Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.13071
Revised: May 3, 2014
Accepted: June 13, 2014
Published online: September 28, 2014
Processing time: 229 Days and 9.8 Hours
NANOG has been extensively researched since its discovery by Chambers et al. NANOG is a homeodomain transcription factor and an essential regulator of embryonic stem cell (ESC) self-renewal, which inhibits differentiation. Cancer stem cells (CSCs) are a small subset of cells that are thought to drive uncontrolled tumor growth; CSCs retain the tumor capabilities of self-renewal and propagation. The existence of CSCs was recently shown by direct experimental evidence. NANOG is expressed in CSCs and ESCs, although it remains unclear whether ESCs and CSCs share similar mechanisms in the regulation of physical and biological processes. Several studies suggest that the expression level of NANOG is high in cancer tissues and low or absent in normal tissues. High levels of NANOG expression are associated with advanced stages of cancer and a poor prognosis, indicating that it plays a vital role in tumor transformation, tumorigenesis, and tumor metastasis. NANOG is part of a complex regulatory network that controls cell fate determination, proliferation, and apoptosis. NANOG cooperates with other regulators, such as microflora, transcription factors, and kinases, in cancer cells. NANOG might have a promising future in anti-cancer and other therapeutic treatments, which could improve human health.
Core tip: This review article differs from previous reviews by concentrating on the relationship between NANOG and cancer. This review contains the following five sections: the structure of NANOG, NANOG and cancer stem cells, signal pathways associated with NANOG in cancer, NANOG in specific human tumors, and the role of NANOG in anti-cancer therapy. Each section contains novel insights and comprehensively reviews the current literature, which will be very useful to the journal’s readers.
- Citation: Sun AX, Liu CJ, Sun ZQ, Wei Z. NANOG: A promising target for digestive malignant tumors. World J Gastroenterol 2014; 20(36): 13071-13078
- URL: https://www.wjgnet.com/1007-9327/full/v20/i36/13071.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.13071
NANOG was discovered by Chambers et al[1] based on its ability to maintain the self-renewal of mouse embryonic stem cells (ESCs) independently of the cytokine leukemia inhibitory factor (LIF). NANOG was identified by comparing expressed sequence tag libraries from mouse ESCs with various somatic tissues[2]. Research on NANOG has received significant attention since its discovery. As a homeodomain-containing transcription factor, NANOG is a key transcription factor involved in the maintenance of pluripotency and self-renewal in undifferentiated ESCs[1-7]. The NANOG protein is encoded by the only open reading frame of the 2184-nucleotide NANOG cDNA[2]. In addition to the embryonic NANOG gene, eleven NANOG pseudogenes have been reported in the human genome[8]. However, only the NANOG homeobox pseudogene 8 (NANOGP8) has a complete open reading frame able to transcribe and translate functional NANOG protein[8-10]. NANOG is hypothesized to be an important regulatory factor associated with the pluripotency of ESCs, whereas NANOGP8 plays a role in tumorigenesis. However, the comprehensive expression patterns of NANOG and NANOGP8 in human cancers have not been fully elucidated. NANOG is expressed from both NANOG and NANOGP8 in colorectal cancers[11]. The human NANOG protein consists of 305 amino acids and could be divided into N-terminal (amino acid 1-95), homeobox domain (amino acid 96-155), and C-terminal (amino acid 156-305) regions[2,12]. The N-terminus is tightly regulated through phosphorylation or other posttranslational modifications of serine, threonine, and proline. The N-terminus also functions as a structural motif for the transcriptional activity of NANOG[12,13]. The C-terminus contains two potent transactivation subdomains[12,13]. The N- and C-terminal regions contain nuclear localization sequences. The homeobox domain in the central region contains a DNA-binding motif and is reported to harbor a potent nuclear export motif[14] that allows the NANOG protein to be transported into and out of the nucleus.
Cancer stem cells (CSCs) are a small subset of cells that are thought to drive uncontrolled tumor growth and retain the potential for tumor self-renewal and propagation. Although the origins of CSCs are debated, the existence of these cells has been proven by direct experimental evidence[15]. Bussolati et al[16] found a tumor-initiating stem cell population in renal carcinomas. CSCs possess several stem cell properties, such as clonogenic ability, the expression of NANOG and Oct-4 stem cell markers, and the absence of epithelial differentiation markers. CSCs have been isolated in the following tumor types: glioblastoma, melanoma, prostate carcinoma, colon carcinoma, head and neck squamous cell carcinoma, breast carcinoma, ovarian carcinoma, bladder carcinoma, lung carcinoma, and pancreatic carcinoma[17-29]. Studies have traced the cell lineages within a growing tumor in glioblastomas[30], intestinal adenomas[31], and squamous skin tumors[32]. CSCs are identified as unique cells with the potential to expand the CSC pool and the potential to differentiate into heterogeneous non-tumorigenic cells that constitute the bulk of the tumor[33]. This minority population of cells retains the self-renewal and propagation potential of the tumor, whereas the vast majority of cells are non-tumorigenic daughter cells of CSCs[34]. Singh et al[35] isolated CSCs in human brain tumors. Tumors that could be serially transplanted were produced when these authors injected as few as 100 cells into mouse brains. However, injecting as many as 105 non-CSCs cells did not result in the development of a tumor. Therefore, the CSC hypothesis provides an attractive cellular mechanism to account for the therapeutic refractoriness and dormant behavior exhibited by many solid tumors.
Liao et al[36] determined that NANOG regulates the self-renewal of breast CSCs. Nanog expression is correlated with aggressiveness in poorly differentiated breast cancer and enhances the tumorigenicity of tumor cells by promoting the self-renewal of CSC subpopulations. The knockdown of NANOG significantly inhibited the growth of breast CSCs. Many years of study on cell transplantation suggests that NANOG plays a vital role in tumor transformation, tumorigenesis, and tumor metastasis via regulating the CSC population[37]. Several studies have shown that NANOG, in combination with other regulators, modifies chromatin structure and forms a key regulatory network controlling the identity, differentiation, self-renewal, and pluripotency of ESCs[38-40]. Although a deeper understanding of the mechanisms of NANOG circuitry in CSCs is needed, CSCs display preferential overexpression of NANOG normally enriched in ESCs. Thus, similar mechanisms might be involved in the regulation of CSCs[41]. The finding that NANOG might play a crucial role in CSC and ESC signaling networks makes it a potentially ideal target for cancer treatment.
Examination of its expression in clinical studies revealed that NANOG is overexpressed in a variety of cancers, including gastrointestinal (GI) tumors. Yang et al[42] found that the expression of the NANOG protein was higher in esophageal cancer tissues and was positively correlated with histological grade and lymphatic metastases. Furthermore, when three cell lines were treated with cisplatin to evaluate drug sensitivity, the authors found that sensitivity to cisplatin was decreased by increased NANOG expression. Their study demonstrated that NANOG could promote tumor cell proliferation, invasion, and resistance. Inhibiting NANOG expression inhibits these behaviors by stalling cells in the G0/G1 phase and inducing apoptosis. Using reverse transcription-polymerase chain reaction and real-time quantitative polymerase chain reactions, Chen et al[43] demonstrated that the expression of NANOG was higher in gastric cancer tissues than in paracancerous tissues. The expression of NANOG was positively correlated with the histological grade of the tumor, suggesting that NANOG might serve as a novel marker for the diagnosis and prognosis of gastric carcinoma.
Side population (SP) cells are thought to include CSCs with self-renewal capacity and high tumorigenicity[44]. Uchino et al[45] found that NANOG was expressed specifically in SP cells of human GI cancer cells. Nucleotide sequencing revealed that NANOGP8 was expressed in GI cancer cells, whereas Nanog was not expressed in these cells. The transfection of NANOGP8 into GI cancer cell lines promoted cell proliferation, whereas its inhibition by anti-NANOG siRNA suppressed the proliferation. These data suggested that NANOGP8 is involved in GI cancer development in a subset of patients and that it presumably acts by supporting CSC proliferation in these patients.
Zhang et al[46] determined that NANOG was expressed in hepatocellular carcinoma (HCC) tissue and paracancerous tissue; however, there was almost no expression of NANOG in normal liver tissue. NANOG expression was positively correlated with the clinical stage and histological grade of the patients, suggesting that NANOG is associated with the development of HCC. A study by Shan et al[47] suggested that NANOG plays a crucial role in maintaining the self-renewal of CSCs through the insulin-like growth factor (IGF) 1R-signaling pathway, and NANOG could be a novel biomarker for CSCs in HCC. The authors successfully isolated NANOG-positive cells, using the NANOG promoter as a reporter system, and determined that NANOG-positive cells exhibited enhanced self-renewal ability, clonogenicity, and tumor initiation capacity. Thus, NANOG expression predicts a worse clinical outcome in HCC. In addition, this study showed that NANOG-positive CSCs were resistant to therapeutic agents and had a high capacity for tumor invasion and metastasis. The knockdown of NANOG in NANOG-positive CSCs reduced the self-renewal capacity, decreased the expression of stem cell related genes, and increased the expression of mature hepatocyte-related genes. The overexpression of NANOG in NANOG-negative cells could restore self-renewal. There are many factors and regulatory networks involved in cancer development. Shan et al[47] found that IGF2 and IGF-receptor were upregulated in NANOG-positive CSCs. The knockdown of NANOG in NANOG-positive CSCs inhibited the expression of IGF1R, and NANOG overexpression in NANOG-negative cells increased the expression of IGF1R. A specific inhibitor of IGF1R signaling could significantly inhibit self-renewal and NANOG expression in NANOG-positive cells, suggesting that IGF1R signaling participates in NANOG-mediated self-renewal in NANOG-positive cells.
There are a limited number of studies investigating the role of NANOG in pancreatic carcinoma. Pancreatic cancer is well known for being difficult to diagnose at early stages and has a poor recurrence-free prognosis. Lu et al[48] isolated pancreatic CSCs from the PANC-1 cell line using flow cytometry. They found that NANOG was highly expressed in human pancreatic cancer tissues, and that NANOG knockdown reduced the proliferation, migration, invasion, chemoresistance, and tumorigenesis of pancreatic CSCs. The authors determined that NANOG affects the biological characteristics of pancreatic CSCs and that its overexpression indicated a worse prognosis. Therefore, NANOG might serve as a potential marker of prognosis and be a novel therapeutic target for pancreatic cancer.
Meng et al[49] studied the expression and regulatory effects of NANOG in colorectal cancer (CRC). The authors found that its overexpression was strongly correlated with poor prognoses, lymph node metastases, and Dukes classification for CRC. Univariate and multivariate survival analyses indicated that NANOG expression was a potential prognostic factor for CRC. Studies using gain-of-function approaches revealed that lentivirus-mediated NANOG overexpression promoted the proliferation, motility, and migration of human CRC cells. Previous authors found that NANOG induced the epithelial-mesenchymal transition. Xu et al[50] observed that NANOG expression was related to histological grade, lymph node metastases, TNM stage, and liver metastases in CRC. A Spearman correlation analysis showed that NANOG expression is linearly correlated with liver metastases. These results suggest that the NANOG protein might be a potential biomarker for postoperative liver metastases of CRC and could be a potential early liver metastasis screening factor in CRC. Shi et al[51] demonstrated that NANOG knockdown could suppress proliferation, colony formation, and in vivo tumorigenicity in CRC; NANOG knockdown could increase the sensitivity of the CRC cell line SW620 to fluorouracil (5-FU). These data suggest that NANOG might regulate the aggressiveness of CRC cells.
In addition to its expression in GI tumors, NANOG is expressed in human breast, cervix, oral, kidney, prostate, lung, gastric, brain, and ovarian cancers[52-63]. An elevated expression of NANOG was positively associated with late stage progression and a poorer prognosis in oral cancer patients. The exact role of NANOG in tumor development remains unclear. A recent study showed that the expression of NANOG mRNA did not have any clinical relevance in 79 fresh clinical CRC samples[64]. Although previous studies have demonstrated that NANOG is highly expressed in a variety of tumors and that its increased expression indicates a poor prognosis in patients, comprehensive and systematic studies examining the role of NANOG in human clinical tumor samples are lacking. The role of NANOG in tumor development remains unclear. Whether NANOG is a marker for CSCs in carcinomas or a biomarker representing CSCs requires further study. Additionally, the mechanism of how it participates in CSC biology requires further research.
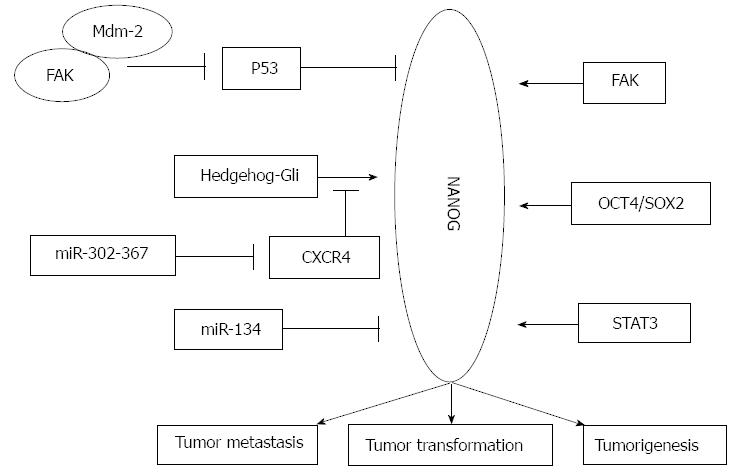
NANOG is not an isolated factor in the regulatory process of tumor development, and there are many regulators involved in tumor transformation, tumorigenesis, and tumor metastasis (Figure 1). For example, other transcription factors, microRNAs, and kinases have been reported to mediate the silencing or overexpression of NANOG and to regulate stemness, malignant transformation, and CSC-like phenotypes in cancer cells.
The p53 protein is a key mediator regulating programmed cell apoptosis and cell cycle related pathways; it activates cell-cycle checkpoints and promotes cell senescence. p53 has a well-accepted role in tumor suppression. During the last decade, a direct involvement of p53 in the stemness regulatory network has been investigated in stem cell and cancer research; p53 could reduce self-renewal and enhance differentiation in ESCs and cancer cells. The suppressive effect of p53 on reprogramming and cancer stemness could partially depend on its negative regulation of NANOG[65-68]. It has been reported that p53 could bind to the NANOG promoter and suppresses its expression after DNA damage[66]. Hedgehog is another essential regulator of stemness properties; hedgehog promotes self-renewal in ESCs and promotes cancer progression. Po et al[69] showed that the loss of p53 activated the Hedgehog-Gli pathway; the Hedgehog-Gli pathway was responsible for NANOG upregulation through p53-independent signaling by the binding of Gli transcription factors to the NANOG promoter. The growth and tumorigenicity of glioma stem cells was regulated by the p53-independent Hedgehog-Gli pathway. Zbinden et al[70] demonstrated a positive regulatory loop between NANOG and sonic hedgehog signaling. The knockdown of NANOG decreased the endogenous activity of Gli1, and NANOG mRNA levels were modulated by the hedgehog pathway. Their research showed that the Hedgehog and p53 pathways are cross-linked and could cross-regulate NANOG expression since p53 directly suppresses NANOG and Hedgehog[66,69].
Focal adhesion kinase (FAK) is a tyrosine kinase that plays a significant role in tumor survival[71]. Many tumors overexpress FAK mRNA and protein[72]. FAK is important for cell adhesion, proliferation, motility, invasion, and angiogenesis[73]. FAK and p53 are involved in the regulation of survival/apoptotic signaling in cancer cells through p53-regulated FAK promoter activity and through FAK-p53 protein binding[74-78]. Golubovskaya[65] reported that NANOG binds the FAK promoter and upregulates FAK expression. Additionally, FAK could bind and phosphorylate NANOG. FAK could bind Mdm-2 and activate p53 degradation[77], and p53 negatively regulates both NANOG and FAK.
The LIF/STAT3 pathway is critical for NANOG function in the maintenance of ESC pluripotency. It was reported that 14 of the 22 STAT3 target genes contributing to the maintenance of an undifferentiated state were also regulated by NANOG in mouse ESCs. Torres et al[79] examined the functional interactions of NANOG with the Stat3 and NF-κB pathways and found that NANOG and Stat3 bind to and synergistically activate Stat3-dependent promoters. NANOG could bind to NF-κB proteins and inhibit the transcriptional activity of NF-κB proteins to increase the expression of pluripotency markers. Bourguignon et al[80] found that NANOG-STAT3 could activate common targets in head and neck squamous cell carcinomas. Additionally, the LIF-induced phosphorylation of STAT3 directs the binding of STAT3 to the NANOG gene enhancer and upregulates NANOG expression. Zhou et al[81] found that the hepatitis C virus (HCV) core protein could upregulate NANOG expression by enforced expression of the phosphorylated Stat3 protein. The phosphorylated Stat3 directly binds to the NANOG promoter and enhances cell growth and cell cycle progression. The knockdown of NANOG blocked the cell cycle at the G0/G1 phases. These findings provide insight into the mechanism of hepatocarcinogenesis by HCV infection. The finding that the LIF/STAT3 pathway promotes NANOG expression in ESCs and cancer cells suggests that NANOG is an important mediator of the LIF-dependent pathway. STAT3 might also regulate NANOG expression through epigenetic modifications[82]. Nettersheim et al[83] showed that the OCT3/4-SOX2-mediated expression of NANOG could be silenced by methylation of promoter CpG-sites in testicular germ cell tumors. The global methylation of DNA decreases from fetal spermatogonia to mature sperm. CpGs in the NANOG promoter were hypomethylated in spermatogonia and hypermethylated in sperm. The selective repression of NANOG suggested that pluripotency must be suppressed to prevent malignant transformation. The methylation of CpGs in the NANOG promoter of germ cell tumors and derived cell lines are correlated to the differentiation state.
MicroRNAs are associated with the pathogenesis of many cancers. There are reports identifying microRNAs that target NANOG and mediate the malignant progression in cancer cells or CSCs. MicroRNA-21 (miR-21) appears to play a critical role in tumor cell survival, chemoresistance, and progression. Bourguignon LY found that NANOG and Stat-3 signaling promote miR-21 expression in CD44-activated head and neck squamous cell carcinoma cells by downregulating tumor suppressor proteins and upregulating proteins that inhibit apoptosis[80]. Fareh et al[84] showed that the miR302-367 cluster is strongly induced during serum-mediated stemness suppression in glioma-initiating cells. The stable expression of the miR302-367 cluster is sufficient to suppress the stemness characteristics, self-renewal, and cell infiltration into host brain tissue through inhibition of the CXCR4 pathway. The inhibition of CXCR4 leads to disruption of the sonic hedgehog-GLI-NANOG network, which is involved in self-renewal and the expression of the embryonic stem cell-like signature. Their research demonstrated that the miR302-367 cluster could efficiently trigger a cascade of inhibitory events leading to the disruption of glioma-initiating stem-like cells with tumorigenic properties. MiR-134 was first found to directly target NANOG in mouse ESCs and glioblastoma cells. The overexpression of miR-134 reduced proliferation, invasiveness, and migration capability, and promoted apoptosis of glioblastoma cell lines by directly suppressing NANOG expression[85]. A global gene expression profile screen of NANOG siRNA-transfected embryonal carcinoma cells suggested that NANOG is involved in the cell cycle-signaling pathway[86]. Several cell cycle- and p53-related signaling pathway genes were downregulated by NANOG knockdown. These results suggest a role of NANOG in the cell cycle and in survival[87].
The majority of the regulatory mechanisms related to NANOG are focused on transcription; however, there are accumulating data showing the importance of post-transcriptional and translational regulation of NANOG. Moretto-Zita et al[88] first reported that the stability of NANOG in mouse and human ESCs is regulated by an evolutionarily conserved post-translational mechanism. The mechanisms through which NANOG regulates cell proliferation and tumor growth require further investigation.
The overexpression of NANOG predicts tumor progression and a poor prognosis in several cancers. NANOG is associated with tumor development[89], and NANOG/NANOGP8 expression is associated with gastric carcinogenesis[90]. Moreover, extensive loss-of-function analyses revealed that RNAi-mediated NANOG knockdown inhibits tumor development[91]. The low expression level of NANOG is a promising survival prognosis marker in oral squamous cell carcinoma patients. NANOG is an important factor that affects the biological behavior of CSCs. There is evidence showing that the elimination of CSCs with the potential for self-renewal and tumor propagation should be a target of cancer drug development. However, CSCs are particularly resistant to conventional chemotherapy and radiotherapy compared with non-CSCs. Previous studies demonstrated the important contribution of pluripotent transcription factors to CSC function. NANOG exhibits significant clinical potential because it serves as a valuable marker of tumorigenesis[92]. Therefore, NANOG might have a promising future in anti-cancer and other therapeutic treatments, which could improve human health. Drugs targeting NANOG activity might be efficacious. However, further work is needed to design individualized therapies for cancer patients. These therapies should focus on functional analyses that define the transcription factors determining CSC phenotypes. Such studies might reveal precise regulatory mechanisms and identify new components of the transcriptional regulatory networks relevant to tumor transformation, tumorigenesis, and metastasis.
P- Reviewer: Gassler N, Iwasaki Y, Ricci-Vitiani L, Tasci I S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Du P
| 1. | Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. [PubMed] |
| 2. | Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631-642. [PubMed] |
| 3. | Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035-1043. [PubMed] |
| 4. | Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Wang J, Levasseur DN, Orkin SH. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA. 2008;105:6326-6331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42-49. [PubMed] |
| 9. | Zhang J, Wang X, Li M, Han J, Chen B, Wang B, Dai J. NANOGP8 is a retrogene expressed in cancers. FEBS J. 2006;273:1723-1730. [PubMed] |
| 10. | Fairbanks DJ, Fairbanks AD, Ogden TH, Parker GJ, Maughan PJ. NANOGP8: evolution of a human-specific retro-oncogene. G3 (Bethesda). 2012;2:1447-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Ibrahim EE, Babaei-Jadidi R, Saadeddin A, Spencer-Dene B, Hossaini S, Abuzinadah M, Li N, Fadhil W, Ilyas M, Bonnet D. Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1- and TCF-dependent mechanisms. Stem Cells. 2012;30:2076-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Oh JH, Do HJ, Yang HM, Moon SY, Cha KY, Chung HM, Kim JH. Identification of a putative transactivation domain in human Nanog. Exp Mol Med. 2005;37:250-254. [PubMed] |
| 13. | Do HJ, Lim HY, Kim JH, Song H, Chung HM, Kim JH. An intact homeobox domain is required for complete nuclear localization of human Nanog. Biochem Biophys Res Commun. 2007;353:770-775. [PubMed] |
| 14. | Park SW, Do HJ, Huh SH, Sung B, Uhm SJ, Song H, Kim NH, Kim JH. Identification of a putative nuclear export signal motif in human NANOG homeobox domain. Biochem Biophys Res Commun. 2012;421:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339-9344. [PubMed] |
| 16. | Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696-3705. [PubMed] |
| 17. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320-4333. [PubMed] |
| 19. | Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153-3161. [PubMed] |
| 20. | O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [PubMed] |
| 21. | Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973-978. [PubMed] |
| 22. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [PubMed] |
| 23. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1007] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 24. | Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Chang HY, van de Rijn M. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016-14021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 494] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 25. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [PubMed] |
| 26. | Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, de Narvajas AA, Gomez TS, Simeone DM, Bujanda L. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 27. | Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883-190; discussion 1883-190;. [PubMed] |
| 28. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [PubMed] |
| 29. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2140] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 30. | Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1518] [Cited by in RCA: 1754] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 31. | Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 861] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 32. | Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 569] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 33. | Albers AE, Chen C, Köberle B, Qian X, Klussmann JP, Wollenberg B, Kaufmann AM. Stem cells in squamous head and neck cancer. Crit Rev Oncol Hematol. 2012;81:224-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Bernstein A. Cancer stem cells: the centrality of translational research to cancer control. CMAJ. 2007;176:29-30. [PubMed] |
| 35. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [PubMed] |
| 36. | Liao WY, Liaw CC, Huang YC, Han HY, Hsu HW, Hwang SM, Kuo SC, Shen CN. Cyclohexylmethyl Flavonoids Suppress Propagation of Breast Cancer Stem Cells via Downregulation of NANOG. Evid Based Complement Alternat Med. 2013;2013:170261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol. 2014;20:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431-440. [PubMed] |
| 39. | Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311-2322. [PubMed] |
| 40. | Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947-956. [PubMed] |
| 41. | Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2057] [Cited by in RCA: 2021] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 42. | Yang L, Zhang X, Zhang M, Zhang J, Sheng Y, Sun X, Chen Q, Wang LX. Increased Nanog expression promotes tumor development and Cisplatin resistance in human esophageal cancer cells. Cell Physiol Biochem. 2012;30:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Chen Z, Xu WR, Qian H, Zhu W, Wang S. Detection of stem cell marker Nanog and diagnostic significance for human gastric cancer. Linchuang Jianyan Zazhi. 2009;27:6-9. |
| 44. | Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506-513. [PubMed] |
| 45. | Uchino K, Hirano G, Hirahashi M, Isobe T, Shirakawa T, Kusaba H, Baba E, Tsuneyoshi M, Akashi K. Human Nanog pseudogene8 promotes the proliferation of gastrointestinal cancer cells. Exp Cell Res. 2012;318:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Zhang M, Wang SX. Expression of OCT4, SOX2 and NANOG and Its Clinical Significance in in Hepatocellular Carcinoma. Hebei Yike Daxue Xuebao. 2012;11. |
| 47. | Shan J, Shen J, Liu L, Xia F, Xu C, Duan G, Xu Y, Ma Q, Yang Z, Zhang Q. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56:1004-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 48. | Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, Lu J, Fan X, Zhu S, Wang Y. Knockdown of Oct4 and Nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 2013;340:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding YQ, Li J. Over-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9:295-302. [PubMed] |
| 50. | Xu F, Dai C, Zhang R, Zhao Y, Peng S, Jia C. Nanog: a potential biomarker for liver metastasis of colorectal cancer. Dig Dis Sci. 2012;57:2340-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Shi Z, Bai R, Fu ZX, Zhu YL, Wang RF, Zheng S. Induced pluripotent stem cell-related genes influence biological behavior and 5-fluorouracil sensitivity of colorectal cancer cells. J Zhejiang Univ Sci B. 2012;13:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433-10444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 53. | Amsterdam A, Raanan C, Schreiber L, Freyhan O, Schechtman L, Givol D. Localization of the stem cell markers LGR5 and Nanog in the normal and the cancerous human ovary and their inter-relationship. Acta Histochem. 2013;115:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104:2255-2265. [PubMed] |
| 55. | Hart AH, Hartley L, Parker K, Ibrahim M, Looijenga LH, Pauchnik M, Chow CW, Robb L. The pluripotency homeobox gene NANOG is expressed in human germ cell tumors. Cancer. 2005;104:2092-2098. [PubMed] |
| 56. | Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48-56. [PubMed] |
| 57. | Lin T, Ding YQ, Li JM. Overexpression of Nanog protein is associated with poor prognosis in gastric adenocarcinoma. Med Oncol. 2012;29:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 58. | Guo Y, Liu S, Wang P, Zhao S, Wang F, Bing L, Zhang Y, Ling EA, Gao J, Hao A. Expression profile of embryonic stem cell-associated genes Oct4, Sox2 and Nanog in human gliomas. Histopathology. 2011;59:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Pan Y, Jiao J, Zhou C, Cheng Q, Hu Y, Chen H. Nanog is highly expressed in ovarian serous cystadenocarcinoma and correlated with clinical stage and pathological grade. Pathobiology. 2010;77:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 61. | Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085-4095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 513] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 62. | Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, Ferlinz A, Shannon M, Oosterhuis JW, Looijenga LH. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl. 2011;34:e160-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | Zhou X, Zhou YP, Huang GR, Gong BL, Yang B, Zhang DX, Hu P, Xu SR. Expression of the stem cell marker, Nanog, in human endometrial adenocarcinoma. Int J Gynecol Pathol. 2011;30:262-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Saiki Y, Ishimaru S, Mimori K, Takatsuno Y, Nagahara M, Ishii H, Yamada K, Mori M. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann Surg Oncol. 2009;16:2638-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Golubovskaya VM. FAK and Nanog cross talk with p53 in cancer stem cells. Anticancer Agents Med Chem. 2013;13:576-580. [PubMed] |
| 66. | Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165-171. [PubMed] |
| 67. | Moon JH, Kwon S, Jun EK, Kim A, Whang KY, Kim H, Oh S, Yoon BS, You S. Nanog-induced dedifferentiation of p53-deficient mouse astrocytes into brain cancer stem-like cells. Biochem Biophys Res Commun. 2011;412:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1078] [Cited by in RCA: 1018] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 69. | Po A, Ferretti E, Miele E, De Smaele E, Paganelli A, Canettieri G, Coni S, Di Marcotullio L, Biffoni M, Massimi L. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J. 2010;29:2646-2658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Zbinden M, Duquet A, Lorente-Trigos A, Ngwabyt SN, Borges I, Ruiz i Altaba A. NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO J. 2010;29:2659-2674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 71. | Golubovskaya VM. Targeting focal adhesion kinase in cancer-part I. Anticancer Agents Med Chem. 2010;10:713. [PubMed] |
| 72. | Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance WG. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9:215-222. [PubMed] |
| 73. | Golubovskaya VM, Cance WG. FAK and p53 protein interactions. Anticancer Agents Med Chem. 2011;11:617-619. [PubMed] |
| 74. | Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008;47:373-382. [PubMed] |
| 75. | Golubovskaya VM, Conway-Dorsey K, Edmiston SN, Tse CK, Lark AA, Livasy CA, Moore D, Millikan RC, Cance WG. FAK overexpression and p53 mutations are highly correlated in human breast cancer. Int J Cancer. 2009;125:1735-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem. 2005;280:25008-25021. [PubMed] |
| 77. | Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 78. | Cance WG, Golubovskaya VM. Focal adhesion kinase versus p53: apoptosis or survival? Sci Signal. 2008;1:pe22. [PubMed] |
| 79. | Torres J, Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat Cell Biol. 2008;10:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 80. | Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31:149-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 81. | Zhou JJ, Chen RF, Deng XG, Zhou Y, Ye X, Yu M, Tang J, He XY, Cheng D, Zeng B. Hepatitis C virus core protein regulates NANOG expression via the stat3 pathway. FEBS Lett. 2014;588:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, Carter MG, Amano T, Park J, Kish S. Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells. 2012;30:2645-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 83. | Nettersheim D, Biermann K, Gillis AJ, Steger K, Looijenga LH, Schorle H. NANOG promoter methylation and expression correlation during normal and malignant human germ cell development. Epigenetics. 2011;6:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Fareh M, Turchi L, Virolle V, Debruyne D, Almairac F, de-la-Forest Divonne S, Paquis P, Preynat-Seauve O, Krause KH, Chneiweiss H. The miR 302-367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19:232-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 85. | Niu CS, Yang Y, Cheng CD. MiR-134 regulates the proliferation and invasion of glioblastoma cells by reducing Nanog expression. Int J Oncol. 2013;42:1533-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 86. | Shanske S, Moraes CT, Lombes A, Miranda AF, Bonilla E, Lewis P, Whelan MA, Ellsworth CA, DiMauro S. Widespread tissue distribution of mitochondrial DNA deletions in Kearns-Sayre syndrome. Neurology. 1990;40:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Choi SC, Choi JH, Park CY, Ahn CM, Hong SJ, Lim DS. Nanog regulates molecules involved in stemness and cell cycle-signaling pathway for maintenance of pluripotency of P19 embryonal carcinoma stem cells. J Cell Physiol. 2012;227:3678-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Moretto-Zita M, Jin H, Shen Z, Zhao T, Briggs SP, Xu Y. Phosphorylation stabilizes Nanog by promoting its interaction with Pin1. Proc Natl Acad Sci USA. 2010;107:13312-13317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 89. | Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 90. | Zhang J, Wang X, Chen B, Xiao Z, Li W, Lu Y, Dai J. The human pluripotency gene NANOG/NANOGP8 is expressed in gastric cancer and associated with tumor development. Oncol Lett. 2010;1:457-463. [PubMed] |