Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.16902
Revised: April 15, 2014
Accepted: August 13, 2014
Published online: December 7, 2014
Processing time: 280 Days and 17 Hours
Acute pancreatitis is a nonbacterial disease of the pancreas. The severe form of this ailment is characterized by high mortality. Whether acute pancreatitis develops as the severe type or resolves depends on the intensity of the inflammatory process which is counteracted by the recruitment of innate defense mechanisms. It has been shown that the hormones ghrelin, leptin and melatonin are able to modulate the immune function of the organism and to protect the pancreas against inflammatory damage. Experimental studies have demonstrated that the application of these substances prior to the induction of acute pancreatitis significantly attenuated the intensity of the inflammation and reduced pancreatic tissue damage. The pancreatic protective mechanisms of the above hormones have been related to the mobilization of non-specific immune defense, to the inhibition of nuclear factor kappa B and modulation of cytokine production, to the stimulation of heat shock proteins and changes of apoptotic processes in the acinar cells, as well as to the activation of antioxidant system of the pancreatic tissue. The protective effect of ghrelin seems to be indirect and perhaps dependent on the release of growth hormone and insulin-like growth factor 1. Leptin and ghrelin, but not melatonin, employ sensory nerves in their beneficial action on acute pancreatitis. It is very likely that ghrelin, leptin and melatonin could be implicated in the natural protection of the pancreatic gland against inflammatory damage because the blood levels of these substances increase in the initial phase of pancreatic inflammation. The above hormones could be a part of the innate resistance system which might remove noxious factors and could suppress or attenuate the inflammatory process in the pancreas.
Core tip: The pathogenesis of acute pancreatitis is not clear and treatment of this disease is unspecific. Since the severe form of acute pancreatitis often leads to death or to pancreatic insufficiency, the understanding of the mechanisms involved in pancreatic protection appears to be an important problem. Experimental data have shown that pancreatitis severity could be attenuated by various hormones. Herein, we review the results of our research studies and others, as well as data from clinical observations concerning the protective effects of ghrelin, leptin and melatonin on acute pancreatitis. We also present the hypothetical mechanisms responsible for the beneficial influence of these substances on pancreatic inflammation.
- Citation: Jaworek J, Konturek SJ. Hormonal protection in acute pancreatitis by ghrelin, leptin and melatonin. World J Gastroenterol 2014; 20(45): 16902-16912
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/16902.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.16902
Acute pancreatitis is a sterile inflammatory disease of the pancreatic tissue. This ailment has been classified as mild, moderate or severe[1]. Mild pancreatitis is the most common, local pancreatic inflammation which resolves by itself. Moderate or severe acute pancreatitis with organ failure requires prolonged and expensive hospitalization, is characterized by high mortality and often leads to endocrine or exocrine dysfunction of the pancreas[2,3]. Despite the intensive research and clinical studies of the last decades, the pathogenesis of acute pancreatitis still remains unclear and the treatment of this disease is not specific. It is believed that the key determinant of this disease is the intra-acinar activation of digestive enzymes (mainly trypsin) and autodigestion of the pancreatic gland which initiates local (mild pancreatitis) or systemic (moderate and severe form of this disease) inflammation[4].
The cellular mechanisms responsible for zymogen activation are not fully understood. One possible explanation could be an impaired autophagy process resulting from lysosomal dysfunction and imbalance between two lysosomal enzymes: cathepsin B, which cleaves trypsinogen into active trypsin, and cathepsin L, responsible for trypsin degradation. The abnormal function of mitochondria and ATP depletion suppresses apoptosis and promotes acinar necrosis[5]. Damaged acinar cells release toxic substances, known as damage associated molecular patterns (DAMP), to the extracellular space leading to the propagation of the sterile inflammatory process[6]. Another hypothesis shows that activation of the nuclear NFκB, observed in the early step of acute pancreatitis, could be related to the cleavage of trypsinogen into active trypsin. However, the relationship between these phenomena has not been elucidated[7]. Recently, an interesting theory concerning the role of the pancreatic duct cells in the pathogenesis of acute pancreatitis has been presented. It has been explained that the reduction of bicarbonate secretion by duct cells leads to the acidification of the luminal space and contributes to the intracellular zymogen activation[8].
The inflammatory process in the pancreas is counteracted by activation of innate defense systems which could remove injurious agents to suppress or reduce pancreatic inflammation. Stimulation of the immune cells, production of protective substances and anti-inflammatory cytokines, modulation of the apoptotic signaling pathway and activation of antioxidant defense systems are among the mechanisms implicated in the cellular and tissue resistance[9-12]. Identification of the factors which trigger the defense systems against acute pancreatitis could be of great importance for the creation of new therapeutic strategies in this disease.
Previous experimental studies on acute pancreatitis have evidenced that non-specific immunity and innate defense systems could be activated in several ways and by various factors, such as stimulation of nitric oxide synthase (NOS) and generation of nitric oxide (NO), thermal preconditioning and administration of low doses of biologically active substances or endotoxins[12-14]. Some hormones have been shown to protect the pancreas against the development of acute pancreatitis and to attenuate the course of this disease. Suppression of pancreatic inflammation and a marked reduction of tissue inflammatory damage have been observed as the result of the application of the hormones ghrelin, leptin and melatonin.
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor, was originally isolated from the rat stomach by Kojima et al[15]. The main source of ghrelin in the organisms of rats and dogs appears to be X/A cells of the oxyntic mucosa, which represent 20% of the mucosal cell population, whereas in humans, ghrelin has been found in the P/D1 cell type[15,16]. Besides the stomach, ghrelin has been widely expressed in the additional tissues of the gastrointestinal tract (duodenum, jejunum, colon, pancreas), in the central nervous system (hypothalamus, cortex, brain stem, pituitary) and in other organs (kidney, heart, lung, testis, immune cells)[16-20]. In the pancreas, ghrelin-producing cells represent an independent cell population of the pancreatic islets and ghrelin is possibly involved in glucose homeostasis[21]. The ghrelin system (ghrelin and its receptor) also exists in the pancreatic acinar cells[22]. An experimental study has shown that ghrelin could be implicated in the stimulation of pancreatic enzyme secretion[23].
Part of ghrelin peptides undergoes posttranslational acylation, dependent on the enzyme ghrelin-O-acyl-transferase (GOAT), and circulating ghrelin consists of two forms: desacyl ghrelin (90%) and acyl ghrelin (10%)[24]. Both forms of ghrelin are agonists of the ghrelin receptor; however, under physiological conditions, only acylated ghrelin is able to activate the intracellular signaling cascade of this receptor[15,25]. The desacyl ghrelin has previously been believed to be a degradation product of acyl ghrelin but recent observations have shown that desacyl ghrelin is able to produce biological effects independent of those of acylated peptide. It has been reported that desacyl ghrelin prevented the activation of apoptosis and fibrosis of cardiomyocytes and protected endothelial cells from apoptosis induced by oxidative stress[26,27]. Administration of desacyl ghrelin to rats modulated body temperature and produced arterial dilatation, probably via activation of the parasympathetic nervous system[28]. On the other hand, desacyl ghrelin could antagonize the activation of the acylated form. All these observations suggest that desacyl ghrelin might be a separate hormone which interacts with its own specific, as yet unknown, receptor[29].
The ghrelin receptor (GHS-Rs) is a G-protein coupled receptor characterized by transmembrane domains[23]. GHS-R has been identified as two spliced variants: functional ghrelin receptor type 1 (GHS-R1a) and non-functional, unspliced GHS-R1b. Both GHS-R1s have been predominantly expressed in the pituitary but this receptor has also been detected in the pancreas, spleen, heart, adrenal and thyroid glands[30,31].
Ghrelin has been shown to produce a wide range of biological effects in the organism, such as: (1) release of prolactin, adrenocorticotropic and growth hormones; (2) control of appetite and food intake; (3) stimulation of gastric and pancreatic secretion and gastrointestinal motility; (4) modulation of cardiovascular and reproductive functions; (5) increase of neoglucogenesis and adipogenesis; (6) stimulation of bone formation; and (7) modulation of immune functions[32]. Nevertheless, the physiological involvement of ghrelin in most of above functions is not completely clear.
The anti-inflammatory effects of ghrelin have been shown in many tissues, including the pancreas[33]. Ghrelin protects gastric mucosa against acute ulceration and accelerates the healing of chronic gastric and duodenal ulcers[34]. Ghrelin suppresses inflammation in sepsis and inflammatory bowel disease, reduces inflammatory pain and attenuated chronic liver injury[35-38]. The presence of ghrelin receptors on human peripheral lymphocytes, neutrophils and on the leukemic T, B and myeloid cell lines indicates that ghrelin is able to directly affect the functions of immune cells[20]. Indeed, ghrelin could inhibit production of anti-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), interleukin 1β (IL-1β), interleukin 6 (IL-6) and interleukin 8 (IL-8)[33,38-40]. It has been demonstrated that the downregulation of proinflammatory cytokines by ghrelin is mediated by MAPK phosphatase-1 enzyme involved in the innate immune response[41]. In addition, ghrelin has been found to reduce the phagocytic activity of macrophages in vivo and in vitro and to decrease the production of high mobility box 1 protein (HMGB1)[42,43]. The anti-inflammatory effect of ghrelin could also be attributed to the activation of NOS and to enhanced production and release of NO[38]. Controversial reports have been presented concerning the effect of ghrelin on nuclear factor kappa B (NF-κB). In human B cells, ghrelin promotes NF-κB, whereas in the pancreas, ghrelin inhibits activation of this substance[44,45].
Numerous studies have shown that administration of ghrelin to animals prior to the induction of acute pancreatitis protected pancreatic tissue against damage and attenuated the inflammation[10,30,45-47]. Ghrelin reduced the morphological signs of acute pancreatic inflammation, diminished blood levels of IL-1β, decreased plasma lipase and improved DNA synthesis in rats subjected to caerulein-induced pancreatitis; however, pancreatic blood flow in these animals was unaffected by ghrelin[30]. The favorable effect of ghrelin on the pancreas has been also demonstrated in acute necrotizing L-arginine pancreatitis as well as in taurocholate-induced pancreatitis and is attributed to the inhibition of NFκ-B expression and the blockade of the inflammatory signal transduction pathway[44,45]. In addition, ghrelin has been demonstrated to lessen pancreatitis-associated lung injury, to reduce sequestration of neutrophils in the lung, to limit production of the proinflammatory cytokines, such as IL-6, and TNFα, and to inhibit pulmonary substance P expression[47,48].
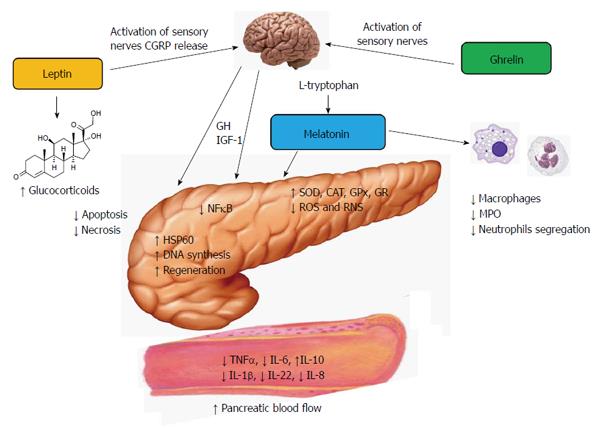
Ghrelin could exert its positive effect on the pancreas via the central mechanism because the pancreatic protective effect of ghrelin was observed following application of this peptide into the cerebral ventricles of rats subjected to caerulein-induced pancreatitis. This protection was correlated with the release of growth hormone (GH) and was completely reversed by deactivation of sensory nerves with capsaicin[10]. Hypophysectomy cancelled out the pancreatic protection evoked by ghrelin and eliminated GH and insulin-like growth factor 1 (IGF-1) from the serum[46]. The above results present the evidence that the protective effect of ghrelin on the pancreas is indirect and depends on the activation of sensory nerves and the release of GH and IGF-1.
A recent report has shown the therapeutic effect of ghrelin in the course of caerulein-induced pancreatitis. Administration of ghrelin after the development of acute pancreatitis reduced the intensity of pancreatic inflammation and accelerated the regeneration of the gland. This effect was mainly related to the reduction of IL1β and to the stimulation of pancreatic cell proliferation[49].
It was demonstrated that in animals with acute pancreatitis, serum levels of endogenous ghrelin at 24 and 48 h were significantly increased compared to the control[47]. Clinical studies have shown that ghrelin levels measured at the first, third and fifth day of hospitalization in patients with acute pancreatitis were higher than in healthy individuals[50]. It was also reported that serum ghrelin levels were markedly elevated in patients with high risk factors for severe forms of acute pancreatitis[51]. It is very likely that ghrelin is released in high amounts in response to the initiation of the inflammatory process in the pancreas to suppress the inflammation and protect the pancreatic tissue.
All the above observations indicate that ghrelin could be implicated in the natural protection of the pancreatic tissue through the activation of the innate immune system to prevent the development of the inflammatory process in the pancreas. The pancreatic protective effect of ghrelin appears to be indirect and depends on the release of GH and IGF-1 by ghrelin (Figure 1).
Leptin is one of the adipokines, cytokine-like hormones which are produced in white adipose tissue. Besides adipocytes, leptin has been also detected in other organs, such as the stomach, muscles and bones[52,53]. This 16-kDa protein is encoded by the ob gene and was first recognized as an inhibitor of appetite, a stimulator of energy expenditure and a regulator of body weight[54]. Leptin is well known as one of the main modulators of energy balance but is also implicated in the regulation of neuroendocrine and secretory functions of the body. Indeed, leptin has been shown to affect gastric and pancreatic secretions, insulin release, to protect the gastric mucosa against noxious agents and to influence the inflammatory process in the pancreas[55,56]. Leptin could be also involved in the modulation of several processes of the body, such as reproduction, angiogenesis and bone metabolism, but recent studies have focused on the role of leptin in immunity[57-59].
Leptin exerts its effect through its specific receptor (Ob-R) which belongs to the class I of the cytokine family, together with receptors for IL-6 and IL-12. Ob-R is represented by six isoforms of different length but only full-length Ob-Rb is able to activate the signal transduction pathway[60]. Ob-R is present in the hypothalamus, pituitary, kidney, lung, bone marrow, enterocytes, reproductive system and pancreatic acinar cells, as well as all types of immune cells[24,60,61].
Leptin received particular attention as the main modulator of immune functions and inflammatory processes in the organism[59]. The reports concerning the effect of leptin on the immune processes are contradictory. In some studies, leptin has been shown to trigger inflammatory responses and to play an important role in chronic inflammation[62,63]. Other studies documented the anti-inflammatory effects of leptin[64,65].
It has been reported that leptin improved the function of macrophages, modulates lymphocyte proliferation, increases the ratio of T naïve/T memory cells and stimulates the production of pro-inflammatory cytokines, such as TNFα, IL-1 and IL-6[66]. Leptin production increases in acute inflammation and sepsis. It was proposed that leptin blood levels could be a predictive factor of the increased survival and attenuation of inflammation[67]. Additional evidence for the beneficial role of leptin in sepsis comes from the observation that the administration of exogenous leptin increased survival in septic mice[68].
Our previous reports have demonstrated that the application of leptin to rats prior to the induction of acute caerulein-induced pancreatitis significantly reduced the severity of pancreatic inflammation[69]. This beneficial effect of leptin has been observed following intraperitoneal as well as intracerebroventricular administration of this adipokine. The effect of leptin on acute pancreatitis was completely abolished by the deactivation of sensory nerves with capsaicin. These nerves employed calcitonin gene-related peptide (CGRP) as one of the neuromediators[70]. The pharmacological blockade of sensory nerves with CGRP 8-37, an antagonist of CGRP, resulted in the total reversion of the favorable effects of leptin on acute pancreatitis[69]. This observation indicates that sensory nerves and CGRP play a part in the protection of the pancreatic gland afforded by leptin against acute damage. Subsequent studies have shown that leptin treatment ameliorates lung injuries in rats with the caerulein model of acute pancreatitis[71]. In addition, treatment with leptin accelerated pancreatic tissue repair in animals subjected to ischemic pancreatitis[72]. The positive effects of this adipokine on acute pancreatitis have been also observed in the model of acute pancreatitis evoked by ischemia/reperfusion and documented by histological assessment and significant reduction of TNFα and Il-1β blood levels[71,72]. The beneficial effect of leptin on acute pancreatitis could be attributed to the activation of the NOS/NO system and release of NO in the pancreas, to the improvement of pancreatic microcirculation and, at least in part, to the release of glucocorticoids which provide an unspecific attenuation of the inflammatory processes[61,62,69,72] (Figure 1). A study on the pancreatic acinar cell line AR42J revealed that leptin is able to increase the gene expression of heat shock protein 60 (HSP60) in these cells[73]. HSP60 belongs to the group of chaperone proteins, stimulated by high temperature, oxidative stress or inflammation. Activation of HSP60 by leptin could limit mitochondrial and nuclear injury, might prevent the endoplasmic reticulum from the damage and could reduce formation of autophagosomes[73,74].
More evidence concerning the important role of leptin in acute pancreatitis comes from experiments on congenitally obese mice with a deficient leptin system. In this study, obese knockout mice with deficits of leptin (LepOb mice) or leptin receptor (LepDb mice) developed acute pancreatitis in the more severe form than the acute pancreatitis demonstrated in wild-type animals[75]. This observation clearly shows that the leptin system is implicated in the innate immune defense against acute pancreatic inflammation, yet leptin is not a sole player in the modulation of pancreatic resistance. Other adipokines, such as adiponectin, visfatin and resistin, could also play a role in the modulation of acute pancreatitis severity[59,76].
In contrast to the reports presenting the anti-inflammatory effects of leptin, recent publications have shown that leptin up-regulated the expression of toll-like receptor 2 (TLR-2) on human monocytes and increased TNFα expression stimulated by endotoxins. This activity of leptin may potentiate innate immunity and inflammation in obese hyperleptinemic patients[77]. Studies on obese (hyperleptinemic) rats with acute necrotizing pancreatitis indicated that in these animals the expression of pro-inflammatory IL-6 was higher, whereas the signal of anti-inflammatory IL-10 was lower than in the group of lean animals[78]. Some authors concluded that obesity and hyperleptinemia could be a risk factor for severe acute pancreatitis[76,79].
Changes of leptin level in acute pancreatitis have been the subject of numerous experimental and clinical studies. Many investigators have noted the considerable increases of leptin blood levels in acute pancreatitis in rats and humans[47,64,75,80]. Nevertheless, the leptin blood levels did not correlate with pancreatitis severity[47,80-82]. The recent comprehensive review of Karpavicius et al[83] analyzed the prognostic value of leptin and other adipokines in predicting the course of acute pancreatitis. The authors concluded that leptin levels increase in acute pancreatitis; however, these levels are not in parallel with the severity of acute pancreatitis and could not be used as a prognostic value of the course and possible complications of this disease[83].
The results of the above studies lead to the conclusion that leptin could be considered a factor which potentiates non-specific immune defense and stimulates inflammatory reaction. It has been shown that leptin blood levels increase in acute pancreatitis, yet these levels have not been related to the severity of this disease. It is very likely that leptin could activate the initial phase of the innate immune response and thus trigger the immune defense in the early stages of inflammation. Such a mobilization of the immune system might suppress the acute inflammation near the beginning and prevent the development of serious disease. Nevertheless, with the development of intensive inflammation, leptin could continuously activate the immune cells and interleukin production to combat the pathogens or factors responsible for inflammation. Constant stimulation of the immune system by leptin might favor the progression of an inflammatory state.
It is worth remembering that other adipokines besides leptin also participate in the modulation of inflammatory process and an imbalance between leptin and other adipokines could deregulate the immune response.
Melatonin (5-methoxy-N-acetyltryptamine) is an indoleamine, produced from amino acid L-tryptophan in the four steps reaction with serotonin as a direct melatonin precursor[84]. This indoleamine is released from the pineal gland in the regular nocturnal/diurnal rhythm, with the peak at night[85]. Melatonin was discovered first in the pineal gland and is best known as the pineal hormone but this substance has also been found in numerous mammalian tissues, such as the retina, Harderian gland, brain and in the gastrointestinal system, which appears to be the richest source of melatonin in the organism[86-88]. Gastrointestinal melatonin originates from the enteroendocrine cells of the gut mucosa, from food ingested and from bile[87,89,90]. Food stimulates the release of melatonin in a manner independent of the light/dark cycle[88,91].
Melatonin could exert its biological effect via its specific receptors MT1, MT2 and MT3 on the cell membranes, but it could also directly enter into the cell because of its high solubility in lipids[92]. Melatonin membrane receptors MT1 and MT2 belong to 7-transmembrane G-protein-coupled receptors and both could be antagonized by luzindole[93]. Melatonin receptor MT3 is an enzyme quinone reductase 2 known as a potent antioxidant[93,94]. Melatonin could perhaps activate nuclear orphan receptors RZR/ROR but the role of these receptors is not known[94].
Melatonin receptors have been detected in many tissues, such as the central nervous system, cardiovascular system, immune cells, retina and the gastrointestinal system[94,95]. Both melatonin and its receptors have been identified in the human pancreatic tissue in the islet of Langerhans and this indoleamine has been suggested as one of the modulators of insulin secretion[95].
Melatonin has been reported to produce a wide range of biological effects but its physiological role in the organism is still unknown. This substance was first recognized as part of the biological clock because of its rhythmic diurnal/nocturnal secretions[84,96]. Subsequent studies have shown a very special role of melatonin as a vitally important tissue protector and modulator of immune response. Melatonin enhances tissue defense against oxidative damage because it is a potent activator of antioxidant enzymes and a direct scavenger of radical oxygen (ROS) and nitrogen (RNS) species. ROS and RNS are produced in mitochondria and under normal conditions they are neutralized by antioxidant enzymes as well as by natural non-enzymatic scavengers, such as melatonin, glutathione, vitamins C and E and others[97,98]. Oxidative stress and inflammation resulted in the massive production of these free radicals which could not be counteracted by antioxidant defense of tissue, leading to tissue damage[99,100]. Melatonin also activates the second line of tissue defense which is dependent on the antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) or glutathione reductase (GR)[101-103].
Besides its antioxidant properties, melatonin could strengthen the immune defense through the modulation of the signal transduction pathways involved in inflammatory processes. Melatonin inhibits the nuclear binding of NF-kappaB and in this manner it could reduce the production of pro-inflammatory cytokines (TNFα and interleukins IL-1β, IL-6, IL-8)[104]. This indoleamine has been reported to reduce prostaglandin synthesis, to modulate cell apoptosis and necrosis, and to affect the process of angiogenesis[105-107].
Experimental studies have shown that the administration of melatonin to rats prior to the induction of acute pancreatitis protected the pancreas from the development of acute inflammation and significantly diminished pancreatic tissue damage[108-114]. Melatonin radically reduced the morphological signs of inflammation, such as edema, leukocyte infiltration and the vacuolization of the acinar cells[10,111,115-117]. The blood levels of amylase and lipase, the indicator enzymes of acute pancreatitis severity, as well as blood levels of pro-inflammatory cytokine TNFα, were significantly reduced in the animals pretreated with melatonin and subjected to acute pancreatitis[10,106,110,113,115-118]. In contrast, the blood level of anti-inflammatory IL-10 was increased markedly in these animals[10,116].
It is worth noting that the favorable effects of melatonin on acute pancreatitis were paralleled by its precursor, L-tryptophan. Application of L-tryptophan to animals produced the significant and dose-dependent increase of melatonin blood level. This observation indicates that the anti-inflammatory effects of L-tryptophan are probably dependent on the conversion of this amino acid to melatonin. The above favorable effects of melatonin or L-tryptophan have been observed in rats subjected to caerulein-induced pancreatitis as well as in those with pancreatitis induced by ischemia-reperfusion[108]. Nevertheless, the beneficial effects of melatonin on acute pancreatitis have been demonstrated in different models of pancreatic inflammation, such as L-arginine pancreatitis, pancreatitis induced by taurocholic acid, and by obstruction of the pancreatic duct[110,117,119]. In severe acute pancreatitis, pretreatment with melatonin prevented multiorgan failure and significantly attenuated tissue damage[119]. Melatonin has been shown to increase the nucleic acid content and rate of DNA synthesis in the pancreas and thus to improve pancreatic regeneration in animals subjected to acute pancreatitis[120].
The mechanism of the pancreatic protective action of melatonin is complex. Melatonin works as a direct and indirect antioxidant and effectively reduced the amount of lipid peroxidation products in pancreatic tissue, which is accompanied by the increase of antioxidant enzymes, such as SOD or GPx[98,121]. Melatonin also slows down apoptosis and necrosis in the pancreas and turns down leukocyte infiltration in the pancreatic tissue subjected to acute inflammation. The blood levels of pro-inflammatory interleukin, such as TNFα, IL-1β, IL-6 and IL-22, were reduced, whereas anti-inflammatory IL-10 increased in response to melatonin application[10,105,116,119]. Among the other mechanisms of the pancreatic protection afforded by melatonin is the improvement of pancreatic blood flow, which ameliorates the tissues from the toxic inflammatory products and mediators[108,113]. Melatonin lowers the activity of macrophages and decreases the production of enzyme myeloperoxidase and generation of prostaglandin[104,105]. Melatonin has also been shown to trigger the production of heat shock protein 60 (HSP60) in the pancreatic acinar AR42J cells. Activation of HSP60 by melatonin could protect the intracellular structures against inflammatory damage and could reduce the acinar cell injury[122] (Figure 1).
Melatonin seems to be a part of the natural protective mechanisms against the development of pancreatic inflammation and low melatonin production is associated with an increased risk of severe acute pancreatitis[118,123,124]. Recent reports indicate that the melatonin blood level in humans is closely related to the severity of pancreatitis. It has been proposed that the evaluation of disease severity could be assessed by measuring the blood levels of melatonin[125].
It is very likely that melatonin might be a part of native protection against the development of acute pancreatitis and a low melatonin blood level could be associated with an increased risk of the severe form of this disease. Perhaps melatonin could be used in the prevention of acute pancreatitis in individuals with an increased risk of pancreatic inflammation.
Ghrelin, leptin and melatonin given prior to the induction of experimental acute pancreatitis resulted in the significant attenuation of pancreatitis severity and protected pancreatic tissue against inflammatory damage. The mechanisms of the beneficial influence of the above substances are related to the mobilization of non-specific immune defense, to the inhibition of nuclear NF-κB and the modulation of cytokine production, to the stimulation of heat shock protein, as well as to the activation of the antioxidant system. Experimental and clinical data have shown that blood levels of ghrelin, leptin and melatonin increase in the initial phase of pancreatic inflammation. This observation indicates that these hormones could be a part of the innate resistance system which might remove noxious factors and could suppress or attenuate the inflammatory process in the pancreas.
P- Reviewer: Rakonczay Z, Sharma SS, Yago MD S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Sarr MG, Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Tsiotos GG, Vege SS. The new revised classification of acute pancreatitis 2012. Surg Clin North Am. 2013;93:549-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Andersson B, Appelgren B, Sjödin V, Ansari D, Nilsson J, Persson U, Tingstedt B, Andersson R. Acute pancreatitis--costs for healthcare and loss of production. Scand J Gastroenterol. 2013;48:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Chlupáč J, Filová E, Riedel T, Houska M, Brynda E, Remy-Zolghadri M, Bareille R, Fernandez P, Daculsi R, Bourget C. Attachment of human endothelial cells to polyester vascular grafts: pre-coating with adhesive protein assemblies and resistance to short-term shear stress. Physiol Res. 2014;63:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427-1430. [PubMed] |
| 5. | Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Hegyi P, Pandol S, Venglovecz V, Rakonczay Z. The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Weber H, Hühns S, Jonas L, Sparmann G, Bastian M, Schuff-Werner P. Hydrogen peroxide-induced activation of defense mechanisms against oxidative stress in rat pancreatic acinar AR42J cells. Free Radic Biol Med. 2007;42:830-841. [PubMed] |
| 10. | Jaworek J. Ghrelin and melatonin in the regulation of pancreatic exocrine secretion and maintaining of integrity. J Physiol Pharmacol. 2006;57 Suppl 5:83-96. [PubMed] |
| 11. | McClave SA. Drivers of oxidative stress in acute pancreatitis: the role of nutrition therapy. JPEN J Parenter Enteral Nutr. 2012;36:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Lomberk G, Urrutia R. Primers on molecular pathways--caspase pathway. Pancreatology. 2009;9:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Jaworek J, Bonior J, Tomaszewska R, Jachimczak B, Kot M, Bielański W, Pawlik WW, Sendur R, Stachura J, Konturek PC. Involvement of cyclooxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide. J Physiol Pharmacol. 2001;52:107-126. [PubMed] |
| 14. | Meng K, Liu Q, Dou Y, Huang Q. Prior peritoneal lavage with hot 0.9 % saline induces HSP70 expression and protects against cerulein-induced acute pancreatitis in rats. Mol Biol Rep. 2013;40:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] |
| 16. | Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, Raimondo F, Cocchi D, Solcia E. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem Cell Biol. 2002;117:511-519. [PubMed] |
| 17. | Volante M, Allia E, Fulcheri E, Cassoni P, Ghigo E, Muccioli G, Papotti M. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol. 2003;162:645-654. [PubMed] |
| 18. | Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett. 2000;486:213-216. [PubMed] |
| 19. | Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284-4291. [PubMed] |
| 20. | Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept. 2006;134:126-131. [PubMed] |
| 21. | Granata R, Ghigo E. Products of the ghrelin gene, the pancreatic β-cell and the adipocyte. Endocr Dev. 2013;25:144-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lai KC, Cheng CH, Leung PS. The ghrelin system in acinar cells: localization, expression, and regulation in the exocrine pancreas. Pancreas. 2007;35:e1-e8. [PubMed] |
| 23. | Jaworek J, Nawrot-Porabka K, Leja-Szpak A, Konturek SJ. Brain-gut axis in the modulation of pancreatic enzyme secretion. J Physiol Pharmacol. 2010;61:523-531. [PubMed] |
| 24. | Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR. Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab. 2005;90:2205-2211. [PubMed] |
| 25. | Staes E, Absil PA, Lins L, Brasseur R, Deleu M, Lecouturier N, Fievez V, Rieux Ad, Mingeot-Leclercq MP, Raussens V. Acylated and unacylated ghrelin binding to membranes and to ghrelin receptor: towards a better understanding of the underlying mechanisms. Biochim Biophys Acta. 2010;1798:2102-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Pei XM, Yung BY, Yip SP, Ying M, Benzie IF, Siu PM. Desacyl ghrelin prevents doxorubicin-induced myocardial fibrosis and apoptosis via the GHSR-independent pathway. Am J Physiol Endocrinol Metab. 2014;306:E311-E323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Shimada T, Furuta H, Doi A, Ariyasu H, Kawashima H, Wakasaki H, Nishi M, Sasaki H, Akamizu T. Des-acyl ghrelin protects microvascular endothelial cells from oxidative stress-induced apoptosis through sirtuin 1 signaling pathway. Metabolism. 2014;63:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Inoue Y, Nakahara K, Maruyama K, Suzuki Y, Hayashi Y, Kangawa K, Murakami N. Central and peripheral des-acyl ghrelin regulates body temperature in rats. Biochem Biophys Res Commun. 2013;430:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Delhanty PJ, Neggers SJ, van der Lely AJ. Mechanisms in endocrinology: Ghrelin: the differences between acyl- and des-acyl ghrelin. Eur J Endocrinol. 2012;167:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 30. | Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1383] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 31. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 758] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 32. | Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo). 2013;2013:518909. [PubMed] |
| 33. | Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Stachura J, Konturek SJ, Konturek PC. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561-573. [PubMed] |
| 34. | Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Deboer MD. Use of ghrelin as a treatment for inflammatory bowel disease: mechanistic considerations. Int J Pept. 2011;2011:189242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Cheyuo C, Jacob A, Wang P. Ghrelin-mediated sympathoinhibition and suppression of inflammation in sepsis. Am J Physiol Endocrinol Metab. 2012;302:E265-E272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Sibilia V, Pagani F, Mrak E, Dieci E, Tulipano G, Ferrucci F. Pharmacological characterization of the ghrelin receptor mediating its inhibitory action on inflammatory pain in rats. Amino Acids. 2012;43:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Kabil NN, Seddiek HA, Yassin NA, Gamal-Eldin MM. Effect of ghrelin on chronic liver injury and fibrogenesis in male rats: possible role of nitric oxide. Peptides. 2014;52:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Beynon AL, Brown MR, Wright R, Rees MI, Sheldon IM, Davies JS. Ghrelin inhibits LPS-induced release of IL-6 from mouse dopaminergic neurones. J Neuroinflammation. 2013;10:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Hou Y, An J, Hu XR, Sun BB, Lin J, Xu D, Wang T, Wen FQ. Ghrelin inhibits interleukin-8 production induced by hydrogen peroxide in A549 cells via NF-kappaB pathway. Int Immunopharmacol. 2009;9:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 41. | Jacob A, Rajan D, Pathickal B, Balouch I, Hartman A, Wu R, Zhou M, Wang P. The inhibitory effect of ghrelin on sepsis-induced inflammation is mediated by the MAPK phosphatase-1. Int J Mol Med. 2010;25:159-164. [PubMed] |
| 42. | Tümer C, Bilgin HM, Obay BD, Diken H, Taşdemir E, Sermet A. Effect of ghrelin administration on phagocytic activity in acute cold-restraint stress exposed rats. Regul Pept. 2007;138:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180:8369-8377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Sung EZ, Da Silva NF, Goodyear SJ, McTernan PG, Arasaradnam RP, Nwokolo CU. Ghrelin promotes nuclear factor kappa-B activation in a human B-lymphocyte cell line. Mol Biol Rep. 2011;38:4833-4838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Zhou X, Xue C. Ghrelin inhibits the development of acute pancreatitis and nuclear factor kappaB activation in pancreas and liver. Pancreas. 2009;38:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Dembiński A, Warzecha Z, Ceranowicz P, Cieszkowski J, Pawlik WW, Tomaszewska R, Kuśnierz-Cabala B, Naskalski JW, Kuwahara A, Kato I. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res. 2006;16:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Kerem M, Bedirli A, Pasaoglu H, Unsal C, Yilmaz TU, Ofluoglu E, Sahin TT. Role of ghrelin and leptin in predicting the severity of acute pancreatitis. Dig Dis Sci. 2007;52:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Zhou X, Xue C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am J Med Sci. 2010;339:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Warzecha Z, Ceranowicz P, Dembinski A, Cieszkowski J, Kusnierz-Cabala B, Tomaszewska R, Kuwahara A, Kato I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J Physiol Pharmacol. 2010;61:419-427. [PubMed] |
| 50. | Daniel P, Leśniowski B, Jasińska A, Pietruczuk M, Małecka-Panas E. Usefulness of assessing circulating levels of resistin, ghrelin, and IL-18 in alcoholic acute pancreatitis. Dig Dis Sci. 2010;55:2982-2987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Lee SH, Kim YD, Kong YH, Han KH, Jeong WJ, Lee SJ, Cheon GJ. The relevance of serum ghrelin concentration to severity of acute pancreatitis. Gut Liver. 2010;4:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y. The stomach is a source of leptin. Nature. 1998;394:790-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 769] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 53. | Gambardella C, Ferrando S, Gallus L, Ravera S, Bianchini P, Ramoino P, Fasulo S, Tagliafierro G. Leptin-like immunoreactivity in the muscle of juvenile sea bass (Dicentrarchus labrax). Microsc Res Tech. 2010;73:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 54. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [PubMed] |
| 55. | Konturek PC, Konturek SJ, Brzozowski T, Jaworek J, Hahn EG. Role of leptin in the stomach and the pancreas. J Physiol Paris. 2001;95:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Gonzalez A, Merino B, Marroquí L, Ñeco P, Alonso-Magdalena P, Caballero-Garrido E, Vieira E, Soriano S, Gomis R, Nadal A. Insulin hypersecretion in islets from diet-induced hyperinsulinemic obese female mice is associated with several functional adaptations in individual β-cells. Endocrinology. 2013;154:3515-3524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Ajala OM, Ogunro PS, Elusanmi GF, Ogunyemi OE, Bolarinde AA. Changes in serum leptin during phases of menstrual cycle of fertile women: relationship to age groups and fertility. Int J Endocrinol Metab. 2013;11:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Baldock P. Reciprocal regulation of bone and energy metabolism. Horm Res Paediatr. 2011;76 Suppl 1:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Procaccini C, De Rosa V, Galgani M, Carbone F, La Rocca C, Formisano L, Matarese G. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 60. | Gorska E, Popko K, Stelmaszczyk-Emmel A, Ciepiela O, Kucharska A, Wasik M. Leptin receptors. Eur J Med Res. 2010;15 Suppl 2:50-54. [PubMed] |
| 61. | Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437-446. [PubMed] |
| 62. | Scotece M, Conde J, López V, Lago F, Pino J, Gómez-Reino JJ, Gualillo O. Adiponectin and leptin: new targets in inflammation. Basic Clin Pharmacol Toxicol. 2014;114:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong ML, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol. 2012;56:597-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 64. | Konturek PC, Jaworek J, Maniatoglou A, Bonior J, Meixner H, Konturek SJ, Hahn EG. Leptin modulates the inflammatory response in acute pancreatitis. Digestion. 2002;65:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Cakir B, Bozkurt A, Ercan F, Yeğen BC. The anti-inflammatory effect of leptin on experimental colitis: involvement of endogenous glucocorticoids. Peptides. 2004;25:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72:330-338. [PubMed] |
| 67. | Bornstein SR, Licinio J, Tauchnitz R, Engelmann L, Negrão AB, Gold P, Chrousos GP. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Tschöp J, Nogueiras R, Haas-Lockie S, Kasten KR, Castañeda TR, Huber N, Guanciale K, Perez-Tilve D, Habegger K, Ottaway N. CNS leptin action modulates immune response and survival in sepsis. J Neurosci. 2010;30:6036-6047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Jaworek J, Bonio J, Leja-Szpa A, Nawrot K, Tomaszewska MR, Stachura J, Pawlik WW, Konturek SJ. Sensory nerves in central and peripheral control of pancreatic integrity by leptin and melatonin. J Physiol Pharmacol. 2002;53:51-74. [PubMed] |
| 70. | Jaworek J, Konturek SJ, Szlachcic A. The role of CGRP and afferent nerves in the modulation of pancreatic enzyme secretion in the rat. Int J Pancreatol. 1997;22:137-146. [PubMed] |
| 71. | Gultekin FA, Kerem M, Tatlicioglu E, Aricioglu A, Unsal C, Bukan N. Leptin treatment ameliorates acute lung injury in rats with cerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:2932-2938. [PubMed] |
| 72. | Warzecha Z, Dembiński A, Ceranowicz P, Jaworek J, Konturek PC, Dembiński M, Bilskl J, Konturek SJ. Influence of leptin administration on the course of acute ischemic pancreatitis. J Physiol Pharmacol. 2002;53:775-790. [PubMed] |
| 73. | Bonior J, Jaworek J, Konturek SJ, Pawlik WW. Leptin is the modulator of HSP60 gene expression in AR42J cells. J Physiol Pharmacol. 2006;57 Suppl 7:135-143. [PubMed] |
| 74. | Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 75. | Zyromski NJ, Mathur A, Pitt HA, Lu D, Gripe JT, Walker JJ, Yancey K, Wade TE, Swartz-Basile DA. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G552-G558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15:5260-5265. [PubMed] |
| 77. | Epps CW, Castillo JA, Schmidt-Küntzel A, du Preez P, Stuart-Hill G, Jago M, Naidoo R. Contrasting historical and recent gene flow among African buffalo herds in the Caprivi Strip of Namibia. J Hered. 2013;104:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Segersvärd R, Tsai JA, Herrington MK, Wang F. Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis. Obesity (Silver Spring). 2008;16:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Segersvärd R, Sylván M, Herrington M, Larsson J, Permert J. Obesity increases the severity of acute experimental pancreatitis in the rat. Scand J Gastroenterol. 2001;36:658-663. [PubMed] |
| 80. | Yavuz N, Unal E, Memisoglu K, Krand O, Kiziler AR, Aydemir B, Kusaslan R, Dogan M, Gunes P, Titiz I. Plasma leptin levels in rats with pancreatitis. Tohoku J Exp Med. 2004;204:243-248. [PubMed] |
| 81. | Duarte-Rojo A, Lezama-Barreda A, Ramirez-Iglesias MT, Peláez-Luna M, Robles-Díaz G. Is leptin related to systemic inflammatory response in acute pancreatitis? World J Gastroenterol. 2006;12:4392-4396. [PubMed] |
| 82. | Tukiainen E, Kylanpaa ML, Ebeling P, Kemppainen E, Puolakkainen P, Repo H. Leptin and adiponectin levels in acute pancreatitis. Pancreas. 2006;32:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Karpavicius A, Dambrauskas Z, Sileikis A, Vitkus D, Strupas K. Value of adipokines in predicting the severity of acute pancreatitis: comprehensive review. World J Gastroenterol. 2012;18:6620-6627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61:383-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 85. | Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2011;17:3888-3898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 158] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 86. | Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960;235:1992-1997. [PubMed] |
| 87. | Bubenik GA, Pang SF, Hacker RR, Smith PS. Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of food. J Pineal Res. 1996;21:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Hardeland R, Poeggeler B. Melatonin and synthetic melatonergic agonists: actions and metabolism in the central nervous system. Cent Nerv Syst Agents Med Chem. 2012;12:189-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Messner M, Huether G, Lorf T, Ramadori G, Schwörer H. Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sci. 2001;69:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Kvetnoy IM, Ingel IE, Kvetnaia TV, Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV. Gastrointestinal melatonin: cellular identification and biological role. Neuro Endocrinol Lett. 2002;23:121-132. [PubMed] |
| 91. | Bubenik GA, Brown GM. Pinealectomy reduces melatonin levels in the serum but not in the gastrointestinal tract of rats. Biol Signals. 1997;6:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995;19:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 93. | Ahmed R, Mahavadi S, Al-Shboul O, Bhattacharya S, Grider JR, Murthy KS. Characterization of signaling pathways coupled to melatonin receptors in gastrointestinal smooth muscle. Regul Pept. 2013;184:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 500] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 95. | Bazwinsky-Wutschke I, Bieseke L, Mühlbauer E, Peschke E. Influence of melatonin receptor signalling on parameters involved in blood glucose regulation. J Pineal Res. 2014;56:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 96. | Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 97. | Reiter RJ, Paredes SD, Korkmaz A, Jou MJ, Tan DX. Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip Toxicol. 2008;1:137-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 98. | Martínez-Cruz F, Osuna C, Guerrero JM. Mitochondrial damage induced by fetal hyperphenylalaninemia in the rat brain and liver: its prevention by melatonin, Vitamin E, and Vitamin C. Neurosci Lett. 2006;392:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Gałecka E, Mrowicka M, Malinowska K, Gałecki P. [Chosen non-enzymatic substances that participate in a protection against overproduction of free radicals]. Pol Merkur Lekarski. 2008;25:269-272. [PubMed] |
| 100. | Shi C, Andersson R, Zhao X, Wang X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology. 2005;5:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Ochoa JJ, Díaz-Castro J, Kajarabille N, García C, Guisado IM, De Teresa C, Guisado R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J Pineal Res. 2011;51:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Shagirtha K, Muthumani M, Prabu SM. Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci. 2011;15:1039-1050. [PubMed] |
| 103. | Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1151] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 104. | Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 105. | Chen HM, Chen JC, Ng CJ, Chiu DF, Chen MF. Melatonin reduces pancreatic prostaglandins production and protects against caerulein-induced pancreatitis in rats. J Pineal Res. 2006;40:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Ganguly K, Sharma AV, Reiter RJ, Swarnakar S. Melatonin promotes angiogenesis during protection and healing of indomethacin-induced gastric ulcer: role of matrix metaloproteinase-2. J Pineal Res. 2010;49:130-140. [PubMed] [DOI] [Full Text] |
| 107. | Li X, Zhang M, Tang W. Effects of melatonin on streptozotocin-induced retina neuronal apoptosis in high blood glucose rat. Neurochem Res. 2013;38:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 108. | Jaworek J, Leja-Szpak A, Bonior J, Nawrot K, Tomaszewska R, Stachura J, Sendur R, Pawlik W, Brzozowski T, Konturek SJ. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J Pineal Res. 2003;34:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Jaworek J, Nawrot-Porabka K, Leja-Szpak A, Bonior J, Szklarczyk J, Kot M, Konturek SJ, Pawlik WW. Melatonin as modulator of pancreatic enzyme secretion and pancreatoprotector. J Physiol Pharmacol. 2007;58 Suppl 6:65-80. [PubMed] |
| 110. | Gülben K, Ozdemir H, Berberoğlu U, Mersin H, Yrkin F, Cakýr E, Aksaray S. Melatonin modulates the severity of taurocholate-induced acute pancreatitis in the rat. Dig Dis Sci. 2010;55:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 111. | Muñoz-Casares FC, Padillo FJ, Briceño J, Collado JA, Muñoz-Castañeda JR, Ortega R, Cruz A, Túnez I, Montilla P, Pera C. Melatonin reduces apoptosis and necrosis induced by ischemia/reperfusion injury of the pancreas. J Pineal Res. 2006;40:195-203. [PubMed] |
| 112. | Qi W, Tan DX, Reiter RJ, Kim SJ, Manchester LC, Cabrera J, Sainz RM, Mayo JC. Melatonin reduces lipid peroxidation and tissue edema in cerulein-induced acute pancreatitis in rats. Dig Dis Sci. 1999;44:2257-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Baykal A, Iskit AB, Hamaloglu E, Guc MO, Hascelik G, Sayek I. Melatonin modulates mesenteric blood flow and TNFalpha concentrations after lipopolysaccharide challenge. Eur J Surg. 2000;166:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Sun X, Shao Y, Jin Y, Huai J, Zhou Q, Huang Z, Wu J. Melatonin reduces bacterial translocation by preventing damage to the intestinal mucosa in an experimental severe acute pancreatitis rat model. Exp Ther Med. 2013;6:1343-1349. [PubMed] |
| 115. | Szabolcs A, Reiter RJ, Letoha T, Hegyi P, Papai G, Varga I, Jarmay K, Kaszaki J, Sari R, Rakonczay Z. Effect of melatonin on the severity of L-arginine-induced experimental acute pancreatitis in rats. World J Gastroenterol. 2006;12:251-258. [PubMed] |
| 116. | Jaworek J, Konturek SJ, Leja-Szpak A, Nawrot K, Bonior J, Tomaszewska R, Stachura J, Pawlik WW. Role of endogenous melatonin and its MT2 receptor in the modulation of caerulein-induced pancreatitis in the rat. J Physiol Pharmacol. 2002;53:791-804. [PubMed] |
| 117. | Brown SL, Birch DA, Kancherla V. Bullying perspectives: experiences, attitudes, and recommendations of 9- to 13-year-olds attending health education centers in the United States. J Sch Health. 2005;75:384-392. [PubMed] [DOI] [Full Text] |
| 118. | Jaworek J, Konturek SJ, Tomaszewska R, Leja-Szpak A, Bonior J, Nawrot K, Palonek M, Stachura J, Pawlik WW. The circadian rhythm of melatonin modulates the severity of caerulein-induced pancreatitis in the rat. J Pineal Res. 2004;37:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Huai JP, Sun XC, Chen MJ, Jin Y, Ye XH, Wu JS, Huang ZM. Melatonin attenuates acute pancreatitis-associated lung injury in rats by modulating interleukin 22. World J Gastroenterol. 2012;18:5122-5128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Sidhu S, Pandhi P, Malhotra S, Vaiphei K, Khanduja KL. Melatonin treatment is beneficial in pancreatic repair process after experimental acute pancreatitis. Eur J Pharmacol. 2010;628:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Reiter RJ, Tan DX, Rosales-Corral S, Manchester LC. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem. 2013;13:373-384. [PubMed] |
| 122. | Bonior J, Jaworek J, Konturek SJ, Pawlik WW. Increase of heat shock protein gene expression by melatonin in AR42J cells. J Physiol Pharmacol. 2005;56:471-481. [PubMed] |
| 123. | Belyaev O, Herzog T, Munding J, Bolik B, Vosschulte A, Uhl W, Müller CA. Protective role of endogenous melatonin in the early course of human acute pancreatitis. J Pineal Res. 2011;50:71-77. [PubMed] [DOI] [Full Text] |
| 124. | Jaworek J, Zwirska-Korczala K, Szklarczyk J, Nawrot-Porąbka K, Leja-Szpak A, Jaworek AK, Tomaszewska R. Pinealectomy aggravates acute pancreatitis in the rat. Pharmacol Rep. 2010;62:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Jin Y, Lin CJ, Dong LM, Chen MJ, Zhou Q, Wu JS. Clinical significance of melatonin concentrations in predicting the severity of acute pancreatitis. World J Gastroenterol. 2013;19:4066-4071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |