Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12637
Revised: April 10, 2014
Accepted: May 12, 2014
Published online: September 21, 2014
Processing time: 233 Days and 19.8 Hours
AIM: To identify pharmaceuticals for the prophylaxis of anastomotic leakage (AL), we systematically reviewed studies on anastomosis repair after colorectal surgery.
METHODS: We searched PubMed and EMBASE for articles published between January 1975 and December 2012. We included studies in English with the primary purpose of promoting healing of anastomoses made in the colon or rectum under uncomplicated conditions. We excluded studies on adverse events from interventions, nutritional interventions or in situ physical supporting biomaterials. The primary outcome was biomechanical strength or AL. We performed meta-analyses on therapeutic agents investigated by three or more independent research groups using the same outcome. The DerSimonian-Laird method for random effects was applied with P < 0.05.
RESULTS: Of the 56 different therapeutic agents assessed, 7 met our inclusion criteria for the meta-analysis. The prostacyclin analog iloprost increased the weighted mean of the early bursting pressure of colonic anastomoses in male rats by 60 mmHg (95%CI: 30-89) vs the controls, and the immunosuppressant tacrolimus increased this value by 29 mmHg (95%CI: 4-53) vs the controls. Erythropoietin showed an enhancement of bursting pressure by 45 mmHg (95%CI: 14-76). The anabolic compound growth hormone augmented the anastomotic strength by 21 mmHg (95%CI: 7-35), possibly via the up-regulation of insulin-like growth factor-1, as this growth factor increased the bursting pressure by 61 mmHg (95%CI: 43-79) via increased collagen deposition. Hyperbaric oxygen therapy increased the bursting pressure by 24 mmHg (95%CI: 13-34). Broad-spectrum matrix metalloproteinase inhibitors increased the bursting pressure by 48 mmHg (95%CI: 31-66) on postoperative days 3-4. In the only human study, the AL incidence was not significantly reduced in the 103 colorectal patients treated with aprotinin (11.7%) compared with the 113 placebo-treated patients (9.7%).
CONCLUSION: This systematic review identified only one randomized clinical trial and seven therapeutic agents from pre-clinical models that could be explored further for the prophylaxis of AL after colorectal surgery.
Core tip: Anastomotic leakage after colorectal surgery is an ongoing challenge and results in high morbidity and mortality. Currently, there is no pharmaceutical compound specifically indicated for the improvement of anastomotic healing. This situation is remarkable considering the many interventions that have been assessed under experimental conditions. This study reviewed 56 therapeutic agents investigated in 75 separate studies. Iloprost, tacrolimus, erythropoietin, growth hormone, insulin-like growth factor-1, hyperbaric oxygen and matrix metalloproteinase inhibitor therapies reproducibly improved anastomosis stability in experimental models. These therapies, alone or in combination, should be explored further.
- Citation: Øines MN, Krarup PM, Jorgensen LN, Ågren MS. Pharmacological interventions for improved colonic anastomotic healing: A meta-analysis. World J Gastroenterol 2014; 20(35): 12637-12648
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12637.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12637
Surgical resection with primary anastomosis is a standard treatment for colorectal cancer and benign diseases, such as diverticulitis, ulcerative colitis and ischemia or for the reversal of an ostomy.
Surgical techniques have been optimized to restore intestinal continuity without compromising blood supply. Nevertheless, the incidence of anastomotic leakage (AL) is 3%-6% after colonic resection and 10%-12% after rectal resection[1,2]. AL increases short-term morbidity, permanent stoma rates and mortality. Furthermore, AL contributes to the recurrence of malignant disease[3]. Risk factors for AL include age older than 60 years, male sex, low serum albumin, prolonged surgery, increased intraoperative blood loss and blood transfusions.
Although the negative impact of corticosteroids[4] and non-steroidal anti-inflammatory drugs[5] have been well documented, there have been no pharmaceutical compounds specifically indicated for the improvement of anastomotic healing. This is remarkable considering the many interventions that have been assessed under experimental conditions. It is even more surprising that this extremely valuable source of scientific data has not been assessed, either critically or systematically in terms of suitability for the prophylaxis of AL in patients.
One reason for this lack of data could be that spontaneous dehiscence and AL have been extremely rare in experimental models. Therefore, we have had to rely on surrogate outcomes of anastomotic repair. The most common measures are the bursting pressure (BPR) and the breaking strength (BST). BPR measurements reflect the resistance to increased intraluminal pressure, and BST indicates increased longitudinal load. BPR and BST are minimal in the early postoperative period, reflecting the fragility of the anastomosis at this stage, but they increase rapidly beginning on postoperative day 3[6-8]. Clinically, AL symptoms usually appear at postoperative days 5-8, while biochemical markers indicate that the intestinal connection dehisces earlier[9].
The aim of the present study was to provide a systematic and comprehensive review of pharmacological stimulation of anastomotic healing to identify compounds potentially capable of preventing AL.
This systematic review followed the PRISMA guidelines[10].
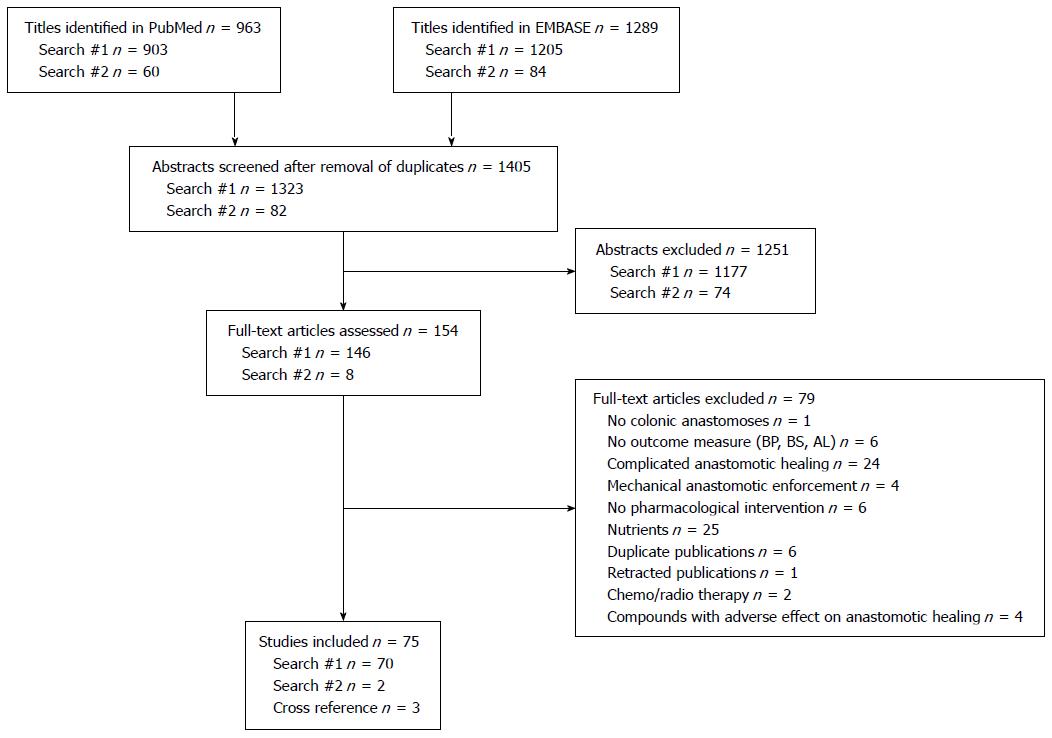
The PubMed and EMBASE databases were searched for articles published between January 1975 and December 2012 using two different syntaxes: search 1, anastomo and (colon or rect) and (strength or pressure); and search 2, anastomo and (colon or rect) and leak not (strength or pressure) and experimental. The references of the included studies were searched manually.
We included controlled studies published in English that primarily investigated a therapeutic agent with the purpose of promoting colonic anastomotic healing under uncomplicated conditions, measured as BPR, BST or AL.
We excluded studies on interventions assessed under complicated conditions, such as intestinal ischemia, generalized peritonitis, colitis, large bowel obstruction, jaundice, diabetes, radiation, malnutrition or the presence of an ostomy. Furthermore, studies with the primary aim of investigating the adverse events associated with therapeutic agents were excluded. The influences of nutritional interventions were similarly excluded. Finally, the effects of mechanical enforcement, such as fibrin sealants, omental pedicle grafts or carboxymethylcellulose coatings, of anastomoses were recently reviewed and thus were excluded here[11].
The titles of the articles were retrieved and screened. Subsequently, the abstracts or the full texts of potentially relevant publications were scrutinized for eligibility. At least two authors decided whether a paper qualified. Disagreements were resolved by discussion among the four authors. The abstracted data included the investigated compound, dosage, route of administration, species, sex, sample size, assessment day and primary outcome of the study.
Therapeutic agents investigated by at least 3 independent research groups using the same primary outcome were subjected to meta-analysis. Pooled estimates were calculated using the inverse-variance weighting method with the DerSimonian-Laird random-effects model. Heterogeneity among the studies was determined using I2 tests. Analyses were conducted using Review Manager, version 5.1 (The Cochrane Collaboration). The level of statistical significance was P < 0.05.
We included 75 studies (Figure 1) that were performed in rats (n = 68), rabbits (n = 4), guinea pigs (n = 1), dogs (n = 1) and humans (n = 1). The most frequently reported outcome was BPR (n = 62), followed by BST (n = 19), whereas only one study used AL.
We identified 56 different compounds that were sub-grouped into immunomodulators (n = 20), hormones and growth factors (n = 14), miscellaneous (n = 15) and proteinase inhibitors (n = 7).
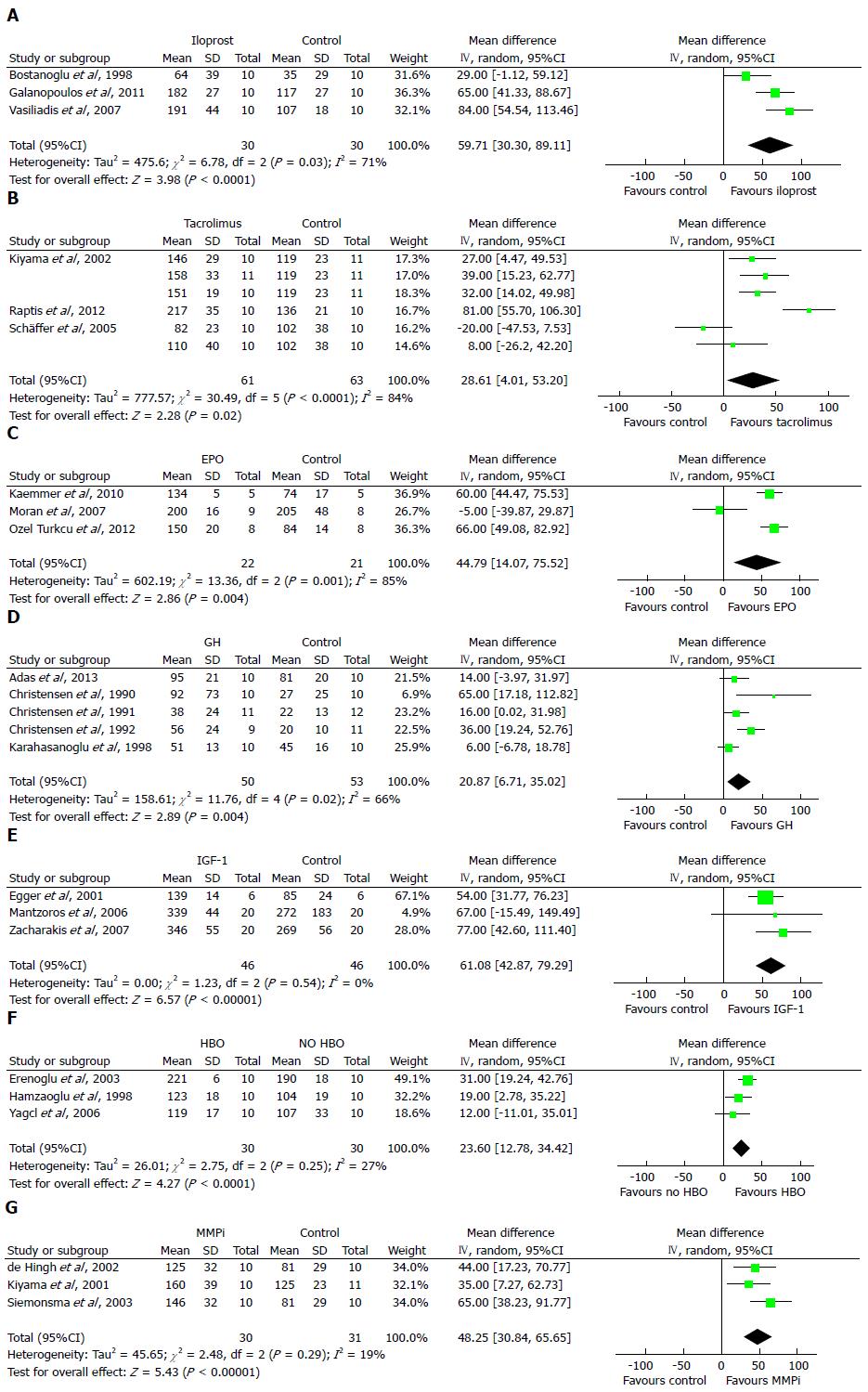
Of the 20 different immunomodulating compounds identified in the search, data for iloprost[12-14] and tacrolimus[15-17] were subjected to meta-analysis (Table 1). This analysis demonstrated that iloprost increased the weighted mean of the early bursting pressure by 60 mmHg (95%CI: 30-89, P < 0.0001) vs the controls (Figure 2A). The corresponding figure for tacrolimus was 29 mmHg (95%CI: 4-53, P = 0.02) (Figure 2B).
| Study | Compound | Species | Sex | Sample size1 | Dosage (mg/kg) | Route | Test | Test day | Effects2 |
| Bostanoğlu et al[12] | Iloprost | Rat | Male | 40 | 0.002 | IP | BPR | 3/7 | ↑82/NS |
| Galanopoulos et al[13] | Iloprost | Rat | Male | 40 | 0.002 | IP | BPR | 4/8 | ↑56/NS |
| Vasiliadis et al[14] | Iloprost | Rat | Male | 20 | 0.002 | IP | BPR | 5/8 | ↑78/NS |
| Kiyama et al[15] | Tacrolimus | Rat | Male | 42 | 0.01/0.1/1.0 | SC | BPR | 4 | ↑23/↑33/↑27 |
| Raptis et al[16] | Tacrolimus | Rat | Male | 40 | 0.1 | SC | BPR | 4/8 | ↑60/↑43 |
| Schäffer et al[17] | Tacrolimus | Rat | Male | 24 | 2.0 | SC | BPR | 5 | NS |
| Study | Compound | Species | Sex | Sample size1 | Dosage (mg/kg) | Route | Test | Test day | Effect2 |
| de Hingh et al[79] | BB-94 | Rat | Male | 60 | 30 | IP | BPR/BST | 1 | NS/NS |
| BPR/BST | 3 | ↑54/↑27 | |||||||
| BPR/BST | 7 | NS/NS | |||||||
| Kiyama et al[80] | BE16627B | Rat | Male | 21 | About 10 | SC | BPR | 4 | ↑28 |
| Pasternak et al[81] | Doxycycline | Rat | Male | 40 | NA | LO | BST | 3 | ↑17 |
| Siemonsma et al[82] | Doxycycline | Rat | Male | 80 | About 40 | SC | BPR/BST | 1 | NS/NS |
| SC | BPR/BST | 3 | ↑93/↑27 | ||||||
| PO | BPR/BST | 3 | ↑36/NS | ||||||
| SC | BPR/BST | 5 | NS/NS | ||||||
| Syk et al[7] | BB-1101 | Rat | Male | 48 | 30 | SC | BST | 1/3/7 | NS/↑48/NS |
| Ågren et al[8] | AG3340 | Rat | Male | 120 | 10 | SC | BST | 3 | ↑47 |
| GM6001 | Rat | Male | 10/100 | SC | BST | 3 | ↑79/↑88 |
Recombinant human granulocyte macrophage-colony stimulating factor increased BPR on days 3 and 7 in one study[18], but this treatment was found to be ineffective in two other studies[19,20]. Interleukin-2 decreased both BPR and BST in male rats[21]. Both parthenolide and resveratrol increased BPR on day 7 but not on days 3 or 5 in one study[22,23], while resveratrol increased BPR on both day 3 and day 7 in another study[22,23].
| Study | Compound | Species | Sex | Sample size1 | Dosage2 | Route | Test | Test day | Effects3 |
| Faruquzzaman et al[34] | EPO | Guinea pig | Male | 20 | 500 | SC | BPR | 7 | NS |
| Fatouros et al[19] | EPO | Rat | Male | 30 | 500 | SC | BST | 7 | ↑37 |
| Kaemmer et al[35] | EPO | Rat | Male | 20 | 5000 | SC | BPR | 3/5 | NS/↑82 |
| Moran et al[36] | EPO | Rat | Male | 20 | 500 | SC | BPR | 7 | NS |
| Ozel Turkcu et al[37] | EPO | Rat | Male | 16 | 500 | IM | BPR | 7 | ↑93 |
| Adas et al[38] | GH | Rat | Male | 20 | 2.0 | SC | BPR | 4 | ↑16 |
| Christensen et al[39] | GH | Rat | Female | 72 | 2.0 | SC | BPR | 2/4/6 | ↑104/↑232/NS |
| Christensen et al[40] | GH | Rat | Female | 72 | 2.0 | SC | BPR | 2/4/6 | ↑55/NS/NS |
| Christensen et al[41] | GH | Rat | Female | 50 | 0.125/0.5/2.0/8.0 | SC | BPR | 4 | NS/NS/↑270/↑430 |
| Christensen et al[42] | GH | Rat | Female | 36 | 2.04/5/6 | SC | BST | 4 | NS/↑34/↑59 |
| Karahasanoglu et al[43] | GH | Rat | Male | 20 | 2.0 | SC | BPR | 4 | ↑11 |
| Egger et al[44] | IGF-1 | Rat | Male | 76 | 1.0 | IP | BPR | 2/4/6 | ↑62/↑67/↑61 |
| Mantzoros et al[45] | IGF-1 | Rat | Female | 40 | 2.0 | IP | BPR | 7 | ↑25 |
| Petersen et al[46] | IGF-1 | Rat | Female | 26 | about 2.5 | SC | BST | 3 | NS |
| Zacharakis et al[47] | IGF-1 | Rat | Male | 40 | 2.0 | IP | BPR | 7 | ↑29 |
Immunomodulating interventions assessed in single studies that reported increased anastomotic integrity included prostaglandin E1[24], cholera toxin[25], bacteria from rat colon[26] and noxythyoline[27]. CD18 antibody[28], Escherichia coli[26], Lactobacillus acidophilus[26], Lactobacillus helveticus[29], Streptococcus thermophiles[29], mesalamine[30], p38 mitogen-activated kinase protein inhibitor[8], activated protein C[31], pyrrolidine dithiocarbamate[32] and trapidil[33] showed no statistically significant effects on anastomotic biomechanical strength.
In this category, sufficient data for the meta-analysis were available for erythropoietin (EPO)[19,34-37], growth hormone (GH)[38-43] and insulin-like growth factor (IGF)-1[44-47] (Table 2). The meta-analyses showed that EPO increased BPR by 45 mmHg (95%CI: 14-76, P = 0.004) (Figure 2C), GH increased BPR by 21 mmHg (95%CI: 7-35, P = 0.004) (Figure 2D) and IGF-1 increased BPR by 61 mmHg (95%CI: 43-79, P < 0.00001) vs the controls (Figure 2E).
In addition to these substances, full-length or truncated keratinocyte growth factor increased anastomotic strength on postoperative days 2-7[44,48]. Compounds investigated in isolated studies reporting enhancement of anastomotic healing included triiodothyronine[49], epidermal growth factor[50], vascular endothelial growth factor[51], leptin[52] and platelet-rich-plasma[53]. In contrast, no significant effects on anastomotic strength were found with locally applied platelet-derived growth factor-BB[54], long-acting glucagon-like peptide-2[55], melatonin[56], octreotide[57] or ranitidine[58].
Although the results from animal studies on oxygen therapy were inconsistent[59-62] (Table 3), hyperbaric oxygen significantly increased BPR by 24 mmHg (95%CI: 13-34, P < 0.0001) in the meta-analysis (Figure 2F). However, the sole human study on oxygen therapy that we retrieved was recently retracted by the journal that published it[63].
Gentamicin, administered systemically and locally, increased BPR on day 5 but not on day 3 in two separate studies[64,65]. Kanamycin in combination with erythromycin was more effective than kanamycin alone in increasing BS of colonic anastomoses on day 7 in dogs[66]. The calcium channel blocker nifedipine increased BPR on days 3 and 7 in one study[67]. Phenytoin increased BPR on days 3 and 7, either by oral or rectal administration at clinically relevant dosages[68]. Male rats that received pharmacological doses of vitamin A for five days preoperatively and for six days postoperatively showed increased BPR[69]. Simvastatin orally administered to rats resulted in increased BPR on days 3 and 7[70]. Zinc intraperitoneally administered to rabbits increased BPR on day 7[71] but showed no significant effect on BST on days 3 or 7 in male rats given an equivalent zinc dosage[72]. Heparin had no effect on BST, but low-molecular-weight heparin increased BST at day 7[73]. A dextran derivative with heparin-like properties increased BPR by more than two-fold on day 2 but not at later time points in two separate studies[74,75]. Albumin[76], colloid[77] and hydroxyethyl starch[76,78] had no significant impacts on anastomotic strength.
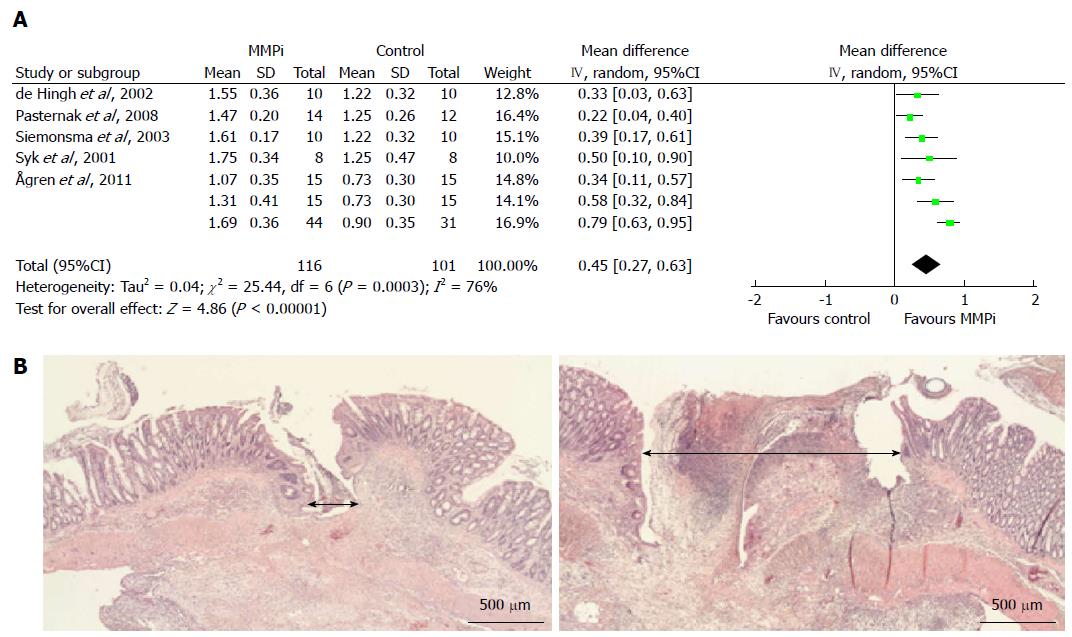
Matrix metalloproteinase (MMP) inhibitors, including AG3340[8], BB-94[79], BB-1101[7], BE166227B[80], doxycycline[81,82] and GM6001[8], consistently resulted in improved anastomotic strength on postoperative days 3-4 but not later (Table 4). The meta-analysis of the three studies using the outcome of BPR showed a weighted mean increase in BPR of 48 mmHg (95%CI: 31-66 mmHg, P < 0.00001) vs the vehicle. The studies that used BST as an outcome measurement demonstrated a significant increase in the weighted mean of BST of 0.45 N (95%CI: 0.27-0.63, P < 0.00001) (Figure 3A). In a study on GM6001, light microscopic examination revealed a pronouncedly smaller longitudinal wound gap compared with vehicle-treated animals (Figure 3B)[8].
Aprotinin increased BPR in rabbits at postoperative days 4 and 7[83,84]. In addition, aprotinin treatment increased the absolute change in collagen concentration from day 0 more than in the controls[84]. In sharp contrast, aprotinin attenuated BPR in rats on day 15[85]. In the single human study, 103 patients were randomized to aprotinin and 113 to the placebo[86]. The incidence of AL in the placebo group (9.7%) was not significantly reduced with aprotinin (11.7%), which was administered in a dosage similar to that given in the experimental studies[83,84]. Post hoc analyses indicated reduced AL in the aprotinin-treated patients who underwent low anterior rectal resections, while those subjected to left hemicolectomy or sigmoid colectomy had more AL[86].
We reviewed the data on 56 different therapeutic agents intended to promote anastomotic wound healing with the purpose of identifying interventions with prophylactic potential in colorectal AL. Approximately half of these agents were assessed in one study only. To obtain more robust conclusions, we performed a meta-analysis of the products that were tested by three or more independent research groups. Meta-analyses were undertaken for iloprost, tacrolimus, EPO, GH, IGF-1, hyperbaric oxygen and MMP inhibitors. These therapies reproducibly improved anastomotic stability in uncomplicated pre-clinical models. Thus, exploration of these agents, alone or in combination, would be the next step in the search for effective interventions for AL prophylaxis.
Anastomotic healing follows the chronological phases of tissue repair, which are largely regulated by cytokines and growth factors[6,87,88]. The initial hemostatic response results in a fibrin/fibronectin matrix that temporarily seals and connects the two bowel ends. Subsequently, inflammatory cell infiltration contributes to loss of the existing collagen of the adjacent submucosa by tissue-destructive proteinases, notably MMPs[89]. From postoperative day 3, the provisional matrix is gradually converted into granulation tissue, which contains many new blood vessels, macrophages and fibroblasts[89]. The collagen synthesis rate then increases dramatically and peaks on days 6-7[90]. BST, but not BPR, was correlated with the increase in collagen deposited in the anastomosis during the first week[90].
Anastomotic repair can be improved via different non-overlapping mechanisms, including inhibition of the degradation of submucosal collagen, promotion of angiogenesis and acceleration of granulation tissue deposition and epithelialization.
The prostacyclin analog iloprost enhanced anastomotic strength, possibly through increased neoangiogenesis and intestinal blood perfusion[13,14]. Tacrolimus also improved anastomotic healing, and light microscopy revealed reduced inflammatory cell infiltration and preserved morphology of the two colonic ends in the tacrolimus group[15]. However, tacrolimus is an immunosuppressive drug that targets T-cell activation and interleukin-2 transcription[15-17]. Based on these results, iloprost is a potential candidate for further exploration, while tacrolimus is not due to its general immunosuppressive effects.
Although the main indication for EPO is anemia, its non-hematopoietic properties could have positive effects on anastomotic healing[91]. EPO treatment also enhanced anastomotic strength under normal situations. Interestingly, improved BPR coincided with reduced MMP-8 expression in anastomotic wounds[35]. A more obvious place for EPO therapy would perhaps be under complicated situations, such as ischemia.
The beneficial effect of GH on anastomotic strength was reproduced on postoperative days 2-4 when administered at a dosage of 2.0 mg/kg per day or more[38-43]. The positive effects could be ascribed to earlier deposition and reorganization of neocollagen in anastomotic wound gaps[41,42]. Similarly, overexpression of GH profoundly stimulated early granulation tissue formation in wounds[92]. One caveat is that most of the studies were performed in female animals. GH treatment was seemingly less effective in male rats, possibly due to the negative influence of testosterone on wound healing[93]. GH stimulated hepatic synthesis of IGF-1, which is believed to mediate the local effects of GH[46]. Exogenous IGF-1 also raised anastomotic collagen levels and anastomotic strength when administered intraperitoneally[44-47]. However, the systemic adverse effects of GH and IGF-1 and the possible danger of using mitogenic substances in colorectal cancer patients might disqualify these agents from further exploration in clinical trials. To circumvent the risk of harm, IGF-1 could be delivered locally[94].
Oxygen therapy for anastomotic wound healing is theoretically attractive. In a series of elegant experiments, Shandall et al[95] demonstrated a strong positive correlation between tissue oxygen tension and the breaking strength of colon anastomoses, largely due to increased hydroxyproline. The results here were inconsistent, although there was a statistically significant, but mediocre, increase in BPR with hyperbaric oxygen therapy[59-61]. On the other hand, hyperbaric oxygen therapy increased intra-abdominal adhesions[96]. Interestingly, a more clinically useful regimen, with 50% oxygen at atmospheric pressure, was ineffective[61]. This outcome agrees with the findings of the PROXI trial, in which no significant effect of supplemental oxygen on anastomotic dehiscence was observed[97]. The PROXI trial was excluded here because AL was not the primary outcome, and patients undergoing emergency surgery were also enrolled.
MMPs comprise a 23-member family of human zinc-dependent endopeptidases[98]. While extracellular matrix remodeling by MMPs is part of the normal physiological response to injury[98], increased activity of MMPs could be deleterious to anastomotic strength due to excessive collagen degradation[89]. Synthetic MMP inhibitors unequivocally improved anastomotic integrity. Kiyama et al[80] attributed the increased mechanical strength to more collagen fibers in the wound gap connecting the large bowel ends. Morphologically, the smaller wound gap observed after administration of GM6001 strongly suggested that MMP inhibitors protected the existent submucosal collagen network from degradation[8]. Intuitively, this observation also indicated a decreased risk of leakage of the intraluminal content into the peritoneal cavity[8]. Interestingly, GM6001 treatment did not increase the formation of intra-abdominal adhesions[99].
The pathogenesis of AL is multifactorial. The studies in our review used surrogate outcomes that could not be directly translated into clinical AL. Furthermore, there was only one study conducted in patients who were subjected to colorectal surgery.
Neither the quality nor publication bias of the included studies was evaluated here because the studies were too small in sample size and too few in number[100].
The efficacy of a therapeutic agent depends on the conditions in which the anastomoses are constructed. We chose to focus on studies investigating anastomotic healing under uncomplicated conditions because these cases are representative of the majority of patients with colorectal cancer[101-103].
To conclude, despite these limitations, our review indicated several promising therapeutic agents for the prevention of AL.
We thank Drs. Thijs Hendriks and Michael Schäffer for providing primary data for the meta-analysis. Drs. Andrea Nelson and Ingvar Syk commented on the manuscript.
Anastomotic leakage (AL) is a very serious and common complication that typically follows surgical removal of colorectal cancer. Any prophylactic intervention would thus have an enormous socioeconomic impact. However, to date, there have been no pharmaceutical interventions available, despite this urgent medical need. This situation is remarkable, considering the many interventions that have been assessed under experimental conditions.
The study was the first to systematically review studies on compounds that could potentially improve anastomotic wound healing and prevent AL.
The search identified 56 different therapeutic agents. Sufficient published data for a meta-analysis were available for iloprost, tacrolimus, erythropoietin, growth hormone, insulin-like growth factor-1, hyperbaric oxygen therapy and broad-spectrum matrix metalloproteinase inhibitors.
This review identified 7 therapeutic substances from pre-clinical studies. These interventions were considered promising enough that they could be explored further, alone or in combination, for AL prophylaxis.
Anastomotic leakage occurs when surgically joined intestinal ends dehisce, resulting in contamination of the abdominal cavity with intestinal contents, leading to peritonitis or sepsis.
This study reviewed 56 therapeutic agents investigated in 75 studies, and it provides important information about possible pharmaceutical candidates. The results could be helpful in improving colonic anastomotic healing.
P- Reviewer: Regimbeau JM, Yu B S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 506] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Krarup PM, Jorgensen LN, Andreasen AH, Harling H. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis. 2012;14:e661-e667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 206] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Eriksen TF, Lassen CB, Gögenur I. Treatment with corticosteroids and the risk of anastomotic leakage following lower gastrointestinal surgery: a literature survey. Colorectal Dis. 2014;16:O154-O160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Klein M, Gögenur I, Rosenberg J. Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ. 2012;345:e6166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum. 1990;33:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 244] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Syk I, Ågren MS, Adawi D, Jeppsson B. Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Br J Surg. 2001;88:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Ågren MS, Andersen TL, Andersen L, Schiødt CB, Surve V, Andreassen TT, Risteli J, Franzén LE, Delaissé JM, Heegaard AM. Nonselective matrix metalloproteinase but not tumor necrosis factor-α inhibition effectively preserves the early critical colon anastomotic integrity. Int J Colorectal Dis. 2011;26:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, Carratalá A, Garcia-Granero E. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum. 2013;56:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9247] [Cited by in RCA: 8863] [Article Influence: 553.9] [Reference Citation Analysis (0)] |
| 11. | Pommergaard HC, Achiam MP, Rosenberg J. External coating of colonic anastomoses: a systematic review. Int J Colorectal Dis. 2012;27:1247-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Bostanoğlu S, Dinçer S, Keskin A, Bostanoğlu A, Dursun A, Serim C. Beneficial effect of Iloprost on impaired colonic anastomotic healing induced by intraperitoneal 5-fluorouracil infusion. Dis Colon Rectum. 1998;41:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Galanopoulos G, Pramateftakis MG, Raptis D, Mantzoros I, Kanellos D, Angelopoulos S, Koliakos G, Zaraboukas T, Lazaridis C. The effects of iloprost on colonic anastomotic healing in rats. Tech Coloproctol. 2011;15 Suppl 1:S117-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Vasiliadis K, Pramateftakis MG, Blouhos K, Mantzoros I, Koliakos G, Zaraboukas T, Kanellos I, Demetriades H, Alamdari DH, Betsis D. Effect of iloprost on impaired anastomotic healing caused by 5-fluorouracil plus leucovorin. Dis Colon Rectum. 2007;50:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kiyama T, Tajiri T, Tokunaga A, Yoshiyuki T, Barbul A. Tacrolimus enhances colon anastomotic healing in rats. Wound Repair Regen. 2002;10:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Raptis D, Mantzoros I, Pramateftakis MG, Despoudi K, Zaraboukas T, Koliakos G, Kanellos I, Lazarides Ch. The effects of tacrolimus on colonic anastomotic healing in rats. Int J Colorectal Dis. 2012;27:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Schäffer M, Fuchs N, Völker J, Schulz T, Kapischke M, Viebahn R. Differential effect of tacrolimus on dermal and intestinal wound healing. J Invest Surg. 2005;18:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Dinc S, Gulcelik MA, Kuru B, Ergeneci D, Camlibel M, Caydere M, Alagol H. Effects of locally applied recombinant human granulocyte-macrophage colony-stimulating factor on ischemic bowel anastomoses in rat. Eur Surg Res. 2004;36:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Fatouros MS, Vekinis G, Bourantas KL, Mylonakis EP, Scopelitou AS, Malamou-Mitsis VD, Kappas AM. Influence of growth factors erythropoietin and granulocyte macrophage colony stimulating factor on mechanical strength and healing of colonic anastomoses in rats. Eur J Surg. 1999;165:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Demirer S, Sengül N, Inan A, Eroğlu A, Bumin C, Kuterdem E. Effect of recombinant human granulocyte/macrophage colony-stimulating factor on the healing of colonic anastomosis in rats. J Invest Surg. 2001;14:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Tadros T, Wobbes T, Hendriks T. Opposite effects of interleukin-2 on normal and transfusion-suppressed healing of experimental intestinal anastomoses. Ann Surg. 1993;218:800-808. [PubMed] |
| 22. | Cakmak GK, Irkorucu O, Ucan BH, Tascilar O, Emre AU, Karakaya K, Bahadir B, Acikgoz S, Pasaoglu H, Ankarali H. The effects of resveratrol on the healing of left colonic anastomosis. J Invest Surg. 2009;22:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bedirli A, Salman B, Pasaoglu H, Ofluoglu E, Sakrak O. Effects of nuclear factor-κB inhibitors on colon anastomotic healing in rats. J Surg Res. 2011;171:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Cali RL, Smyrk TC, Blatchford GJ, Thorson AG, Christensen MA. Effect of prostaglandin E1 and steroid on healing colonic anastomoses. Dis Colon Rectum. 1993;36:1148-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Kaplan M, Mentes BB, Tatlicioğlu E, Kayhan B, Aybay C. Effect of mucosal immunomodulation with fed cholera toxin on healing of experimental colonic anastomosis. Dis Colon Rectum. 2002;45:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Okada M, Bothin C, Kanazawa K, Midtvedt T. Experimental study of the influence of intestinal flora on the healing of intestinal anastomoses. Br J Surg. 1999;86:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Rosin RD, Exarchakos G, Gilmore OJ, Ellis H. Topical noxythiolin in colonic healing. Br J Surg. 1978;65:603-606. [PubMed] |
| 28. | Törkvist L, Månsson P, Raud J, Larsson J, Thorlacius H. Role of CD18-dependent neutrophil recruitment in skin and intestinal wound healing. Eur Surg Res. 2001;33:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Aguilar-Nascimento JE, Prado S, Zaffani G, Salomão AB, Neves Jde S, Dock-Nascimento DB, Mello PR, Okay TS. Perioperative administration of probiotics: effects on immune response, anastomotic resistance and colonic mucosal trophism. Acta Cir Bras. 2006;21 Suppl 4:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Aslan A, Temiz M, Hakverdi S, Polat G, Tumer C, Temiz A, Canbolant E. Effect of mesalamine on healing in experimental colon anastomosis: a randomised experimental study. Int J Surg. 2008;6:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Teke Z, Sacar M, Yenisey C, Atalay AO, Bicakci T, Erdem E. Activated protein C prevents deleterious effects of remote reperfusion injury caused by intestinal ischemia on wound healing in the left colonic anastomoses: an experimental study in the murine model. Am J Surg. 2008;196:774-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Teke Z, Aytekin FO, Kabay B, Yenisey C, Aydin C, Tekin K, Sacar M, Ozden A. Pyrrolidine dithiocarbamate prevents deleterious effects of remote ischemia/reperfusion injury on healing of colonic anastomoses in rats. World J Surg. 2007;31:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Colak T, Nayci A, Polat G, Polat A, Comelekoglu U, Kanik A, Turkmenoglu O, Aydin S. Effects of trapidil on the healing of colonic anastomoses in an experimental rat model. ANZ J Surg. 2003;73:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Faruquzzaman SK. The healing role of erythropoietin in the obstructive vs nonobstructive left colonic anastomosis. Bratisl Lek Listy. 2009;110:530-535. [PubMed] |
| 35. | Kaemmer DA, Otto J, Binneboesel M, Klink C, Krones C, Jansen M, Cloer C, Oettinger A, Schumpelick V, Klinge U. Erythropoietin (EPO) influences colonic anastomotic healing in a rat model by modulating collagen metabolism. J Surg Res. 2010;163:e67-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Moran M, Ozmen MM, Duzgun AP, Gok R, Renda N, Seckin S, Coskun F. The effect of erythropoietin on healing of obstructive vs nonobstructive left colonic anastomosis: an experimental study. World J Emerg Surg. 2007;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Ozel Turkcu U, Cakmak GK, Demir EO, Bakkal H, Oner MO, Okyay RD, Bassorgun IC, Ciftcioglu MA. The effect of erythropoietin on anastomotic healing of irradiated rats. J Invest Surg. 2012;25:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Adas M, Kemik O, Adas G, Arikan S, Kuntsal L, Kapran Y, Toklu AS. Is combined therapy more effective than growth hormone or hyperbaric oxygen alone in the healing of left ischemic and non-ischemic colonic anastomoses? Clinics (Sao Paulo). 2013;68:1440-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Christensen H, Oxlund H, Laurberg S. Growth hormone increases the bursting strength of colonic anastomoses. An experimental study in the rat. Int J Colorectal Dis. 1990;5:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Christensen H, Oxlund H, Laurberg S. Postoperative biosynthetic human growth hormone increases the strength and collagen deposition of experimental colonic anastomoses. Int J Colorectal Dis. 1991;6:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Christensen H, Flyvbjerg A. Dose-dependent stimulatory effect of human growth hormone on the strength and collagen deposition of colonic anastomoses in the rat. Acta Endocrinol (Copenh). 1992;126:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Christensen H, Oxlund H. Growth hormone increases the collagen deposition rate and breaking strength of left colonic anastomoses in rats. Surgery. 1994;116:550-556. [PubMed] |
| 43. | Karahasanoglu T, Altinli E, Hamzaoglu I, Paksoy M, Yeşildere T, Alemdaroglu K. Effect of growth hormone treatment on the healing of left colonic anastomoses in protein-malnourished rats. Br J Surg. 1998;85:931-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Egger B, Inglin R, Zeeh J, Dirsch O, Huang Y, Büchler MW. Insulin-like growth factor I and truncated keratinocyte growth factor accelerate healing of left-sided colonic anastomoses. Br J Surg. 2001;88:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Mantzoros I, Kanellos I, Angelopoulos S, Koliakos G, Pramateftakis MG, Kanellos D, Zacharakis E, Zaraboukas T, Betsis D. The effect of insulin-like growth factor I on healing of colonic anastomoses in cortisone-treated rats. Dis Colon Rectum. 2006;49:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Petersen TI, Kissmeyer-Nielsen P, Flyvbjerg A, Laurberg S, Christensen H. Effect of insulin-like growth factor I (IGF-I) administration on the healing of colonic anastomoses in rats. Int J Colorectal Dis. 1996;11:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Zacharakis E, Demetriades H, Kanellos D, Sapidis N, Zacharakis E, Mantzoros I, Kanellos I, Koliakos G, Zaraboukas T, Topouridou K. Contribution of insulin-like growth factor I to the healing of colonic anastomoses in rats. J Invest Surg. 2007;20:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Egger B, Tolmos J, Procaccino F, Sarosi I, Friess H, Büchler MW, Stamos M, Eysselein VE. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. Am J Surg. 1998;176:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Karaman K, Bostanci EB, Dincer N, Ulas M, Ozer I, Dalgic T, Ercin U, Bilgihan A, Ginis Z, Akoglu M. Effects of thyroid hormone supplementation on anastomotic healing after segmental colonic resection. J Surg Res. 2012;176:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Sakalloglu AE, Yagmurlu A, Dindar H, Hasirci N, Renda N, Gokcora IH. Effects of low-dose epidermal growth factor loaded gelatin microspheres in colonic anastomosis. Technol Health Care. 2002;10:328-331. |
| 51. | Ishii M, Tanaka E, Imaizumi T, Sugio Y, Sekka T, Tanaka M, Yasuda M, Fukuyama N, Shinozaki Y, Hyodo K. Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. Eur Surg Res. 2009;42:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Tasdelen A, Algin C, Ates E, Kiper H, Inal M, Sahin F. Effect of leptin on healing of colonic anastomoses in rats. Hepatogastroenterology. 2004;51:994-997. [PubMed] |
| 53. | Yol S, Tekin A, Yilmaz H, Küçükkartallar T, Esen H, Caglayan O, Tatkan Y. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008;146:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Saribeyoğlu K, Baca B, Hamzaoğlu I, Pekmezci S, Karahasanoğlu T, Hamzaoğlu H. Does becaplermin (platelet-derived growth factor-BB) reverse detrimental effects of ischemia on colonic anastomosis? Dis Colon Rectum. 2003;46:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Redstone HA, Buie WD, Hart DA, Wallace L, Hornby PJ, Sague S, Holst JJ, Sigalet DL. The effect of glucagon-like Peptide-2 receptor agonists on colonic anastomotic wound healing. Gastroenterol Res Pract. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Ozdogan M, Oruk I, Renda N, Kaynaroglu V, Baykal A. The effect of exogenous melatonin on experimental colonic anastomosis. Acta Chir Belg. 2005;105:302-305. [PubMed] |
| 57. | Colak T, Dag A, Turkmenoglu O, Polat A, Comelekoglu U, Bagdatoglu O, Polat G, Akca T, Sucullu I, Aydin S. The effect of octreotide on healing of injured colonic anastomosis with immediate postoperative intraperitoneal administration of 5-Fluorouracil. Dis Colon Rectum. 2007;50:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Apostolidis SA, Michalopoulos AA, Papadopoulos VN, Paramythiotis D, Zatagias A, Gigis P, Harlaftis N. Effect of ranitidine on healing of normal and transfusion-suppressed experimental anastomoses. Tech Coloproctol. 2004;8 Suppl 1:s104-s107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Erenoğlu C, Uluutku H, Emeksiz S, Akin ML, Foley E, Celenk T. Effect of hyperbaric oxygen on anastomoses created under the influence of 5-FU. Undersea Hyperb Med. 2003;30:321-326. [PubMed] |
| 60. | Hamzaoğlu I, Karahasanoğlu T, Aydin S, Sahin DA, Carkman S, Sariyar M, Alemdaroğlu K. The effects of hyperbaric oxygen on normal and ischemic colon anastomoses. Am J Surg. 1998;176:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Kirk D, Irvin TT. The role of oxygen therapy in the healing of experimental skin wounds and colonic anastomosis. Br J Surg. 1977;64:100-103. [PubMed] |
| 62. | Yagci G, Ozturk E, Ozgurtas T, Gorgulu S, Kutlu OC, Topal T, Cetiner S, Tufan T. Preoperative and postoperative administration of hyperbaric oxygen improves biochemical and mechanical parameters on ischemic and normal colonic anastomoses. J Invest Surg. 2006;19:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Schietroma M, Carlei F, Cecilia EM, Piccione F, Bianchi Z, Amicucci G. Colorectal Infraperitoneal anastomosis: the effects of perioperative supplemental oxygen administration on the anastomotic dehiscence. J Gastrointest Surg. 2012;16:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Binnebösel M, Junge K, Kaemmer DA, Krones CJ, Titkova S, Anurov M, Schumpelick V, Klinge U. Intraperitoneally applied gentamicin increases collagen content and mechanical stability of colon anastomosis in rats. Int J Colorectal Dis. 2009;24:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Subhas G, Bhullar JS, Cook J, Shah A, Silberberg B, Andrus L, Decker M, Mittal VK. Topical gentamicin does not provide any additional anastomotic strength when combined with fibrin glue. Am J Surg. 2011;201:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | LeVeen HH, Wapnick S, Falk G, Olivas O, Bhat D, Gaurdre M, Patel M. Effects of prophylactic antibiotics on colonic healing. Am J Surg. 1976;131:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Ugurlu L, Turan M, Canbay E, Elagöz S, Sen M. Effect of nifedipine on the healing of left colonic anastomoses in rats. Surg Today. 2003;33:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Turan M, Saraydin SU, Canbay E, Karadayi K, Bulut E, Cetinkaya O, Elagöz S, Sen M. Positive effects of phenytoin on experimental colonic anastomoses. Int J Colorectal Dis. 2004;19:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Bark S, Rettura G, Goldman D, Seifter E, Levenson SM, Demetriou AA. Effect of supplemental vitamin A on the healing of colon anastomosis. J Surg Res. 1984;36:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Karadeniz Cakmak G, Irkorucu O, Ucan BH, Emre AU, Bahadir B, Demirtas C, Tascilar O, Karakaya K, Acikgoz S, Kertis G. Simvastatin improves wound strength after intestinal anastomosis in the rat. J Gastrointest Surg. 2009;13:1707-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Tümer AR, Kama NA, Tümer L, Reis E, Müftüoğlu S. Effects of 5-fluorouracil and zinc on healing of colonic anastomoses in rabbits. Eur J Surg. 1999;165:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Ågren MS, Andersen L, Heegaard AM, Jorgensen LN. Effect of parenteral zinc sulfate on colon anastomosis repair in the rat. Int J Colorectal Dis. 2008;23:857-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Mätzsch T, Bergqvist D, Blomquist P, Jiborn H. Influence of standard heparin or low molecular weight heparin on healing of abdominal wounds and colonic anastomoses in rats. Acta Chir Scand. 1987;153:593-598. [PubMed] |
| 74. | Benoit J, Meddahi A, Ayoub N, Barritault D, Sezeur A. New healing agent for colonic anastomosis. Int J Colorectal Dis. 1998;13:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Meddahi A, Benoit J, Ayoub N, Sézeur A, Barritault D. Heparin-like polymers derived from dextran enhance colonic anastomosis resistance to leakage. J Biomed Mater Res. 1996;31:293-297. [PubMed] |
| 76. | Hotz B, Hotz HG, Arndt M, Holmer C, Buhr HJ, Ritz JP. Fluid resuscitation with human albumin or hydroxyethyl starch--are there differences in the healing of experimental intestinal anastomoses? Scand J Gastroenterol. 2010;45:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Marjanovic G, Villain C, Juettner E, zur Hausen A, Hoeppner J, Hopt UT, Drognitz O, Obermaier R. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg. 2009;249:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Wang P, Gong G, Li Y, Li J. Hydroxyethyl starch 130/0.4 augments healing of colonic anastomosis in a rat model of peritonitis. Am J Surg. 2010;199:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | de Hingh IH, Siemonsma MA, de Man BM, Lomme RM, Hendriks T. The matrix metalloproteinase inhibitor BB-94 improves the strength of intestinal anastomoses in the rat. Int J Colorectal Dis. 2002;17:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Kiyama T, Onda M, Tokunaga A, Efron DT, Barbul A. Effect of matrix metalloproteinase inhibition on colonic anastomotic healing in rats. J Gastrointest Surg. 2001;5:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Pasternak B, Rehn M, Andersen L, Agren MS, Heegaard AM, Tengvall P, Aspenberg P. Doxycycline-coated sutures improve mechanical strength of intestinal anastomoses. Int J Colorectal Dis. 2008;23:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Siemonsma MA, de Hingh IH, de Man BM, Lomme RM, Verhofstad AA, Hendriks T. Doxycycline improves wound strength after intestinal anastomosis in the rat. Surgery. 2003;133:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Young HL, Wheeler MH. Effect of intravenous aprotinin (Trasylol) on the healing of experimental colonic anastomoses in the rabbit. Eur Surg Res. 1983;15:18-23. [PubMed] |
| 85. | Uzunköy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000;43:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Sheridan WG, Shandall AA, Alexander-Williams J, Keighley MR, Boulos PB, Young HL. A multicenter trial of the use of the proteolytic enzyme inhibitor aprotinin in colorectal surgery. Dis Colon Rectum. 1989;32:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 87. | Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 88. | Rijcken E, Sachs L, Fuchs T, Spiegel HU, Neumann PA. Growth factors and gastrointestinal anastomotic healing. J Surg Res. 2014;187:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Ågren MS, Andersen TL, Mirastschijski U, Syk I, Schiødt CB, Surve V, Lindebjerg J, Delaissé JM. Action of matrix metalloproteinases at restricted sites in colon anastomosis repair: an immunohistochemical and biochemical study. Surgery. 2006;140:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Oxlund H, Christensen H, Seyer-Hansen M, Andreassen TT. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res. 1996;66:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Sorg H, Harder Y, Krueger C, Reimers K, Vogt PM. The nonhematopoietic effects of erythropoietin in skin regeneration and repair: from basic research to clinical use. Med Res Rev. 2013;33:637-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Thorey IS, Hinz B, Hoeflich A, Kaesler S, Bugnon P, Elmlinger M, Wanke R, Wolf E, Werner S. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J Biol Chem. 2004;279:26674-26684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Rijcken E, Fuchs T, Sachs L, Kersting CM, Bruewer M, Krieglstein CF. Insulin-like growth factor 1-coated sutures improve anastomotic healing in an experimental model of colitis. Br J Surg. 2010;97:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Shandall A, Lowndes R, Young HL. Colonic anastomotic healing and oxygen tension. Br J Surg. 1985;72:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Azevedo LA, Parra RS, Da Rocha JJ, Ramalho LN, Ramalho FS, Féres O. Hyperbaric oxygen on the healing of ischemic colonic anastomosis--an experimental study in rats. Undersea Hyperb Med. 2010;37:405-411. [PubMed] |
| 97. | Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Høgdall C, Lundvall L, Svendsen PE, Mollerup H, Lunn TH, Simonsen I. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 98. | Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 99. | Mirastschijski U, Johannesson K, Jeppsson B, Ågren MS. Effect of a matrix metalloproteinase activity and TNF-alpha converting enzyme inhibitor on intra-abdominal adhesions. Eur Surg Res. 2005;37:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 345] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 101. | Goodyear SJ, Leung E, Menon A, Pedamallu S, Williams N, Wong LS. The effects of population-based faecal occult blood test screening upon emergency colorectal cancer admissions in Coventry and north Warwickshire. Gut. 2008;57:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Cuffy M, Abir F, Audisio RA, Longo WE. Colorectal cancer presenting as surgical emergencies. Surg Oncol. 2004;13:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Iversen LH, Bülow S, Christensen IJ, Laurberg S, Harling H. Postoperative medical complications are the main cause of early death after emergency surgery for colonic cancer. Br J Surg. 2008;95:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |