Published online Jan 28, 2014. doi: 10.3748/wjg.v20.i4.1074
Revised: October 25, 2013
Accepted: November 12, 2013
Published online: January 28, 2014
Processing time: 174 Days and 1.5 Hours
AIM: To evaluate whether granulocyte colony-stimulating factor receptor (G-CSFR) expression before preoperative irradiation can predict the radiosensitivity of rectal cancer.
METHODS: The expression of G-CSFR was examined, using immunohistochemistry, in biopsy specimens from 126 patients with locally advanced rectal adenocarcinoma before preoperative irradiation. Radiosensitivity was then evaluated according to the Rectal Cancer Regression Grading. Endoscopic inspection was used to detect the tumor area in each patient. General patient information, such as age, gender, lymph node status, tumor size and degree of differentiation was recorded. A statistical analysis was then performed to evaluate the correlation between clinical or pathological parameters and G-CSFR expression in tumors.
RESULTS: According to endoscopic inspection, the tumor area ranged from 4 to 48 cm2 (median, 15 cm2). Positive G-CSFR immunoreactions (G-CSFR+) were observed in 85 specimens, and negative (G-CSFR-) in 41. No significant differences were found in age, gender, tumor invasion, lymph node status and tumor size between G-CSFR+ and G-CSFR- patients. G-CSFR expression was positively correlated with poor radiotherapy response (58.8% vs 75.6%, P = 0.014, r = 0.219). The proportion of well-differentiated tumors in G-CSFR+ and G-CSFR- patients was 24.7% and 36.6%, respectively. Sphincter preservation was observed in 57.6% of G-CSFR+ patients and 78.5% of G-CSFR- patients. Significant correlations were found between G-CSFR expression and tumor differentiation (24.7% vs 36.6%, P = 0.019, r = 0.210), as well as sphincter preservation (57.6% vs 78.5%, P = 0.044, r = 0.180).
CONCLUSION: The expression of G-CSFR before preoperative irradiation may predict the radiosensitivity of rectal cancer.
Core tip: It is unknown whether the expression of granulocyte colony-stimulating factor receptor (G-CSFR) can predict tumor response or sphincter preservation in patients with rectal cancer receiving preoperative radiotherapy. This study found that the expression of G-CSFR before preoperative irradiation may predict the radiosensitivity of rectal cancer.
- Citation: Yang XD, Huang P, Wang F, Xu ZK. Expression of granulocyte colony-stimulating factor receptor in rectal cancer. World J Gastroenterol 2014; 20(4): 1074-1078
- URL: https://www.wjgnet.com/1007-9327/full/v20/i4/1074.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i4.1074
Colorectal cancer is one of the most common cancers in humans and the second leading cause of cancer death[1,2]. Each year there are approximately 42000 newly diagnosed cases in the United States alone, and 1 million worldwide[3]. As the main treatment option is confined to surgery, preoperative radiotherapy has been used to improve the outcome of surgery in patients with rectal cancer. Studies have shown that the local recurrence rate was 20%-60% in patients with locally advanced rectal cancer treated with surgery alone, whereas adjuvant radiotherapy improved local control and survival rate[3]. Other studies also showed the benefits of preoperative radiotherapy[4,5]. When comparing the benefits of neo-adjuvant radiotherapy with adjuvant radiotherapy, the former therapy is superior because it decreases tumor bulk, enhances sphincter preservation and reduces acute toxicity. As a result, preoperative pelvic radiotherapy resulted in a 4%-30% pathological complete response and a partial response in 30%-60% of cases[3-5].
Partial response after neo-adjuvant radiation therapy occurs in 46.7% of patients, and of these patients, 17.9% have complete responses. A previous study demonstrated that response to preoperative radiotherapy varies, depending on clinical factors such as tumor stage, total dose, fractionation schedules, concomitant chemotherapy treatment or time between radiation and surgery[6]. The study also suggested that although tumor-node-metastasis classification was useful for staging patients and selecting them for specific treatments, it was not sufficient: patients at the same stage may have different outcomes[6]. Therefore, additional prognostic biomarkers are needed for the clinical management of patients with rectal cancer. Significant research effort has been devoted to developing molecular targeted therapies or searching for molecular markers that are useful either in predicting treatment outcome or in selecting patients for specific molecular targeted therapies, based on particular tumor characteristics[7].
A previous study in our laboratory demonstrated that granulocyte colony-stimulating factor receptor (G-CSFR) was expressed in most human colorectal cancers, was involved in the development of human colorectal cancer and may be associated with more aggressive cancer[8]. However, little data on the effect of G-CSFR expression on response to pelvic radiotherapy in the preoperative setting are available. Thus, in the present study, we determined the expression level of G-CSFR in locally advanced rectal cancer and assessed whether G-CSFR expression could predict tumor response in patients treated with preoperative radiation therapy.
All patients gave informed consent before their inclusion in the study, and the Ethics Committee of the hospital approved all the human studied, which were performed in accordance with the ethical standards. The inclusion criteria were as follows: (1) histologically confirmed diagnosis of adenocarcinoma of the rectum, with the inferior margin of the tumor less than 10 cm from the anal verge; (2) clinically staged as locally advanced rectal cancer; (3) administration of preoperative radiotherapy followed by surgical resection; and (4) available tissue samples of the diagnostic biopsy and tumor specimen for review and immunostaining. From September 2002 to October 2007, 126 patients with locally advanced rectal adenocarcinoma were administered preoperative radiotherapy in the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). All patients were free of distant metastases at the time of diagnosis. Assessment of local extension was based on clinical and radiographic evaluations. Diagnostic studies consisted of colorectal endoscopy, abdominopelvic computed tomography (CT), chest X-ray, endoscopic ultrasonography and routine laboratory studies. The patients were staged according to the American Joint Committee on Cancer Staging.
Consecutive sections (4 μm) of formalin-fixed and paraffin-embedded tissue specimens were used for immunohistochemistry. The sections were stained using a labeled streptavidin-biotin peroxidase method (LSAB2 Kit; Dako Japan Inc., Kyoto, Japan) comprising a mouse monoclonal anti-G-CSFR antibody (Serotec Ltd, United Kingdom; dilution 1:80). The slides were immersed in 0.3% hydrogen peroxide/methanol for 10 min to deplete endogenous peroxidase. Then, nonspecific binding sites were blocked with 0.3% normal goat serum for 10 min. The primary antibody was then applied, and the sections were incubated overnight at 4 °C. After washing with phosphate-buffered saline (PBS, 0.01 mol/L pH 7.4), the secondary antibody (biotinylated goat-anti-mouse IgG) was applied to the tissue sections, which were incubated at room temperature for 10 min. After washing with PBS, a streptavidin peroxidase reagent was applied and the sections were incubated at room temperature for 10 min. Finally, the reaction product was visualized by developing the color following incubation of the slides in a solution of 0.3% hydrogen peroxide and 3-amino-9-ethylcarbazole chromogen. The sections were lightly counterstained with hematoxylin. Negative controls were parallel sections in which the primary antibody was replaced with the same volume of PBS. The immunoreactions were graded as negative (0), weakly positive (1), moderately positive (2), or strongly positive (3), according to the immunostaining intensity. Two independent pathologists who had no pre-knowledge of this study read all the slides. In the case of disagreement between the two pathologists, a third pathologist read the slides, discussed the results with his/her two colleagues, and then made the final decision.
Physical examination, endorectal ultrasound and pelvic CT were used to stage the patients. Preoperative radiotherapy was delivered to the pelvis with 60CO. The clinical target volume included the tumor, the entire rectum, the anterior wall of the sacrum, the posterior wall of the prostate or vagina, and the perirectal, presacral, hypogastric, actuator, and iliac lymph nodes. The standard anterior-posterior/posterior-anterior fields received 45 Gy, and then two opposed lateral boost fields up to 50.4 Gy were used. All patients received conventional fractionation of 1.80 Gy/d, and five fractions per week. Physical examination, endorectal ultrasound and pelvic CT were used to restage the patients four to six weeks later. The patients then received total mesorectal excision by a group of surgeons in our hospital. Subsequently, the pathological response was evaluated in postsurgical specimens and graded according to the method described by Mandard et al[9]. This method was used to assess the pathological response after neo-adjuvant radiotherapy in rectal cancer on a scale of 1-5, based on the presence of residual tumor cells and the extent of fibrosis. The two pathologists who were blinded to the study assessed the grade of tumor response. We considered grade 1, which is defined as the absence of residual tumor cells and fibrosis extending through the layers of the rectum, as “complete pathological response”. We considered grade 2-4, which are characterized by the presence of various amounts of residual tumor cells, as “partial response”, and grade 5, defined as absence of tumor response, as “no response”.
The Kendall’s tau-b and the Spearman test were used to examine the correlation between various clinical or pathological parameters and the expression of G-CSFR in tumors. The correlation was evaluated using the Spearman test. P values less than 0.05 were considered statistically significant. All calculations were performed using SPSS 12.0 for Windows.
General patient information, such as age, gender, lymph node status, tumor size and degree of differentiation are summarized in Table 1. There were 54 men and 72 women, aged from 30 to 90 years (median, 60 years). According to the endoscopic inspection, the tumor area ranged from 4 to 48 cm2 (median, 15 cm2) (Figure 1). The results of G-CSFR immunostaining are also summarized in Table 1. Positive immunoreactions (G-CSFR+) of cytoplasmic staining in tumor cells were observed in 85 of 126 pre-radiation biopsy specimens, while 41 were negative (G-CSFR-). No significant differences were found in age, gender, tumor invasion, lymph node status and tumor size between G-CSFR+ patients and G-CSFR- patients. There were 36 well differentiated tumors, 55 moderately differentiated tumors and 35 poorly differentiated tumors.
| Clinicopathological | Cases | G-CSFR | Pvalue | r | |||
| parameters | 0 | 1 | 2 | 3 | |||
| 41 | 26 | 28 | 31 | ||||
| Age (yr) | |||||||
| < 60 | 67 | 20 | 17 | 15 | 15 | ||
| ≥ 60 | 59 | 21 | 9 | 13 | 16 | NS | |
| Gender | |||||||
| Male | 54 | 18 | 10 | 11 | 15 | ||
| Female | 72 | 23 | 16 | 17 | 16 | NS | |
| Pathological stage | |||||||
| LN- | 72 | 21 | 12 | 20 | 19 | ||
| LN+ | 54 | 20 | 14 | 8 | 12 | NS | |
| T3 | 38 | 13 | 6 | 9 | 10 | ||
| T4 | 88 | 28 | 20 | 19 | 21 | NS | |
| Size (cm2) | |||||||
| > 15 | 66 | 18 | 14 | 15 | 19 | ||
| ≤ 15 | 60 | 23 | 12 | 13 | 12 | NS | |
| Differentiation | |||||||
| Well | 36 | 15 | 6 | 10 | 5 | ||
| Moderate | 55 | 17 | 15 | 13 | 10 | ||
| Poor | 35 | 9 | 5 | 5 | 16 | 0.019 | 0.210 |
| Sphincter preservation | |||||||
| Yes | 81 | 32 | 15 | 17 | 17 | ||
| No | 45 | 9 | 11 | 11 | 14 | 0.044 | 0.180 |
| Distance from anal verge (cm) | |||||||
| 5-10 | 82 | 28 | 17 | 19 | 18 | ||
| < 5 | 44 | 13 | 9 | 9 | 13 | NS | |
| Pathological response | |||||||
| Complete | 37 | 18 | 7 | 5 | 7 | ||
| Partial | 44 | 13 | 10 | 11 | 10 | ||
| No response | 45 | 10 | 9 | 12 | 14 | 0.014 | 0.219 |
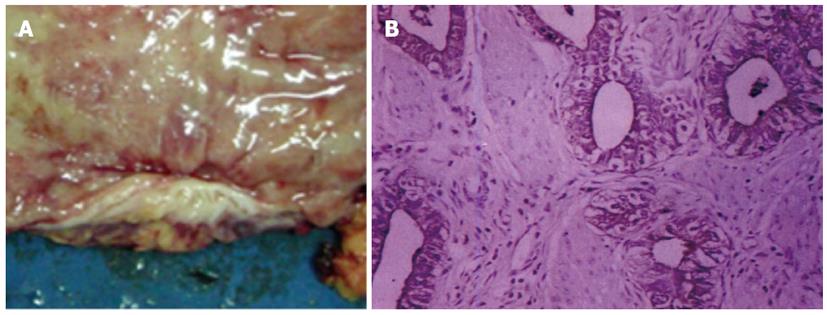
Significant correlations were found for the pathological response, tumor differentiation and the proportion of sphincter preservation between G-CSFR+ patients and G-CSFR- patients. In this study, the proportion of sphincter preservation was 57.6% (49/85) in G-CSFR+ patients, compared to 78.5% (32/41) in G-CSFR- patients (P = 0.044, r = 0.180). The proportion of well differentiated tumors in G-CSFR+ patients was 24.7% (21/85), while that in G-CSFR- patients was 36.6% (15/41) (P = 0.019, r = 0.210). The distance from the anal verge to the inferior margin of the tumor was 5 - 10 cm in 82 patients, whereas the distance was less than 5 cm in 44 patients. Various responses to radiotherapy were seen in the 126 post-radiation rectal cancer specimens. In hematoxylin-stained slides, 81 patients (64.3%, 81/126) responded to pelvic preoperative radiotherapy. Of these patients, 37 (29.4%, 37/126) achieved a complete pathological response, with no residual tumor in the resected specimens, and the cancer cells were completely replaced by fibrosis, necrosis, or calcified tissue (Figure 2). Partial responses were seen in 44 patients (34.9%, 44/126). Response to pelvic radiotherapy was observed in 75.6% (31/41) of G-CSFR- patients and 58.8% (50/85) of G-CSFR+ patients (P = 0.014, r = 0.219). Only 19 (22.4%, 19/85) G-CSFR+ patients achieved complete pathological response, compared with 43.9% (18/41) of G-CSFR- patients. Multivariate analysis showed that G-CSFR status was a significant predictor of complete remission.
Mitotic or clonogenic cell death is considered to be the major mechanism of most solid tumors response to clinical radiotherapy[10]. With increased knowledge of cell cycle regulation, apoptosis and DNA repair, attempts have been made to identify molecular markers capable of predicting the radiation sensitivity of tumors. Markers, including p53, have been studied in cell cycle regulation, apoptosis and resistance to radiation[11,12]. However, it is questionable whether these constituents can be used to predict radiotherapy response. In the present study, we followed patients in an attempt to identify a new specific molecular marker for radiotherapy sensitivity.
Our study is the first to examine the relationship between G-CSFR expression and tumor radiosensitivity in rectal cancer. The results demonstrated that G-CSFR overexpression was significantly associated with a poor response to radiotherapy, although the exact mechanism involved in this relationship remains unknown. Our previous study proved that G-CSFR expression was upregulated in colorectal cancer cells compared with normal mucous membranes[8]. The current findings further reinforce our understanding of the mechanistic role of G-CSF/G-CSFR signaling in colorectal cancer. It is possible that the behavior of colorectal tumors expressing G-CSFR may be influenced by locally expressed G-CSF. G-CSF can be produced either by white blood cells, which frequently infiltrate colorectal tumors, or by cancer cells themselves, as shown in cultured bladder carcinoma and astrocytoma cell lines[13,14]. Downstream G-CSF signaling in non-hematopoietic cells is not well understood, although it has been intensively studied in hematopoietic cells[15]. The proliferative effect of G-CSF in colorectal cancer in vivo and in vitro, and the mechanism involved were not studied. Interestingly in our study, a significant correlation was found between G-CSFR expression on pre-radiation biopsy and tumor differentiation. This indicated that the G-CSF/G-CSFR signaling pathway may be involved in the development of rectal cancer, and that G-CSFR levels may be correlated with either aggressive disease or a poor prognosis. G-CSFR may mediate a cytoprotective response to reduce cell sensitivity to radiation, similar to the mechanism of accelerated proliferation in hematopoietic tumor cells. Therefore, preoperative radiotherapy may be able to eradicate G-CSFR- tumor cells, whereas G-CSFR+ tumor cells may remain active because of their radio-resistance.
In conclusion, our study indicates that there is a significant correlation between G-CSFR expression and tumor radiosensitivity. Examination of G-CSFR expression suggested that pre-radiation biopsy specimens could predict the radiosensitivity of locally advanced rectal cancer before pre-operative irradiation, and that G-CSFR expression was associated with a lack of pathological complete response. Overall, our analysis of G-CSFR expression is helpful in determining a subgroup of high-risk patients suitable for more therapeutic modalities, such as appropriate monoclonal antibodies against G-CSFR.
Colorectal cancer is one of the most common cancers in humans. The expression of granulocyte colony-stimulating factor receptor (G-CSFR) is involved in the development of human colorectal cancer, and may be associated with more aggressive cancer. The authors investigated whether G-CSFR expression could predict tumor response or sphincter preservation in patients with rectal cancer receiving preoperative radiotherapy.
Markers, including p53, transforming growth factor beta1 and epidermal growth factor receptor, have been studied in cell cycle regulation, apoptosis and resistance to radiation. Previous studies have proved that G-CSFR expression was upregulated in colorectal cancer cells, compared to the normal mucous membrane.
This study is the first to examine the relationship between G-CSFR expression and tumor radiosensitivity in rectal cancer. It was found that the expression of G-CSFR before preoperative irradiation may predict the radiosensitivity of rectal cancer. The current findings further reinforce their understanding of the mechanistic role of the G-CSF/G-CSFR signal in colorectal cancer.
G-CSFR expression in pre-radiation biopsy specimens can predict the radiosensitivity of locally advanced rectal cancer before preoperative irradiation. Analysis of G-CSFR expression is helpful in determining a subgroup of high-risk patients suitable for more therapeutic modalities, such as appropriate monoclonal antibodies against G-CSFR.
G-CSFR, also known as cluster of differentiation 114, is a cell-surface receptor for granulocyte colony-stimulating factor and comprises an extracellular ligand-binding portion, a transmembrane domain and a cytoplasmic portion that is responsible for signal transduction.
The authors evaluated the correlation between clinical or pathological parameters and the G-CSFR expression of tumors. The results presented were very interesting and suggest that the expression of G-CSFR before preoperative irradiation may predict radiosensitivity of rectal cancer.
P- Reviewers: Crea F, De Nardi P, Leitman M, Langner C, Hiraki M, Kim YJ, Triantafyllou K S- Editor: Gou SX L- Editor: Stewart GJ E- Editor: Wang CH
| 1. | Walker JJ, Brewster DH, Colhoun HM, Fischbacher CM, Lindsay RS, Wild SH. Cause-specific mortality in Scottish patients with colorectal cancer with and without type 2 diabetes (2000-2007). Diabetologia. 2013;56:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Liu L, Lemmens VE, De Hingh IH, de Vries E, Roukema JA, van Leerdam ME, Coebergh JW, Soerjomataram I. Second primary cancers in subsites of colon and rectum in patients with previous colorectal cancer. Dis Colon Rectum. 2013;56:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Wilkinson N, Scott-Conner CE. Surgical therapy for colorectal adenocarcinoma. Gastroenterol Clin North Am. 2008;37:253-267, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Penopoulos V, Handolias M, Avgerinos A, Maris T, Ilias T, Issopoulos N, Christianopoulos G, Betsis D, Vrettou E, Kitis G. A short course of preoperative radiotherapy improves prognosis of operable rectal carcinoma: a case control study. Hepatogastroenterology. 2008;55:1280-1287. [PubMed] |
| 5. | Park IJ, Kim HC, Yu CS, Kim TW, Jang SJ, Kim JC. Effect of adjuvant radiotherapy on local recurrence in stage II rectal cancer. Ann Surg Oncol. 2008;15:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, Lim SB, Choi HS, Jeong SY, Park JG. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Gosens MJ, Dresen RC, Rutten HJ, Nieuwenhuijzen GA, van der Laak JA, Martijn H, Tan-Go I, Nagtegaal ID, van den Brule AJ, van Krieken JH. Preoperative radiochemotherapy is successful also in patients with locally advanced rectal cancer who have intrinsically high apoptotic tumours. Ann Oncol. 2008;19:2026-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Yang X, Liu F, Xu Z, Chen C, Wu X, Li G, Li J. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J. 2005;81:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 10. | Wang J, Yi J. Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer Biol Ther. 2008;7:1875-1884. [PubMed] |
| 11. | Rajkumar T, Samson M, Rama R, Sridevi V, Mahji U, Swaminathan R, Nancy NK. TGFbeta1 (Leu10Pro), p53 (Arg72Pro) can predict for increased risk for breast cancer in south Indian women and TGFbeta1 Pro (Leu10Pro) allele predicts response to neo-adjuvant chemo-radiotherapy. Breast Cancer Res Treat. 2008;112:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Ahmed WA, Suzuki K, Imaeda Y, Horibe Y. Ki-67, p53 and epidermal growth factor receptor expression in early glottic cancer involving the anterior commissure treated with radiotherapy. Auris Nasus Larynx. 2008;35:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Quentmeier H, Zaborski M, Drexler HG. The human bladder carcinoma cell line 5637 constitutively secretes functional cytokines. Leuk Res. 1997;21:343-350. [PubMed] |
| 14. | Kikuchi T, Nakahara S, Abe T. Granulocyte colony-stimulating factor (G-CSF) production by astrocytoma cells and its effect on tumor growth. J Neurooncol. 1996;27:31-38. [PubMed] |
| 15. | Sarkar CA, Lauffenburger DA. Cell-level pharmacokinetic model of granulocyte colony-stimulating factor: implications for ligand lifetime and potency in vivo. Mol Pharmacol. 2003;63:147-158. [PubMed] |