Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12691
Revised: May 4, 2014
Accepted: June 13, 2014
Published online: September 21, 2014
Processing time: 171 Days and 20.5 Hours
Patients with esophageal squamous cell carcinoma generally present at an advanced stage at the time of diagnosis. The most common sites of visceral metastasis are the lung, liver and bone, but brain and bone marrow involvement is exceedingly rare. Herein, we report a 62-year-old man with a 4-wk history of progressive low back pain with radiation to bilateral lower legs, dysphagia and body weight loss. Esophageal squamous cell carcinoma with regional lymph node, liver and bone metastases was diagnosed. He underwent concurrent chemoradiotherapy and got a partial response. Four months later, he complained of headache, diplopia and severe hearing impairment in the left ear. There was no evidence for bacterial, fungal, tuberculous infection or neoplastic infiltration. Magnetic resonance imaging of the brain demonstrated thickening and enhancement of bilateral pachymeninges and multiple enhancing masses in bilateral skull. Dural metastasis was diagnosed and he received whole brain irradiation. In addition, laboratory examination revealed severe thrombocytopenia and leucopenia, and bone marrow study confirmed the diagnosis of metastatic squamous cell carcinoma. This is the first described case of esophageal squamous cell carcinoma with dural and bone marrow metastases. We also discuss the pathogenesis of unusual metastatic diseases and differential diagnosis of pachymeningeal thickening.
Core tip: The first case of esophageal squamous cell carcinoma with dural and bone marrow metastases is reported. It can help us recognize the unusual metastatic pattern and better understand the pathogenesis of the rare entity.
- Citation: Chen YH, Huang CH. Esophageal squamous cell carcinoma with dural and bone marrow metastases. World J Gastroenterol 2014; 20(35): 12691-12695
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12691
Patients with esophageal squamous cell carcinoma generally have a poor outcome, and the majority of them present at an advanced stage at the time of diagnosis. Lymphatic and hematogenous metastases are well-documented in patients with esophageal squamous cell carcinoma[1-3]. It has been observed that the lung, liver, bone, pleura and adrenal gland are the most common sites of visceral metastasis, but brain and bone marrow involvement is exceedingly rare in esophageal squamous cell carcinoma. Recently, advances in neuroimaging, such as computed tomography (CT) and magnetic resonance imaging (MRI), and the increased survival of these patients have led to more frequent and earlier detection of brain metastasis. In three studies from Japan, it was found that squamous cell carcinoma was the predominant histological type and the incidence of brain metastasis from esophageal cancer was about 1.5%[4-6]. Although some studies have mentioned esophageal cancer with brain metastasis, dural metastasis has not been reported in the past literature. Bone marrow metastasis is another poor prognostic factor and usually occurs in advanced or terminal stage in cancer treatment. Herein we present, to our knowledge, the first case of esophageal squamous cell carcinoma with dural and bone marrow metastases.
A 62-year-old man was admitted with progressive low back pain with radiation to bilateral lower legs for one month. He was a smoker and had a history of alcohol consumption. He also complained of dysphagia and a body weight loss of 5 kg within 2 mo. Physical examination was remarkable. Endoscopy identified two ulcerative tumors in the middle and lower third of the esophagus from 25 to 31 cm and from 34 to 37 cm, respectively. CT of the chest showed esophageal cancer with regional lymph node, liver and bone metastases. A biopsy was positive for squamous cell carcinoma. He underwent concurrent chemoradiotherapy with cisplatin/5-fluorouracil (5-FU). Radiotherapy was applied via conventional parallel-opposed anterior-posterior fields covering the entire esophagus and mediastinum using 10-MV photon in 2 Gy daily fractions, five days per week to a total dose of 36 Gy. Then, additional 14 Gy in 7 fractions of irradiation was applied via intensity-modulated radiotherapy (IMRT) to limit the spinal cord dose under 45 Gy cumulatively. The clinical target volume of IMRT covered the gross tumor volume with a 4 cm margin in the cranio-caudal direction and a 1 cm lateral margin. The metastatic bone lesion was not involved in the field of radiotherapy initially because it was asymptomatic in clinical presentation. Chemotherapy consisted of four cycles of cisplatin (4 h drip infusion; 75 mg/m2) on day 1 and 5-FU (continuous infusion; 1000 mg/m2) on day 1-4 every 4 wk. Follow-up endoscopy showed a near complete response of esophageal tumors and there was regressive change of liver metastases on CT of the chest.
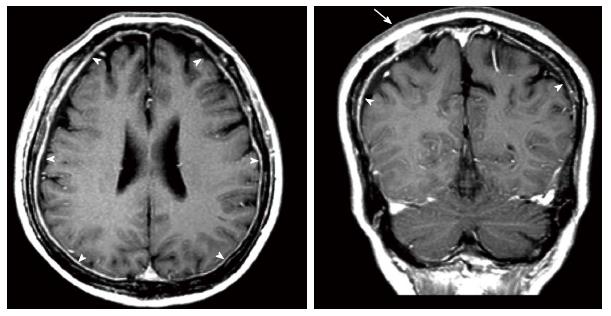
About 4 mo after concurrent chemoradiotherapy, he complained of headache, and the frequency and intensity increased gradually. In addition, the associated symptoms included diplopia and severe hearing impairment in the left ear. No nausea, vomiting, or meningeal sign was mentioned. He took analgesics for symptom relief but without improvement. Cerebrospinal fluid (CSF) study was performed and there was no evidence of bacterial, fungal, tuberculous infection or neoplastic infiltration. MRI of the brain demonstrated thickening and enhancement of bilateral pachymeninges and multiple enhancing masses in bilateral skull (Figure 1). At that moment, the primary esophageal tumor still showed a near complete response, but CT of the abdomen revealed mild progressive change of metastatic liver tumors. He received whole brain irradiation at a total dose of 30 Gy in 10 fractions and the above mentioned symptoms relieved subsequently. Two months later, MRI of the brain demonstrated regressive change of dural metastases and skull lesions.
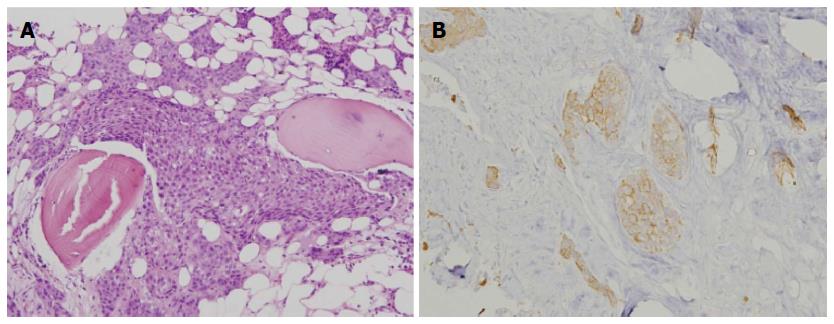
Besides, laboratory examination revealed severe thrombocytopenia (around 20000-30000 per microliter) and leukopenia with left shift of neutrophil maturation. We arranged bone marrow study and histopathologic examination showed clusters of epithelial-like cells. Tumor cells had eosinophilic cytoplasm with hyperchromatic nuclei, and some nuclei had prominent nucleoli with a high nuclear-to-cytoplasmic ratio. Immunohistochemical staining showed expression of high molecular weight cytokeratin, confirming the diagnosis of metastatic squamous cell carcinoma (Figure 2). Afterwards, he received palliative chemotherapy with cisplatin/5-FU for liver, bone and bone marrow metastases, but no obvious response was achieved. Finally, he died due to pneumonia 45 d after the diagnosis of bone marrow metastasis.
The increasing incidence of central nervous system metastases may be largely attributable to the greater availability and use of diagnostic imaging, and the advances in systemic therapy. Carcinomatous infiltration of the dura is found in 8% to 9% of patients with primary extraneural malignancy[7,8]. The most frequently reported primary tumor types metastasizing to the dura mater are prostate, breast, lung and stomach carcinomas[9]. Clinical presentation is often associated with the location of tumor and extent of involvement, the most common symptom is headache, and other symptoms include weakness, numbness, visual disturbance, speech difficulty, seizure, hearing impairment, gait disturbance, and altered mental status. Gadolinium-enhanced brain MRI is the study of first choice for the diagnosis of intracranial dural metastases. Surgical resection is the best treatment when the lesion is single, or multiple lesions but resectable, and the systemic disease is well controlled. Radiotherapy is a treatment option if operation was not indicated. There is no indication of intrathecal chemotherapy in dural metastases. In general, the outcome of dural metastases was poor.
There are two possible mechanisms of dural metastases: direct extension of skull metastases or hematogenous metastases. Direct extension from calvarial metastases was found predominantly in lung, prostate, breast cancers and Ewing sarcoma; in the absence of skull invasion, hematogenous spread mostly occurs through systemic arterial circulation[10]. Most patients had evidence of primary lung cancer or metastatic lung lesion when brain metastasis was diagnosed[11], but the incidence of lung metastases was only 0%-25% in esophageal cancer patients in previous reports[12,13]. One reason is that the lung lesions are too small to be detected[5,14], and the other reason arises from tumor spread through the vertebral venous system[5,13,15]. No lung metastasis was documented in our patient, either. Ideally, clinicians should be alert to brain metastasis despite clinically undetected lung metastases in patients with esophageal squamous cell carcinoma.
Previous reports have tried to find the risk factors for brain metastases in esophageal cancer patients. The most common histological types of esophageal cancer are squamous cell carcinoma and adenocarcinoma. Although the incidence of brain metastasis was higher in adenocarcnoma group than squamous cell carcinoma group, histology does not appear to be a risk factor for the development of brain metastasis[16-18]. On the other hand, one study demonstrated that patients with large esophageal tumors (> 8.63 cm in length) had a statistically greater chance of developing brain metastasis compared to patients with small tumors (< 5.12 cm in length)[16]. The reason may be that the chance of dissemination through the vertebral venous system increased. Our patient’s tumor length was 11 cm (7 cm and 4 cm in two tumors, respectively), and it may be considered as a high risk factor for brain metastasis.
MRI of the brain showed thickening and enhancement of bilateral pachymeninges, and the differential diagnosis included spontaneous intracranial hypotension (SIH), idiopathic hypertrophic cranial pachymeningitis (IHCP), dural metastasis and infection. SIH is a syndrome of low CSF pressure characterized by postural headaches in patients without any history of dural puncture or penetrating trauma. It is thought to result from an occult CSF leak resulting in decreased CSF volume and low CSF pressure[19]. The pattern of headache in our patient was not related to postural change and there was no low CSF pressure in lumbar puncture, so SIH was excluded. IHCP is a rare form of diffuse inflammatory disease that causes thickening of the dura mater, and typically causes progressive cranial nerve palsies, headaches, and cerebellar dysfunction[20,21]. The diagnosis is established by excluding all other granulomatous and infectious diseases[22]. We did CSF study which was negative for infective disease, so IHCP and infection were less likely. According to his underlying disease and clinical presentation, dural metastasis was the most likely diagnosis.
Distant metastasis is the main cause of mortality and morbidity in cancer patients. The bone marrow is a site of cancer cell deposition and dissemination, and could be considered a target organ for metastasis. Tumor cells spread to the bone marrow mainly through hematogenous dissemination, and may be observed while the primary tumor is still in the early stage[23]. At the time of diagnosis, cytopenia was a frequent finding, especially anemia and thrombocytopenia. Chemotherapy is a treatment option, and dose delays and dose reductions are necessary in some cases. The cytopenic complications, such as febrile neutropenia or bleeding events, should be closely monitored. The presence of bone marrow metastasis is an independent predictor of poor prognosis.
In conclusion, to the best of our knowledge, the present case is the first described case of esophageal squamous cell carcinoma with dural and bone marrow metastases. It is important to recognize the unusual metastatic pattern and offer our patients the differential diagnosis of pachymeningeal thickening and enhancement. In the era of cancer treatment, clinicians should be aware of this condition.
A 62-year-old man was admitted with progressive low back pain with radiation to bilateral lower legs, accompanied with dysphagia and body weight loss.
This case was diagnosed with esophageal squamous cell carcinoma with dural and bone marrow metastases.
Spontaneous intracranial hypotension, idiopathic hypertrophic cranial pachymeningitis and infection were mentioned in the differential diagnosis.
The laboratory findings revealed that severe thrombocytopenia (around 20000-30000 per microliter) and leukopenia with left shift of neutrophil maturation. CSF study was negative for bacterial, fungal, tuberculous infection or neoplastic infiltration.
Magnetic resonance imaging of the brain showed thickening and enhancement of bilateral pachymeninges and multiple enhancing masses in bilateral skull.
Bone marrow study showed clusters of epithelial-like cells which had eosinophilic cytoplasm with hyperchromatic nuclei, and some nuclei had prominent nucleoli with a high nuclear-to-cytoplasmic ratio. Immunohistochemical staining revealed expression of high molecular weight cytokeratin, confirming the diagnosis of metastatic squamous cell carcinoma.
Whole brain irradiation was performed in this case.
This case is the first described case of esophageal squamous cell carcinoma with dural and bone marrow metastases.
Thickening and enhancement of pachymeninges may be caused by spontaneous intracranial hypotension, idiopathic hypertrophic cranial pachymeningitis, dural metastasis and infection.
The lung, liver, bone, pleura and adrenal gland are the most common sites of visceral metastasis, but brain and bone marrow involvement are exceedingly rare in esophageal squamous cell carcinoma. Magnetic resonance imaging of the brain and cerebrospinal fluid study must be considered if brain metastasis is highly suspected.
This case presented the unusual metastatic pattern and offered the differential diagnosis of pachymeningeal thickening and enhancement. It may help us to understand the pathogenesis of this rare entity, and clinicians should be aware of this condition.
P- Reviewer: Iizuka T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Du P
| 1. | Bosch A, Frias Z, Caldwell WL, Jaeschke WH. Autopsy findings in carcinoma of the esophagus. Acta Radiol Oncol Radiat Phys Biol. 1979;18:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Mandard AM, Chasle J, Marnay J, Villedieu B, Bianco C, Roussel A, Elie H, Vernhes JC. Autopsy findings in 111 cases of esophageal cancer. Cancer. 1981;48:329-335. [PubMed] |
| 3. | Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120-1125. [PubMed] |
| 4. | Kawabata R, Doki Y, Ishikawa O, Nakagawa H, Takachi K, Miyashiro I, Tsukamoto Y, Ohigashi H, Sasaki Y, Murata K. Frequent brain metastasis after chemotherapy and surgery for advanced esophageal cancers. Hepatogastroenterology. 2007;54:1043-1048. [PubMed] |
| 5. | Ogawa K, Toita T, Sueyama H, Fuwa N, Kakinohana Y, Kamata M, Adachi G, Saito A, Yoshii Y, Murayama S. Brain metastases from esophageal carcinoma: natural history, prognostic factors, and outcome. Cancer. 2002;94:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 6. | Yoshida S. Brain metastasis in patients with esophageal carcinoma. Surg Neurol. 2007;67:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Meyer PC, Reah TG. Secondary neoplasms of the central nervous system and meninges. Br J Cancer. 1953;7:438-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592. [PubMed] |
| 9. | Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005;75:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Kleinschmidt-DeMasters BK. Dural metastases. A retrospective surgical and autopsy series. Arch Pathol Lab Med. 2001;125:880-887. [PubMed] |
| 11. | Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Kaneko T, Hirao M, Shimada M, Takayama T, Iwazawa T, Murata K, Inoue M, Terashima T, Mizunoya S, Okagawa K. Postoperative brain metastasis from esophageal carcinomas: report of 4 cases. Kyobu Geka. 1991;44:1013-1017. [PubMed] |
| 13. | Sakata R, Ohiwa Y, Shinmura F, Ariwa R. Intracerebral metastasis of esophageal carcinoma--a case report and review of literature. No Shinkei Geka. 1985;13:647-651. [PubMed] |
| 14. | Vaquero J, Abreu L, Cabezudo JM. Esophageal carcinoma metastatic to the brain. Am J Gastroenterol. 1982;77:541-542. [PubMed] |
| 15. | Batson OV. The Function of The Vertebral Veins And Their Role In The Spread of Metastases. Ann Surg. 1940;112:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1252] [Cited by in RCA: 1149] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 16. | Gabrielsen TO, Eldevik OP, Orringer MB, Marshall BL. Esophageal carcinoma metastatic to the brain: clinical value and cost-effectiveness of routine enhanced head CT before esophagectomy. AJNR Am J Neuroradiol. 1995;16:1915-1921. [PubMed] |
| 17. | Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol. 2011;17:2407-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Weinberg JS, Suki D, Hanbali F, Cohen ZR, Lenzi R, Sawaya R. Metastasis of esophageal carcinoma to the brain. Cancer. 2003;98:1925-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Rando TA, Fishman RA. Spontaneous intracranial hypotension: report of two cases and review of the literature. Neurology. 1992;42:481-487. [PubMed] |
| 20. | Mamelak AN, Kelly WM, Davis RL, Rosenblum ML. Idiopathic hypertrophic cranial pachymeningitis. Report of three cases. J Neurosurg. 1993;79:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Sylaja PN, Cherian PJ, Das CK, Radhakrishnan VV, Radhakrishnan K. Idiopathic hypertrophic cranial pachymeningitis. Neurol India. 2002;50:53-59. [PubMed] |
| 22. | Phanthumchinda K, Sinsawaiwong S, Hemachudha T, Yodnophaklao P. Idiopathic hypertrophic cranial pachymeningitis: an unusual cause of subacute and chronic headache. Headache. 1997;37:249-252. [PubMed] |
| 23. | Pantel K, Woelfle U. Micrometastasis in breast cancer and other solid tumors. J Biol Regul Homeost Agents. 2004;18:120-125. [PubMed] |