Published online Oct 21, 2014. doi: 10.3748/wjg.v20.i39.14442
Revised: June 27, 2014
Accepted: July 22, 2014
Published online: October 21, 2014
Processing time: 221 Days and 21.5 Hours
AIM: To determine whether intra-arterial infusion of triolein emulsion has biochemical and histopathologic effect on rabbit liver.
METHODS: An emulsion of 0.2 mL triolein in 20 mL of saline was infused into either the hepatic arteries of nine rabbits (group 1) or the superior mesenteric arteries of 12 rabbits (group 2). Five rabbits infused with 20 mL of normal saline were used as a control group (group 3). The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured to evaluate liver function in each group just before the infusion, at 2 h, day 1, day 4, and day 7 following infusion. Each rabbit in all of the groups was infused with Evans blue on day 7 to evaluate changes in vascular permeability, and obtain the stained area of the hepatic surface. If the stained area was not available, the anteroinferior portion of the right hepatic lobe was selected. The obtained tissues were examined by light, electron and confocal microscopy. The changes in AST and ALT levels at each time point were calculated and statistically analyzed using a mixed linear model. A P value < 0.05 was regarded as statistically significant.
RESULTS: In group 1 (hepatic artery group), both the AST and ALT serum levels increased significantly on day 1 (P = 0.0016 and P < 0.0001, respectively) compared with the control group, followed by a decrease thereafter. In group 2 (portal vein group), the AST level increased on day 4 (P = 0.0095), while the ALT level increased significantly on day 1 (P < 0.0001), and decreased thereafter, as compared with the control. For the remainder of the examination days, there were no significant changes in the AST and ALT levels (P > 0.05). Only three rabbits in each group showed hepatic surface staining with the Evans blue dye. Light and electron microscopic findings showed no specific changes in the selected hepatic tissues. Confocal microscopic examination with transferase-mediated dUTP nick-end labeling stain revealed lack of hepatocyte apoptosis in any of the groups. There were no differences in the results between group 1 and group 2.
CONCLUSION: Infusion of triolein emulsion into rabbit livers revealed a minimal transient decrease of liver function, and no specific histopathologic changes.
Core tip: Unlike triolein bolus, the infusion of triolein emulsion into a liver did not lead to mechanical obstruction of the vessels. In fact, triolein emulsion transiently and reversibly increased the vascular permeability, while triolein bolus induced obstruction similar to the fat embolism syndrome. This result suggests that trioleincould be applicable for adjuvant treatment for intractable diseases due to blood barriers (blood-brain, blood-retinal, or blood-testis barrier). This study aimed to determine potential side effects of triolein emulsion infusion into the liver with the sinusoidal capillary prior to the application of the technique in the organs with blood barrier.
- Citation: Kim YW, Park YM, Yoon S, Kim HJ, Park DY, Cho BM, Choi SH. Effect of intra-arterial infusion with triolein emulsion on rabbit liver. World J Gastroenterol 2014; 20(39): 14442-14449
- URL: https://www.wjgnet.com/1007-9327/full/v20/i39/14442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i39.14442
The capillaries can be classified into three types according to their structural characteristics as determined by electron microscopy. The type I capillaries, also known as continuous capillaries, are found in many tissues, including muscle, lung, the central nervous system, and skin. The type I endothelial cells contain a large amount of cytoplasm in relation to the nucleus, fine filaments, and numerous pinocytotic vesicles. The pinocytotic vesicles have a diameter of 50-70 nm and are most likely involved in the transport of fluid across the capillary wall, representing the sites of the so-called “large-pore” system of capillary permeability. The endothelial cells are held together by either simple or interdigitated junctions. In most type I endothelial cells, a narrow gap exists between the opposed cell membranes containing some electron-dense material. However, in certain regions, the opposed cell membranes fuse to form tight junctions. The type II capillaries or the fenestral capillaries are commonly found in the intestinal mucosa, many endocrine glands, the renal glomerulus, and the pancreas. Within the fenestral capillary endothelium, the cytoplasm on each side of the nucleus is extremely thin and is perforated at intervals by “pores” ranging in diameter from 30-50 nm. These pores, or circular fenestrations, may be closed by a thin diaphragm. The diaphragm, which is thinner than the cell membrane, has a complex structure, and its chemical composition is unknown. The endothelial cells in these capillaries are separated by gap junctions[1]. The type III or the sinusoids or sinusoidal capillaries are found in the liver, the spleen, the bone marrow, and the lymph nodes. These capillaries have a luminal diameter much greater than that of normal capillaries. In fact, sinusoids may be 30 μm or more in diameter and have irregular, tortuous walls. Furthermore, their walls are not formed by a continuous layer of endothelial cells, as in true capillaries, and wide gaps (approximately 100 nm in diameter) exist between cells. The basal lamina in sinusoids is incomplete, and there is no morphologic barrier between the sinusoids and the perisinusoidal space. Plasma has a direct access to the surface of the liver cell, a structural feature of great functional importance for active metabolic exchange between the liver and the blood[2].
The brain, the eyeball, and the testis are comprised of continuous capillaries and specialized blood-organ barriers (the blood-brain barrier, the blood-ocular barrier, and the blood-testis barrier, respectively) to protect from penetration of harmful agents. These barriers can, however, be obstacles to drug delivery and can decrease therapeutic drug effects[3-5]. There have been studies attempting to open the blood-organ barriers by intra-arterially injected triolein emulsion in the brain[6], the eyeball[7], and the testis[8]. The blood-organ barrier breakdown and increased vascular permeability induced by triolein injection appear as a contrast enhancement on gadolinium-enhanced magnetic resonance images[6,8]. Although the increased vascular permeability appears immediately after triolein injection, and continues for one to three days, it is reversible and reverts to normal after seven days. Furthermore, histologic findings appear to be mostly normal, except for the mild perivascular swelling[6]. Intra-arterial infusion of a triolein emulsion in the skeletal muscles, which have continuous capillaries but no blood-organ barrier, also increases vascular permeability without significant histologic changes[9]. These results suggest an adjunctive role of the triolein emulsion in chemotherapy. It is thought that if a drug is infused after a triolein emulsion injection, not only can one achieve the therapeutic effect with a small amount, but one can also enhance the drugs with a little therapeutic effect. Prior to clinical use of triolein as an adjunctive drug for chemotherapeutic treatment of tumors in the organs with blood barrier (brain, eyeball, or testis), it is necessary to study the histologic or functional changes in the other body organs into which triolein are injected.
The effect of triolein emulsion on sinusoidal capillaries has not been reported. The sinusoidal capillaries do not have a barrier, especially in the liver. Therefore, it is expected that the triolein-induced vascular change will differ from those of continuous capillaries in the brain or eyeball. The purpose of this study was to evaluate biochemical and histopathologic effects of the intra-arterial infusion of triolein emulsion on the liver’s sinusoidal capillaries as a preliminary basis of future studies.
The Animal Research Committee of the Medical Research Institution approved all experiments and surgical procedures. The study included 26 rabbits weighing 2.1-3.7 kg (average, 2.455 kg) anesthetized with intramuscularly-administered ketamine HCL (2.5 mg/kg; Korea United Pharm, Inc., Seoul, South Korea) and xylazine (0.125 mg/kg; Bayer Korea, Seoul, South Korea) and ventilated with room air. The rabbit body temperature was measured with a rectal probe (MGA-III 219; Shibaura Electronics Co. Ltd., Tokyo, Japan) and was maintained at 37.0-37.5 °C.
Following anesthetization, the right femoral artery was isolated and ligated with 4-0 silk ties. An 18-gauge catheter (Insyte; Becton Dickinson, Sandy, UT, United States) was inserted into an artery and a 2.2 F microcatheter (Progreat; Terumo, Tokyo, Japan) with a micro-guide wire (GT 0.016”; Terumo) was passed through the catheter into the lumen of an artery. The micro-catheter tip was positioned in the abdominal aorta under digital subtraction angiography (Multistar TOP; Siemens, Erlangen, Germany).
A 1-mL syringe containing 0.2 mL of triolein (1,2,3-tri [cis-9-octadecenoyl]glycerol, 99% purity, d = 0.91 g/mL; Sigma-Aldrich, St. Louis, MO, United States) and a 20-mL syringe containing 20 mL saline was connected to a 3-way stopcock. The fat emulsion was made by mixing via the stopcock using a vigorous to-and-fro movement of the syringes for 2 min. The rabbits were divided into two groups [hepatic artery group (group 1, n = 9) and portal vein group (group 2, n = 11)] with fat emulsion injected into the hepatic artery and the portal vein, respectively. In the control group (group 3, n = 5), 20 mL of saline was infused into the hepatic artery.
In nine rabbits, the emulsified fat was infused manually into the hepatic artery at a rate of 4 mL/min for 5 min. Blood sampling was performed, and the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) serum levels were checked before and at 2 h, and 1, 4, and 7 d after infusion to assess liver function changes following the infusion of triolein emulsion. The blood sampling before infusion of triolein emulsion was obtained from the femoral artery just before insertion of a 2.2-F micro-catheter as a control. The blood samples taken at 2 h, day 1, and day 4 after the infusion of triolein emulsion were obtained from the ear artery of each rabbit; the sample taken at day 7 after the infusion was taken from the right ventricle of the heart after opening of the thorax.
For the evaluation of the permeability change of the hepatic artery or portal vein, 4% Evans blue (1 mL/kg; Sigma-Aldrich) was injected intravenously to check the presence or absence of a stained hepatic surface. One hour after the injection of 4% Evans blue, the thorax of each rabbit was opened, and a small hole was made in the right ventricular wall through which blood was drained. After inserting an 18-gauge catheter (Insyte) into the left ventricle, saline was injected slowly for 2 min, followed by injection of 10% formalin for 2 min and a complete replacement of blood with clear saline. The abdominal wall was opened to check the presence or absence of staining of the hepatic surface by Evans blue, and a picture of the hepatic surface was taken by a digital camera.
In 12 rabbits, emulsified fat was injected into the portal vein via the superior mesenteric artery. The effect of the triolein emulsion on the liver was compared with the hepatic artery group (group 1). Injection of triolein emulsion into the superior mesenteric artery was performed using the same technique described in group 1.
In the five rabbits designated as controls, 20 mL of saline was infused into the hepatic artery using the same procedure described for group 1.
The rabbits were infused with Evans blue on day 7 post-infusion, and the stained areas of the hepatic surfaces were observed. In the absence of a stained area, the anteroinferior portion of the right hepatic lobe was selected. The obtained tissues were then stained with hematoxylin and eosin (H and E) and examined by light microscopy.
The sample tissues were cut into 1 mm3 cubes for electron microscopic examination. The samples were prefixed with 2.5% glutaraldehyde in phosphate buffer (PB; pH 7.2) for 2 h at 1-4 °C and washed in 0.1 mol/L PB. Next, samples were fixed in 1% OsO4 solution for 2 h and washed in 0.1 mol/L PB. After washing, all specimens were processed according to a standard procedure through upgraded alcohols and propylene oxide, and were then embedded in Epon 812 resin (Polysciences, Warrington, PA, United States). The resin-embedded blocks were cut into 1 μm thick sections and stained with toluidine blue. The areas of interest were selected under a light microscope. Ultrathin sections were cut with a diamond knife using an ultramicrotome (Leica, Wien, Austria) and applied to nickel 150 mesh grids. Samples were stained with uranyl acetate and lead citrate, and examined with a transmission electron microscope (JEM 1200 EX-II; JEOL, Tokyo, Japan). The presence or absence of vasogenic or cytotoxic edema, as well as the integrity of the vascular endothelial wall, were evaluated.
The samples were examined for apoptosis using a terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay. For paraffin sections, the tissues were fixed in 4% paraformaldehyde and neutral-buffered formalin. The specimens were dehydrated in graded ethanol, embedded in paraffin, and cut into 4 μm thick sections on a microtome (Leica, Wetzlar, Germany). The paraffin sections were mounted on 3-aminopropyltriethoxysilane-coated slides and left overnight at 40 °C. After deparaffinization and rehydration, the sections were incubated with proteinase K (20 μg/mL; Roche, Indianapolis, IN, United States) for 15 min at room temperature, and rinsed twice with phosphate-buffered saline (PBS) for 2 min each. Next, the sections were incubated in equilibration buffer for at least 10 s at room temperature, and thereafter in working strength TdT enzyme for 1 h at 37 °C. The sections were shaken about 15 times in a coplin jar containing a working strength stop/wash buffer, incubated at room temperature for 10 min, and then rinsed three times in PBS for 3 min each. Next, the sections were incubated with working strength anti-digoxigenin conjugate for 30 min in a dark room, followed by a 3-time rinse with PBS for 3 min each and mounting with mounting medium. Finally, the slides were analyzed under a Leica TCS confocal laser scanning microscope (Leica).
Statistical analysis of the data for each group was performed by analysis of time-series changes. That is, changes in AST and ALT serum levels between before and 2 h, and 1, 4, and 7 d after triolein injection were measured for each group. Both the changes of median and interquartile range were determined. Statistical analysis of these values was performed using a linear mixed model after logarithmic transformation. A P value < 0.05 was regarded as statistically significant.
The mean AST and ALT serum levels in group 1 measured just before the infusion of triolein emulsion were set as a baseline (46.33 IU/L and 58.78 IU/L, respectively; Table 1). The mean change values for the group 1 in AST and ALT serum levels 2 h after infusion of triolein emulsion showed no statistically significant difference (P > 0.05). However, the medians of mean values of changes in the group 1 AST and ALT serum levels one day after infusion were 90.00 IU/L and 56.00 IU/L (P < 0.0016 and P < 0.0001, respectively), and showed a significant increase as compared with the control group. There was no difference in the mean values of changes for the group 1 AST and ALT serum levels at day 4 and day 7 after infusion as compared with the mean values of changes before infusion (P > 0.05; Tables 2 and 3).
| Rabbit | AST, IU/L | ALT, IU/L | ||||||||
| No. | Pre | 2 h | D1 | D4 | D7 | Pre | 2h | D1 | D4 | D7 |
| Group 1 (n = 9) | ||||||||||
| Mean ± SD | 46.33 ± 13.86 | 61.78 ± 20.50 | 124.33 ± 71.68 | 30.56 ± 5.36 | 58.56 ± 21.86 | 58.78 ± 23.66 | 59.67 ± 21.15 | 116.56 ± 53.35 | 75.78 ± 30.65 | 30.65 ± 15.45 |
| Group 2 (n = 11) | ||||||||||
| Mean ± SD | 45.58 ± 9.11 | 50.08 ± 13.13 | 44.42 ± 16.97 | 31.18 ± 11.99 | 37.18 ± 10.01 | 38.50 ± 14.39 | 38.00 ± 13.62 | 57.92 ± 19.96 | 46.82 ± 16.19 | 43.73 ± 12.72 |
| Group 3 (n = 5) | ||||||||||
| Mean ± SD | 38.20 ± 9.68 | 39.40 ± 6.07 | 45.80 ± 23.41 | 53.80 ± 19.97 | 63.20 ± 4.97 | 52.20 ± 4.21 | 53.80 ± 6.38 | 60.20 ± 11.14 | 62.60 ± 7.16 | 63.80 ± 13.05 |
| AST, IU/L | Median, IU/L | Interquartile range, IU/L | |
| Group 1 (n = 9) P = 0.0016 | 2 h | 18.00 | -5.00-31.00 |
| D1 | 90.001 | 16.00-113.00 | |
| D4 | -14.00 | -28.00-(-4.50) | |
| D7 | 6.00 | -6.00-30.50 | |
| Group 2 (n = 11) P = 0.0095 | 2 h | 6.00 | -5.00-8.00 |
| D1 | -3.00 | -11.00-18.00 | |
| D4 | -14.001 | -21.00-(-8.00) | |
| D7 | -8.00 | -11.00-(-3.00) | |
| Group 3 (n = 5) P = 0.0355 | 2 h | 1.00 | -3.00-5.50 |
| D1 | 0.00 | -3.50-22.50 | |
| D4 | 13.00 | 3.00-29.50 | |
| D7 | 31.001 | 13.00-34.00 |
| ALT, IU/L | Median, IU/L | Interquartile range, IU/L | |
| Group 1 | 2 h | 1.00 | -5.50- 5.00 |
| (n = 9) | D1 | 56.001 | 29.50-84.00 |
| P < 0.0001 | D4 | 20.00 | 4.00-34.00 |
| D7 | 3.00 | -7.00-10.50 | |
| Group 2 | 2 h | 0.00 | -4.00-3.00 |
| (n = 11) | D1 | 16.001 | 12.00-31.00 |
| P < 0.0001 | D4 | 6.00 | 3.00-11.00 |
| D7 | 4.00 | 3.00-11.00 | |
| Group 3 | 2 h | 1.00 | -1.00-4.50 |
| (n = 5) | D1 | 4.00 | 2.00-16.00 |
| P > 0.05 | D4 | 7.00 | 4.00-18.50 |
| D7 | 6.00 | 2.50-23.50 |
The group 2 mean AST and ALT serum levels determined just before infusion of triolein emulsion were taken as baseline values (45.58 IU/L and 38.50 IU/L, respectively; Table 1). As compared with the values before triolein infusion, the group 2 median of mean changes showed statistical significance in AST levels at day 4 (-14.00 IU/L, P = 0.0095) and in ALT levels at day 1 (16.00 IU/L, P < 0.0001) after infusion. For the remaining examination days, there were no significant changes in the group 2 AST and ALT serum levels (P > 0.05; Tables 2 and 3).
Changes in AST and ALT serum levels before and each time after saline injection in the control group are shown in Table 1. There was no significant difference in control group AST and ALT levels between before and each time after saline injection, except for the AST level at day 7 following saline injection (P = 0.0355; Tables 2 and 3).
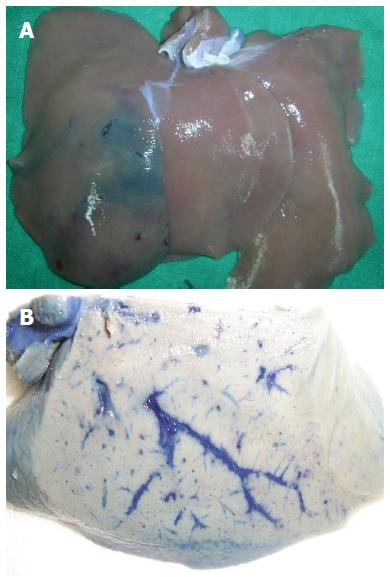
In 3/9 rabbits of group 1, the hepatic surfaces were stained blue with Evans blue (Figure 1A). The stained area measured approximately 1 cm × 1 cm to 5 cm × 2 cm. In the remaining six rabbits, the hepatic surfaces did not show blue staining. The cut surfaces of all of the rabbits revealed blue-stained hepatic vessels (Figure 1B).
One of 12 rabbits in group 2 was excluded due to death on day 4. In three of the remaining 11 rabbits, the hepatic surfaces were stained blue with Evans blue. The cut surfaces of the livers of all 11 rabbits revealed blue-stained hepatic vessels.
The hepatic surface after injection of Evans blue did not reveal blue stain in the control group. The cut surface of the liver in the control group showed blue-stained hepatic vessels.
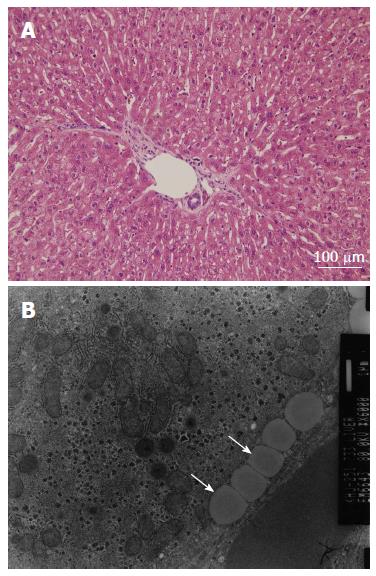
All nine rabbits in group 1 had normal livers according to results from the H and E staining and the light microscopic examination (Figure 2A). The electron microscopic examination showed multiple fat vacuoles within the vascular lumen of the group 1 rabbits with no evidence of interstitial edema. The integrity of the endothelium was well preserved in the rabbits of group 1 (Figure 2B).
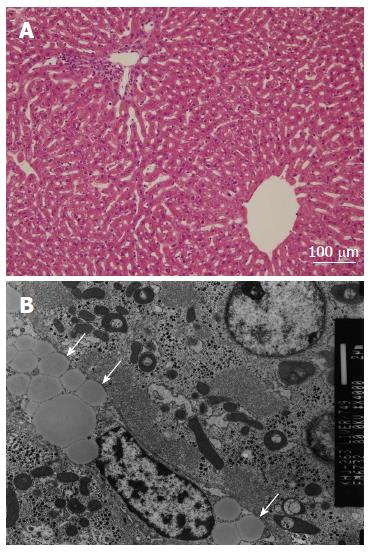
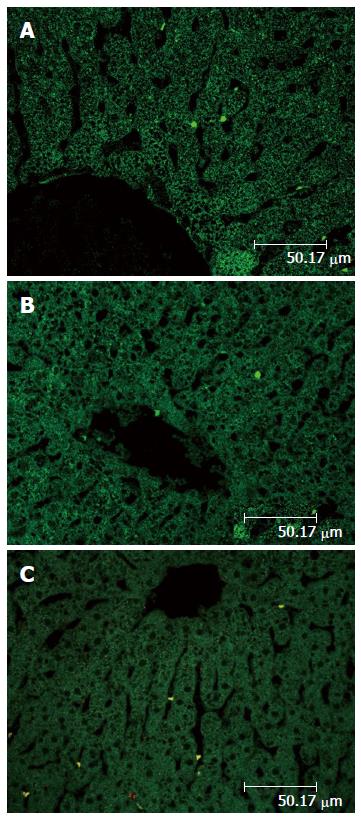
In group 2, all 11 rabbits had normal liver as determined by H and E staining and light microscopy (Figure 3A). These rabbits did have multiple fat vacuoles within the vascular lumen, as determined by the electron microscopic examination, with no evidence of vasogenic or cytotoxic edema. The integrity of the endothelium was well preserved in the rabbits of group 2 (Figure 3B). The confocal microscopic examination after TUNEL staining revealed no evidence of apoptosis in the hepatocytes of both group 1 and group 2 (Figure 4A, B).
The control group rabbits exhibited normal findings in hepatic lobules, hepatocytes, portal veins, bile canaliculi, and sinusoids upon light microscopic examination after H and E staining. The electron microscopy results showed absence of both vasogenic and cytotoxic edema, and a well preserved vascular wall. The confocal microscopy results revealed no evidence of apoptosis of the hepatocytes in the control group (Figure 4C).
In the present study, a triolein emulsion was infused either into the hepatic artery (hepatic artery group; group 1) or the portal vein via superior mesenteric artery (portal vein group; group 2). The changes in hepatic function were evaluated biochemically and histopathologically for seven days. In group 1, AST and ALT levels were increased significantly on day 1 and decreased thereafter. In group 2, the AST level increased on day 4, while the ALT level increased significantly on day 1 and decreased thereafter. When the rabbits were sacrificed and stained with Evans blue on day 7 after triolein infusion, only three rabbits in each group had their hepatic surfaces stained. The light microscopic and electron microscopic findings showed no specific change in the selected hepatic tissue for either of the groups. All of the rabbits, regardless of their grouping, did not show apoptosis in their hepatocytes, as determined by the confocal microscopic examination after TUNEL staining. There were no statistical differences in the results from groups 1 and 2. These results suggest that triolein emulsion (0.2 mL triolein in 20 mL of saline) infused into the rabbit liver does not cause significant changes in hepatic function or structure. Of note, hepatic function can be momentarily affected following triolein infusion, resulting in an increase in AST and ALT levels. This tendency has been reported in previous studies involving triolein emulsions in which infusion of triolein emulsion into the carotid artery led to reversible changes in increased vascular permeability[6]. Thus, increased vascular permeability or tissue edema worsened on the day of examination or day 1, and then decreased in subsequent days, returning to normal by day 7[6]. These findings are similar to the clinical reports on the fat embolism syndrome, in which the patient’s condition worsened on day 1 after onset of fat embolism syndrome only to revert to normal[10]. Thus, triolein emulsion causes symptoms related to increased vascular permeability with symptoms being transient.
When triolein emulsion is infused into a vessel, it can cause increased vascular permeability appearing as lesional enhancement by contrast material on imaging studies[6-8]. The increase in vascular permeability reverts to normal over time and histopathologic examinations reveal no permanent changes. The embolized lesions induced by triolein emulsion show no significant difference in cerebral hemodynamics from the contralateral normal side[11]. Also, on magnetic resonance spectroscopic examination, cerebral fat embolism induced by a triolein emulsion results in no significant changes in the major metabolites of the brain[12]. However, fat emboli, unlike other emboli, are fluid and deformable, with the ability to penetrate capillaries. As the fat emboli penetrate capillaries, they break up into smaller globules. After a temporary hold-up in systemic capillaries, the emboli pass into veins and return to the lung. The cycle gets repeated again and again, resulting in the globules becoming smaller and smaller until they are no longer embolic. As the globules approach micron size, the phagocytic systems in the liver and elsewhere readily remove them from the blood[10]. Thus, fat emboli can reach the heart and lungs under continuous perfusion pressure from the heart, thereby entering the systemic circulation and gaining a potential to affect other organs.
The portal vein and hepatic artery provide the liver with a dual blood supply. Blood flows through the sinusoids and empties into the central vein of each lobule and the hepatic veins, which leave the liver and empty into the inferior vena cava. The portal vein carries venous blood drained from the nutrient rich alimentary tract. Most of the substances from the alimentary tract are transferred to the liver via the portal vein, except lipids that are carried via the lymphatics. The liver breaks down or modifies toxic substances. Histologically, the liver consists of hepatic lobules. A hepatic lobule is 1-2 mm in diameter and consists of a roughly hexagonal arrangement of hepatocytic plates radiating outward from a central vein. Portal triads are regularly distributed at the vertices of the lobule containing a bile duct and branches of the hepatic artery and hepatic portal vein. A portal lobule has at its center a portal triad, with three central hepatic veins peripherally adjacent. The blood of the liver flows through the sinusoids and empties into the central vein of each lobule and the hepatic veins[2]. Since the hepatic damage is related to the vascular supply, it is important to study changes to the hepatocytes adjacent to the sinusoids. Unlike the brain, testis, and orbit, which have blood-organ barriers, the liver has no barriers between the sinusoids and adjacent tissues. Therefore, one may assume that a triolein injection into the liver would result in considerable histological and biochemical changes. Contrary to the expectation, however, no significant changes were found in the present study. The results in this study suggest that the injection of a triolein emulsion into the hepatic artery, or portal vein may not lead to histopathologic changes of the liver if the volume of infused triolein is controlled. Therefore, although used as an adjunctive drug in chemotherapeutic treatment of cancer patients, a triolein emulsion may not damage the liver histopathologically.
Apoptosis, the process of programmed cell death, is a result of irreparable cellular damage. Living organisms use this control mechanism to maintain their internal states within certain limits. The TUNEL reaction identifies the presence of either single-strand or double-stranded breaks in genomic DNA[13]. Presence of a fluorescent color on the confocal microscopic examination after TUNEL staining is indicative of apoptosis. In the present study, there was no evidence of apoptosis of the hepatocytes, suggesting that the triolein emulsion did not affect the homeostasis of hepatocytes.
A limitation of the current study was that only a single triolein volume was used (0.2 mL of triolein in 20 mL of normal saline). The previous studies involving trioleinemulsions[6,11] used 0.05 mL or 1 mL of infused triolein in 20 mL of saline. It is thought that the vascular permeability may be associated with infused triolein volume, but there have been no studies on the correlation between the volume of infused triolein and vascular permeability. Therefore, it is necessary to determine the proper volume of infused triolein that minimizes the effect on the tissue, while increasing the vascular permeability as desired. Another limitation was that the mechanism of a transient rise in the AST level could not be unveiled. The third limitation involved the difficulty in comparing the AST and ALT levels of the experimental groups and the normal ranges due to the low number of rabbits in the control group. The fourth limitation was inability to directly demonstrate to what degree the vascular permeability was increased after infusion of the triolein emulsion into the hepatic artery or portal vein. This limitation can be overcome with computed tomography.
In conclusion, infusion of triolein emulsion (0.2 mL triolein in 20 mL of saline) into rabbit livers resulted in a minimal and transient decrease of hepatic function, and no specific histopathologic changes. These results will be useful in future studies of triolein emulsion in the liver.
Infusion of the triolein emulsion into the arteries transiently increased vascular permeability of the vessels with blood barrier in the brain, testis, and retina without significant histopathologic change. Intra-arterial infusion of a triolein emulsion in the skeletal muscles, which have continuous capillaries and no blood-organ barrier, also increased vascular permeability without significant histologic changes.
A triolein emulsion may be used as an adjunctive drug for chemotherapy. It is thought that if a drug is infused following a triolein emulsion injection, the therapeutic effect can be achieved with a small amount of the drugs. Furthermore, triolein emulsion seems to enhance drugs with little therapeutic effect. Prior to clinical use, as an adjunctive drug for chemotherapy, it is necessary to determine the potential histologic or functional changes triolein may induce in other body organs into which it is injected. The effect of triolein emulsion on sinusoidal capillaries has not been previously reported. Since no barrier exists around sinusoidal capillaries, especially in the liver, one may expect the triolein-induced vascular changes to differ from those of continuous capillaries in the brain or eyeball.
Infusion of triolein emulsion into arteries transiently increases vascular permeability in the vessels with blood barrier in the brain, testis and retina, with no significant histopathologic change. Intra-arterial infusion of a triolein emulsion in the skeletal muscles, which have continuous capillaries and no blood-organ barrier, also increases vascular permeability without significant histologic changes. However, fat emboli are fluid and deformable, and can penetrate capillaries. Thus, fat emboli can reach the heart and lungs under continuous perfusion pressure from the heart, thereby entering the systemic circulation and gaining the potential to affect other organs. There has been no report evaluating the effect of triolein emulsion on the vessel with the sinusoidal type of capillaries, such as in the liver. Infusion of triolein emulsion (0.2 mL triolein in 20 mL of saline) into the rabbit livers caused a minimal and transient decrease of hepatic function, with no specific histopathologic changes.
The study results suggest that an infusion of triolein emulsion produces a minimal and transient effect, without specific histopathologic changes on the sinusoidal capillaries, and these findings may be useful in future triolein emulsion studies for adjuvant chemotherapeutic treatment of tumors.
Triolein, a triglyceride composed of three oleic acids, is the most important constituent fatty acid of the neutral fat in the long bone. It has been reported that triolein is connected with a breakdown of the blood-brain barrier in fat embolism syndrome.
This manuscript investigated the effect of intra-arterial infusion of triolein emulsion on rabbit liver. The authors showed that infusion of triolein emulsion into rabbit livers manifested a minimal transient effect on the alanine aminotransferase and aspartate aminotransferase levels and produced no significant effect on liver histology. These results, although primarily descriptive, may provide a preliminary basis for further studies regarding the intra-arterial infusion of triolein emulsion.
P- Reviewer: Liu ZW, Reshetnyak VI, NakajimaH S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Leeson THLC. The circulatory system. In: Leeson THLC, editor. Histology. Philadelphia, London, Toronto: WB Saunders 1981; 257-282. |
| 2. | Leeson THLC. The Liver. In: Leeson THLC, editor. Histology. Philadelphia, London, Toronto: WB Saunders 1981; 383-397. |
| 3. | Mathieu D, Fortin D. Chemotherapy and delivery in the treatment of primary brain tumors. Curr Clin Pharmacol. 2007;2:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Verbraeken H, Verstraete A, Van de Velde E, Verschraegen G. Penetration of gentamicin and ofloxacin in human vitreous after systemic administration. Graefes Arch Clin Exp Ophthalmol. 1996;234 Suppl 1:S59-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Touroutoglou N, Dimopoulos MA, Younes A, Hess M, Pugh W, Cox J, Cabanillas F, Sarris AH. Testicular lymphoma: late relapses and poor outcome despite doxorubicin-based therapy. J Clin Oncol. 1995;13:1361-1367. [PubMed] |
| 6. | Kim HJ, Lee CH, Kim HG, Lee SD, Son SM, Kim YW, Eun CK, Kim SM. Reversible MR changes in the cat brain after cerebral fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2004;25:958-963. [PubMed] |
| 7. | Lee JE, Jea SY, Oum BS, Kim HJ, Ohn YH. Effect of fat embolism with triolein emulsion on blood-retinal barrier. Ophthalmic Res. 2009;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Kim KN, Kim HJ, Lee SD, Moon TY, Lee SH, Lee JW, Lee TH. Effect of triolein emulsion on the blood-testis barrier in cats. Invest Radiol. 2004;39:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kim HJ, Kim YW, Lee IS, Song JW, Jeong YJ, Choi SH, Choi KU, Suh KT, Cho BM. Intra-arterial delivery of triolein emulsion increases vascular permeability in skeletal muscles of rabbits. Acta Vet Scand. 2009;51:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Sevitt S. The significance and pathology of fat embolism. Ann Clin Res. 1977;9:173-180. [PubMed] |
| 11. | Kim YW, Kim HJ, Cho BM, Moon TY, Eun CK. The study of cerebral hemodynamics in the hyperacute stage of fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2006;27:398-401. [PubMed] |
| 12. | Baik SK, Kim YW, Kim HJ, Lee JW, Cho BM, Kim DH, Choi SH, Lee SH, Chang KH. Proton magnetic resonance spectroscopic findings of cerebral fat embolism induced by triolein emulsion in cats. Acta Radiol. 2008;49:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Formichi P, Battisti C, Bianchi S, Cardaioli E, Federico A. Evidence of apoptosis via TUNEL staining in muscle biopsy from patients with mitochondrial encephaloneuromyopathies. J Submicrosc Cytol Pathol. 2003;35:29-34. [PubMed] |