CRC, as they are upregulated significantly in CRC and have been shown to be regulated by STAT3, which is hyperactivated in approximately 90% of colorectal tumor biopsies[41,53,54]. The TLR4 agonist monophosphoryl lipid A is approved for use in several vaccines as an adjuvant[1,51].
Rosa et al[55] established a KRAS mutated CRC model and showed that an immunomodulatory oligonucleotide sequence in combination with cetuximab had an antitumor effect. This is probably based on the alteration of MAPK phosphorylation, resulting in structural and functional changes in the relationship between epidermal growth factor receptor (EGFR) and TLR9[50,55]. Mutation of the KRAS gene has a critical role in colon cancer and may cause resistance to anti-EGFR therapy, which is the reason why panitumumab and cetuximab therapy do not show a positive effect on the control of proliferation and metastasis in KRAS-mutated colon cancer; this kind of biological therapy could only be useful in the case of patients carrying the wild-type KRAS gene; estrogen receptors may take part in colorectal carcinogenesis, and interaction between TLR9 and estrogen receptors may have further therapeutic importance in CRC, and TLR9 agonist therapy has been tested clinically on the colon[50]. The TLR9 agonist, which is commonly referred to as CpG-ODN, has been added to the arsenal of anti-cancer drugs as monotherapy, or in combination with chemotherapy, radiotherapy, and other immunotherapeutic approaches, as they increase antigen presentation and boost anti-tumor B and T cell responses[56]. TLR9 agonists were reported to show TP53-independent activity within human CRC cells, inhibit their proliferation, promote apoptosis, and improve anti-cancer effects of radiotherapy and chemotherapy[17]. One therapeutic advantage of the use of TLR9 agonists in this tumor model could be to sensitize tumors to the toxic effects of radiation treatment[10,57]. Combined administration of a TLR9 agonist and an IL-10 antagonist is one of the candidates for cancer treatment[8]. Recently, it was shown that TLR ligands may be critical for dendritic cell (DC) activation, and combined TLR activation can lead to better DC maturation status, and also induce more effective antitumor immune responses against colon cancer, showing that it may be a potential strategy to develop more powerful DC cancer vaccines[58].
It was reported that specific small molecule inhibitors of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) reduce immunosuppression to increase the proinflammatory effects of TLR ligands that support antitumor immunity. Multiple strategies to inhibit PIK3CA in DC led to IL-10 and transforming growth factor-β1 suppression but did affect IL12 or IL1B induction by the TLR5 ligand flagellin[59].
TLR4 plays an important role in innate immunity as the first line of host defense. Most human cells express high levels of TLR antagonist proteins and a low level of TLR4. Tumor progression involves TLR4-mediated irregular and uninhibited production of proinflammatory cytokines, immunosuppressive cytokines as well as chemokines; suggesting that the discovery of TLR4 antagonists may be an ideal strategy to treat tumors. TLR4 antagonists were found to pose a risk of compromising host immunity in other studies, so that it is a scientific dilemma whether a TLR4 agonist or antagonist should be targeted as treatment for cancer[60]. The TLR4/LY96 antagonist antibody inhibited colitis-associated neoplasia in a mouse model, and it was shown that TLR regulation can affect the outcome of both acute colitis and its consequences, i.e., cancer. Targeting TLR4 and other TLR’s may ultimately play a role in prevention or treatment of colitis-associated cancer[61].
The developmental process for TLR-targeting products in cancer has not been altogether straightforward, and two of the earliest TLR pioneers have had disappointing results. However, there are a number of promising second-generation products currently in development, and targeting of TLR9 for metastatic CRC in clinical phase II/III trials is being performed by Mologen company[45]. Various TLR agonists are currently under investigation in clinical trials for their ability to orchestrate antitumor immunity[10]. Pollinex Quattro (Allergy Therapeutics Ltd., Worthing, UK) is a vaccine that contains a monophosphoryl lipid adjuvant to stimulate TLR4, combined with ragweed pollen extract for the treatment of seasonal allergic rhinitis[1,62]. Following positive results in phase III trials, Allergy Therapeutics have submitted Pollinex Quattro for regulatory approval in Europe[1]. TLR9 is a key determinant of the innate immune responses in both sterile and infectious injury. Specific TLR9 antagonism reduces tissue damage in a wide range of pathologies, and has been delivered by modification of nucleic acids, a recognized ligand for TLR9, and a novel small-molecule enantiomeric analogue of traditional morphinans which has specific TLR9 antagonist properties and reduces sterile inflammation-induced organ damage[52]. Some of the TLR-based therapeutics under evaluation in CRC are shown in Figure 2 and Table 3.
| Compound | Target (agonist) | Indications | Drug class or trade | Clinical phase |
| BCG[10,71,72] | TLR2/4 | CRC | Synthetic ssRNA | Phase I |
| MPL[10,72] | TLR4 | CRC | Synthetic ssRNA | Phase I |
| CBLB502[1,23,71] | TLR5 | Colon cancer | Flagellin | Phase I |
| Imiquimod (Aldara)[10,71,72] | TLR7 | CRC | Small molecule ssRNA | Phase I/II/III |
| IMO2055[1,10] | TLR9 | CRC | CpG oligonucleotide | Phase I/II |
| MGN1703[16] | TLR9 | CRC | dSLIM | Phase II |
TLRs are very interesting receptors and are highly important in the field of adjuvant, pathogen, and probiotic research. TLRs constitute a link between adaptive (specific) and innate (non-specific) immunity, contributing to the capacity of our immune system to efficiently combat pathogens. They also enable immune cells to discriminate between self and nonself antigens[17]. TLRs are connected to the cell signaling machinery via intracellular adaptor molecules, and stimulation of the TLR/IL1R signaling pathway activates the major inflammatory transcription factor NF-κB1 by allowing its nuclear translocation[63]. Predictably, MYD88 was shown to play a role in tumorigenesis via TLR and IL1 proinflammatory mechanisms[63-65]. TLR-mediated signaling can promote tumor growth, and using a TLR agonist or antagonist in combination with an antigen isolated from tumors may increase the effect of vaccination and evoke specific innate immunity against a tumor[6]. TLR stimulation results in NF-κB1 activation, a key modulator in driving inflammation to cancer and mitogen-activated protein kinases that have been shown to recruit mitotic and prostaglandin endoperoxide synthase 2 (PTGS2)-induced pathways in carcinogenesis[66].
CRC is a major cause of cancer-associated morbidity and mortality worldwide, and is the third most common cancer in men and women; in addition, CRC is the third leading cause of cancer-related deaths, and the incidence of this disease is increasing[67,68]. The role of TLRs in CRC pathology has not been fully elucidated. Bacterial infection stimulates the TLR/MYD88 pathway in tumor tissues, which leads to the induction of PTGS2 in stromal cells, including macrophages, and induction of the PTGS2/PGE(2) pathway in tumor stroma is important for the development and maintenance of an inflammatory microenvironment in gastrointestinal tumors[69]. Persistent TLR-specific activation of NF-κB in CRC, and particularly in tumor-initiating cells, may thus sustain further tumor growth and progression through perpetuation of signaling in inflammatory and tissue repair mechanisms, with consequent self-renewal of pluripotent tumor cells; activation through self-ligands or viral RNA fragments may maintain this inflammatory process, suggesting a key role in cancer progression[66]. Chronic activation of TLRs expressed by tumor cells from CRC and pluripotent PROM1 (CD133)+ colon cancer initiating cells may sustain inflammation responses, mediate resistance to apoptosis, and promote further tumor progression. Therefore, targeting of TLR signaling may be a potential mechanism to abrogate this inflammation-mediated effect in tumor progression[66]. The pathways that are downstream of TLRs and culminate in proliferation and recruitment of inflammatory cells during injury can be usurped to support cancer development[70].
Although much effort has been put forward to determine TLR ligand requirements and receptor activity, many questions remain. However, there are reasons to be optimistic that TLRs represent strong candidates for cancer targeting. Drug candidates are being developed to target CRC or act as vaccine adjuvants. We hope that they can be safely used systemically and have the power to transform chemotherapeutic interventions in CRC in the near future.
P- Reviewer: Konturek PC S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Liu XM
| 1. | Connolly DJ, O’Neill LA. New developments in Toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J Interferon Cytokine Res. 2013;33:467-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Li J, Wang X, Zhang F, Yin H. Toll-like receptors as therapeutic targets for autoimmune connective tissue diseases. Pharmacol Ther. 2013;138:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Ge P, Zhu Y. TLR2 and TLR4 in the brain injury caused by cerebral ischemia and reperfusion. Mediators Inflamm. 2013;2013:124614. [PubMed] |
| 5. | Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362-371. [PubMed] |
| 6. | Patten DA, Collett A. Exploring the immunomodulatory potential of microbial-associated molecular patterns derived from the enteric bacterial microbiota. Microbiology. 2013;159:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Thorpe DW, Stringer AM, Gibson RJ. Chemotherapy-induced mucositis: the role of the gastrointestinal microbiome and toll-like receptors. Exp Biol Med (Maywood). 2013;238:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | So EY, Ouchi T. The application of Toll like receptors for cancer therapy. Int J Biol Sci. 2010;6:675-681. [PubMed] |
| 9. | Thada S, Valluri VL, Gaddam SL. Influence of Toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand J Immunol. 2013;78:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | van Bergenhenegouwen J, Plantinga TS, Joosten LA, Netea MG, Folkerts G, Kraneveld AD, Garssen J, Vos AP. TLR2 & amp; Co: a critical analysis of the complex interactions between TLR2 and coreceptors. J Leukoc Biol. 2013;94:885-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Vaz J, Akbarshahi H, Andersson R. Controversial role of toll-like receptors in acute pancreatitis. World J Gastroenterol. 2013;19:616-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Hackam DJ, Good M, Sodhi CP. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg. 2013;22:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Falck-Hansen M, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis. Int J Mol Sci. 2013;14:14008-14023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 1:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 16. | Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Niedzielska I, Niedzielski Z, Tkacz M, Orawczyk T, Ziaja K, Starzewski J, Mazurek U, Markowski J. Toll-like receptors and the tendency of normal mucous membrane to transform to polyp or colorectal cancer. J Physiol Pharmacol. 2009;60 Suppl 1:65-71. [PubMed] |
| 18. | Belmont L, Rabbe N, Antoine M, Cathelin D, Guignabert C, Kurie J, Cadranel J, Wislez M. Expression of TLR9 in tumor-infiltrating mononuclear cells enhances angiogenesis and is associated with a worse survival in lung cancer. Int J Cancer. 2014;134:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Holldack J. Toll-like receptors as therapeutic targets for cancer. Drug Discov Today. 2014;19:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Podda G, Nyirenda M, Crooks J, Gran B. Innate immune responses in the CNS: role of toll-like receptors, mechanisms, and therapeutic opportunities in multiple sclerosis. J Neuroimmune Pharmacol. 2013;8:791-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Curr Opin Pharmacol. 2011;11:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Arslan F, Houtgraaf JH, Keogh B, Kazemi K, de Jong R, McCormack WJ, O’Neill LA, McGuirk P, Timmers L, Smeets MB. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ Cardiovasc Interv. 2012;5:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Burdelya LG, Brackett CM, Kojouharov B, Gitlin II, Leonova KI, Gleiberman AS, Aygun-Sunar S, Veith J, Johnson C, Haderski GJ. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc Natl Acad Sci USA. 2013;110:E1857-E1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Lucas K, Maes M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 25. | Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12:1676-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Lin YT, Verma A, Hodgkinson CP. Toll-like receptors and human disease: lessons from single nucleotide polymorphisms. Curr Genomics. 2012;13:633-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Sommariva M, De Cecco L, De Cesare M, Sfondrini L, Ménard S, Melani C, Delia D, Zaffaroni N, Pratesi G, Uva V. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 2011;71:6382-6390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8101] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 29. | Xu H, Wu Q, Dang S, Jin M, Xu J, Cheng Y, Pan M, Wu Y, Zhang C, Zhang Y. Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma. PLoS One. 2011;6:e27399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Tang XY, Zhu YQ, Wei B, Wang H. Expression and functional research of TLR4 in human colon carcinoma. Am J Med Sci. 2010;339:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Pimentel-Nunes P, Gonçalves N, Boal-Carvalho I, Afonso L, Lopes P, Roncon-Albuquerque R, Soares JB, Cardoso E, Henrique R, Moreira-Dias L. Decreased Toll-interacting protein and peroxisome proliferator-activated receptor γ are associated with increased expression of Toll-like receptors in colon carcinogenesis. J Clin Pathol. 2012;65:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Pimentel-Nunes P, Teixeira AL, Pereira C, Gomes M, Brandão C, Rodrigues C, Gonçalves N, Boal-Carvalho I, Roncon-Albuquerque R, Moreira-Dias L. Functional polymorphisms of Toll-like receptors 2 and 4 alter the risk for colorectal carcinoma in Europeans. Dig Liver Dis. 2013;45:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Nihon-Yanagi Y, Terai K, Murano T, Matsumoto T, Okazumi S. Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol Immunother. 2012;61:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’hUigin C. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 339] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, Shimada K, Wong MH, Michelsen KS, Arditi M. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS One. 2010;5:e13027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Tsimogiannis KE, Tellis CC, Tselepis AD, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G. Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc. 2012;26:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Slattery ML, Herrick JS, Bondurant KL, Wolff RK. Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int J Cancer. 2012;130:2974-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 39. | Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, Kudo E, Shimada M, Sano T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J, Karttunen TJ. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 41. | Tye H, Jenkins BJ. Tying the knot between cytokine and toll-like receptor signaling in gastrointestinal tract cancers. Cancer Sci. 2013;104:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | O’Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS One. 2012;7:e44176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Eiró N, González L, González LO, Fernandez-Garcia B, Andicoechea A, Barbón E, García-Muñiz JL, Vizoso FJ. Toll-like receptor-4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother. 2013;36:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Eyking A, Ey B, Rünzi M, Roig AI, Reis H, Schmid KW, Gerken G, Podolsky DK, Cario E. Toll-like receptor 4 variant D299G induces features of neoplastic progression in Caco-2 intestinal cells and is associated with advanced human colon cancer. Gastroenterology. 2011;141:2154-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Cammarota R, Bertolini V, Pennesi G, Bucci EO, Gottardi O, Garlanda C, Laghi L, Barberis MC, Sessa F, Noonan DM. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. J Transl Med. 2010;8:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Kamizato M, Nishida K, Masuda K, Takeo K, Yamamoto Y, Kawai T, Teshima-Kondo S, Tanahashi T, Rokutan K. Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol. 2009;44:1172-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Kim HJ, Kim CH, Ryu JH, Joo JH, Lee SN, Kim MJ, Lee JG, Bae YS, Yoon JH. Crosstalk between platelet-derived growth factor-induced Nox4 activation and MUC8 gene overexpression in human airway epithelial cells. Free Radic Biol Med. 2011;50:1039-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5843] [Cited by in RCA: 6721] [Article Influence: 448.1] [Reference Citation Analysis (0)] |
| 49. | Rahman AH, Taylor DK, Turka LA. The contribution of direct TLR signaling to T cell responses. Immunol Res. 2009;45:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Fűri I, Sipos F, Germann TM, Kalmár A, Tulassay Z, Molnár B, Műzes G. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects. World J Gastroenterol. 2013;19:4119-4126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Brunner R, Jensen-Jarolim E, Pali-Schöll I. The ABC of clinical and experimental adjuvants--a brief overview. Immunol Lett. 2010;128:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Hoque R, Farooq A, Malik A, Trawick BN, Berberich DW, McClurg JP, Galen KP, Mehal W. A novel small-molecule enantiomeric analogue of traditional (-)-morphinans has specific TLR9 antagonist properties and reduces sterile inflammation-induced organ damage. J Immunol. 2013;190:4297-4304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Lee H, Song M, Shin N, Shin CH, Min BS, Kim HS, Yoo JS, Kim H. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One. 2012;7:e34318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Lee MJ, Lee JK, Choi JW, Lee CS, Sim JH, Cho CH, Lee KH, Cho IH, Chung MH, Kim HR. Interleukin-6 induces S100A9 expression in colonic epithelial cells through STAT3 activation in experimental ulcerative colitis. PLoS One. 2012;7:e38801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Rosa R, Melisi D, Damiano V, Bianco R, Garofalo S, Gelardi T, Agrawal S, Di Nicolantonio F, Scarpa A, Bardelli A. Toll-like receptor 9 agonist IMO cooperates with cetuximab in K-ras mutant colorectal and pancreatic cancers. Clin Cancer Res. 2011;17:6531-6541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR. Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Li X, Liu D, Liu X, Jiang W, Zhou W, Yan W, Cen Y, Li B, Cao G, Ding G. CpG ODN107 potentiates radiosensitivity of human glioma cells via TLR9-mediated NF-κB activation and NO production. Tumour Biol. 2012;33:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Hong X, Dong T, Hu J, Yi T, Li W, Zhang Z, Lin S, Niu W. Synergistical toll-like receptors activated dendritic cells induce antitumor effects against carcinoembryonic antigen-expressing colon cancer. Int J Colorectal Dis. 2013;28:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Marshall NA, Galvin KC, Corcoran AM, Boon L, Higgs R, Mills KH. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-γ+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Mai CW, Kang YB, Pichika MR. Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: its expression and effects in the ten most common cancers. Onco Targets Ther. 2013;6:1573-1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Fukata M, Shang L, Santaolalla R, Sotolongo J, Pastorini C, España C, Ungaro R, Harpaz N, Cooper HS, Elson G. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2011;17:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 62. | Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498-505. [PubMed] |
| 63. | Kfoury A, Le Corf K, El Sabeh R, Journeaux A, Badran B, Hussein N, Lebecque S, Manié S, Renno T, Coste I. MyD88 in DNA repair and cancer cell resistance to genotoxic drugs. J Natl Cancer Inst. 2013;105:937-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5756] [Article Influence: 239.8] [Reference Citation Analysis (0)] |
| 65. | Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 456] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 66. | Grimm M, Kim M, Rosenwald A, Heemann U, Germer CT, Waaga-Gasser AM, Gasser M. Toll-like receptor (TLR) 7 and TLR8 expression on CD133+ cells in colorectal cancer points to a specific role for inflammation-induced TLRs in tumourigenesis and tumour progression. Eur J Cancer. 2010;46:2849-2857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10452] [Article Influence: 696.8] [Reference Citation Analysis (0)] |
| 68. | Eiró N, González L, González LO, Andicoechea A, Fernández-Díaz M, Altadill A, Vizoso FJ. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol. 2012;32:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012;47:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 914] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 71. | Gambara G, De Cesaris P, De Nunzio C, Ziparo E, Tubaro A, Filippini A, Riccioli A. Toll-like receptors in prostate infection and cancer between bench and bedside. J Cell Mol Med. 2013;17:713-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Vacchelli E, Eggermont A, Sautès-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2013;2:e25238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |