Published online Aug 28, 2014. doi: 10.3748/wjg.v20.i32.11406
Revised: March 14, 2014
Accepted: April 30, 2014
Published online: August 28, 2014
Processing time: 224 Days and 8.1 Hours
AIM: To evaluate preventative effects of glutamine in an animal model of gut ischemia/reperfusion (I/R).
METHODS: Male Wistar rats were housed in a controlled environment and allowed access to food and water ad libitum. Twenty male Wistar rats were divided into four experimental groups: (1) control group (control) - rats underwent exploratory laparotomy; (2) control + glutamine group (control-GLU) - rats were subjected to laparotomy and treated intraperitoneally with glutamine 24 and 48 h prior to surgery; (3) I/R group - rats were subjected to occlusion of the superior mesenteric artery for 30 min followed by 15 min of reperfusion; and (4) ischemia/reperfusion + glutamine group (G + I/R) - rats were treated intraperitoneally with glutamine 24 and 48 h before I/R. Local and systemic injuries were determined by evaluating intestinal and lung segments for oxidative stress using lipid peroxidation and the activity of superoxide dismutase (SOD), interleukin-6 (IL-6) and nuclear factor kappa beta (NF-κB) after mesenteric I/R.
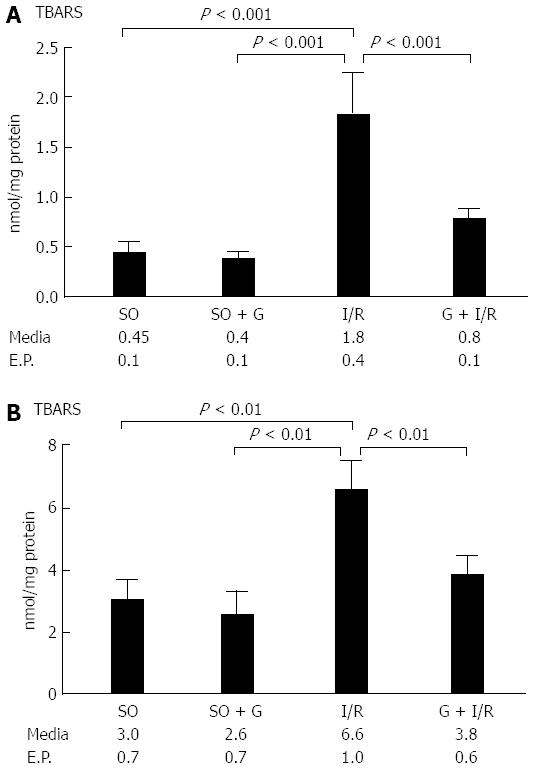
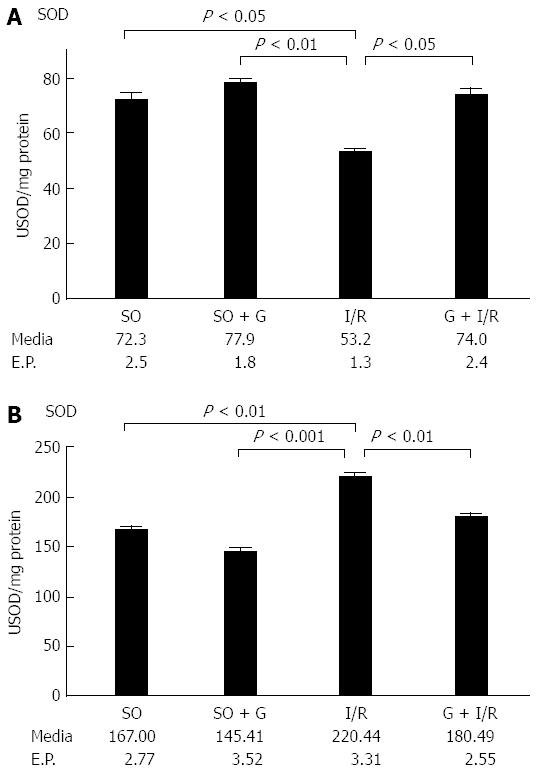
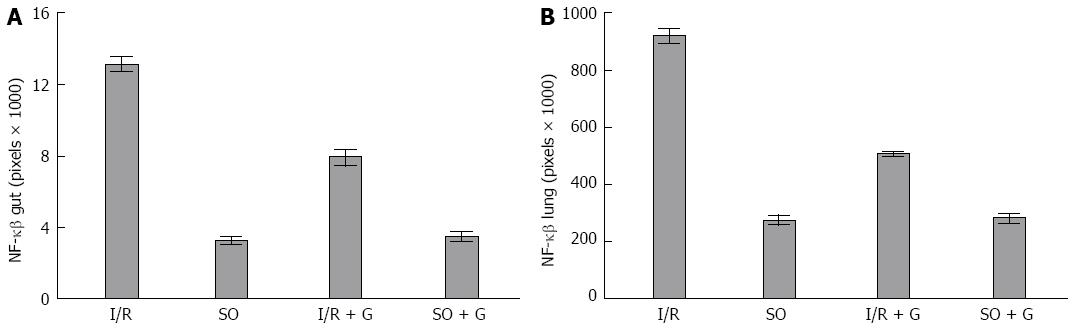
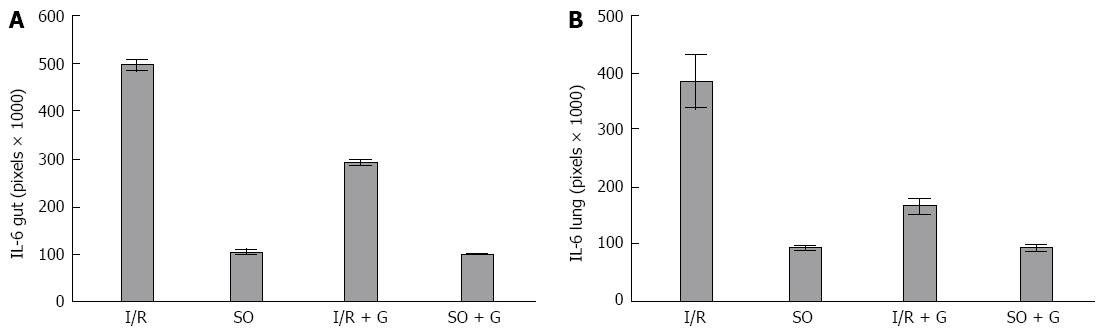
RESULTS: Lipid peroxidation of the membrane was increased in the animals subjected to I/R (P < 0.05). However, the group that received glutamine 24 and 48 h before the I/R procedure showed levels of lipid peroxidation similar to the control groups (P < 0.05). The activity of the antioxidant enzyme SOD was decreased in the gut of animals subjected to I/R when compared with the control group of animals not subjected to I/R (P < 0.05). However, the group that received glutamine 24 and 48 h before I/R showed similar SOD activity to both control groups not subjected to I/R (P < 0.05). The mean area of NF-κB staining for each of the control groups was similar. The I/R group showed the largest area of staining for NF-κB. The G + I/R group had the second highest amount of staining, but the mean value was much lower than that of the I/R group (P < 0.05). For IL-6, control and control-GLU groups showed similar areas of staining. The I/R group contained the largest area of IL-6 staining, followed by the G + I/R animals; however, this area was significantly lower than that of the group that underwent I/R without glutamine (P < 0.05).
CONCLUSION: These results demonstrate that pretreatment with glutamine prevents mucosal injury and improves gut and lung recovery after I/R injury in rats.
Core tip: Ischemia-reperfusion (I/R) leads to oxidative stress, with local and systemic consequences. Many enzymes and interleukins have been implicated in this process, among them interleukin-6 (IL-6) and nuclear factor kappa beta (NF-κB). The exact role of these enzymes is still not clear. Some substances, such as glutamine, have been studied as protective agents against oxidative stress. In an animal experimental model of intestinal I/R we have found that glutamine reduced lipid peroxidation, preserved superoxide dismutase activity, and decreased the expression of IL-6 and NF-κB in both lung and intestine, suggesting a protective role of this amino acid in the setting of intestinal I/R.
- Citation: Zabot GP, Carvalhal GF, Marroni NP, Hartmann RM, Silva VDD, Fillmann HS. Glutamine prevents oxidative stress in a model of mesenteric ischemia and reperfusion. World J Gastroenterol 2014; 20(32): 11406-11414
- URL: https://www.wjgnet.com/1007-9327/full/v20/i32/11406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i32.11406
Ischemic conditions such as arterial occlusions, transplants, mesenteric ischemia and shock occur commonly in medical practice and affect a growing number of individuals of various ages, leading to high morbidity and mortality. However, unlike ischemic injuries, reperfusion injuries alter not only the affected areas but also produce systemic changes, so that the reestablishment of the blood flow to ischemic areas may result in damage to the entire body. The damage to remote organs is termed post-traumatic multiple organ failure (MOF)[1].
Gut ischemia usually results from occlusion of the celiac trunk and/or the superior mesenteric artery by thrombi or emboli and, more frequently, from non-occlusive processes, such as in the case of decreased mesenteric blood flow that occurs in heart failure and sepsis[2]. In the gut, ischemia followed by reperfusion frequently results in MOF, with the gut being the organ that triggers the injury process in distant organs. A systemic inflammatory reaction is initiated from pro-inflammatory substances released by the gut into the lymphatic circulation, with the inflammation mainly affecting lungs, liver and kidneys[3].
Although the details about the molecular mechanisms that determine injuries in ischemic events are not yet well defined, it is known that reactive oxygen species (ROS) play an important role in the pathogenesis of gut injury after an ischemia/reperfusion (I/R) event[4]. Parks and Granger reported that the tissue damage that occurs during reperfusion is greater than the injury that occurs during ischemia. Rupture of the mucosal barrier, bacterial translocation and activation of the inflammatory response, as well as acid-base balance and electrolyte disorders, are observed[5]. Superoxide and hydrogen peroxide are thought to be the main free radicals that contribute to I/R injury. Under normal conditions ROS are neutralized by endogenous antioxidant enzymes, but an excess of free radicals is observed during reperfusion, which results in oxidative stress[6]. Those free radicals originate when oxygen (O2) is reintroduced into the ischemic tissue during reperfusion. Superoxide dismutase (SOD) is an antioxidant enzyme highly specific for superoxide elimination, thus reducing gastrointestinal lesions caused by I/R[7].
Nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB) is a transcription factor that plays a crucial role not only in normal states but also in the coordination of adaptive immune responses by regulating the expression of many cell mediators[8]. This factor, which was first described in 1986 by Sen and Baltimore[9], binds to specific kappa binding sites in the immunoglobulins of B cells. It is now well recognized that NF-κB is expressed in most cell types and that NF-κB consists of a dimer composed of members of the Relish (Rel) family. The NF-κB/Rel family contains five subunits, p50, p52, p65 (RelA), c-Rel and Rel-B. These subunits form homo- and heterodimers in several combinations. Generally, NF-κB is composed of two polypeptides, one of 50-kDa (p50) and one of 65-kDa (p65). In homeostatic cells, NF-κB remains in the cytoplasm in its inactive form, associated with proteins that inhibit the kB site called kB inhibitors (IkB). Seven IkB isoforms have been described: IkBα, IkBβ, IkBγ, IkBε, Bcl-3, p100 and p105. NF-κB is activated by a variety of signals relevant to the etiology and pathophysiology of inflammation[8]. Intracellular and/or extracellular stimuli such as bacterial products (endotoxins, peptidoglycans), viruses and viral components, protozoa, cytokines (tumor necrosis factor-alpha (TNF-α), interleukins), free radicals and/or oxidants are needed to activate NF-κB[8]. In 2002, Haddad[10] suggested that NF-κB activation controls the oxidant/antioxidant balance.
Interleukin (IL)-6 and TNF-α levels are elevated in I/R as well as in MOF. Measurements of plasma levels of these endotoxins are important to assess the systemic effects of gut I/R. ROS promote oxidative stress as a result of the production of inflammatory cytokines, such as IL-6 and TNF-α, in addition to promoting the activation of neutrophils. IL-6 and TNF-α not only directly induce tissue damage but are also potent neutrophil activators. When sequestered in the intestinal tissue, these mediators and their enzymatic products promote increased micro vascular permeability, interstitial and perivascular edema, MOF and pulmonary edema[11].
The damage and loss of mucosal barrier integrity promotes bacterial translocation and the production of cytokines. The next stage is the transport of inflammatory mediators through the intestinal lymphatic system. The lung is the first exposed organ[12]. After resuscitation from hemorrhagic shock, lymph duct ligation prevents remote lung injury, the so-called “gut-lymph hypothesis”[13]. Lymphatic thoracic duct ligation prior to mesenteric I/R protects against lung injuries and modulates serum levels of endotoxins, D-lactate, diamine oxidase and cytokines. MOF causes acute lung injury (ALI) through the production of inflammatory mediators drained through the circulatory system. The gastrointestinal tract has the largest lymphatic system of the body. Mediators released by activated inflammatory cells during an acute event reach the interstitium, which is predominantly drained by lymphatics[13].
Several substances have been used for the treatment and/or prevention of experimental colitis. The experiments aimed to evaluate new drugs for the treatment of inflammatory processes or combinations of drugs to achieve better results[14]. Substances that inhibit or minimize the inflammatory process caused by aggressive agents, such as glutamine, have been used for prevention purposes. Glutamine is an uncharged, polar amino acid that is non-essential or occasionally essential, hydrophilic, and found on the surface of proteins where it interacts with water. Glutamine is the most abundant amino acid in peripheral blood[15]. This substance was initially used prophylactically in patients undergoing radiation therapy, leading to a reduction in the incidence and severity of actinic enteritis[16]. Glutamine also has a major role in the immune defense of the intestinal mucosal barrier due to its participation in the formation of immunoglobulins, especially IgA. Glutamine decreases the inflammatory effects of methotrexate-induced enterocolitis and reduces bacterial translocation in animals with abdominal sepsis[17]. This amino acid acts on macrophage activity, interfering with phagocytosis at inflammatory sites. In addition to the direct protective effects mentioned above, glutamine plays an important role in intestinal inflammatory processes by acting on ROS[14]. Glutamine is a multifunctional amino acid used for the synthesis of urea in the liver, renal aminogenesis, gluconeogenesis, and as the main respiratory fuel for many cells. Low glutamine concentrations are found during catabolic stress and are associated with susceptibility to infections. Glutamine is not only an important energy source for mitochondria but is also a precursor of the brain neurotransmitter glutamate, which then participates in the synthesis of the antioxidant glutathione[15]. Glutamine is thus vital in the regulation of the intracellular oxidative balance[16]. Glutamine has been used as a nutritional supplement in severely debilitated patients to reduce the deleterious effects of oxidative stress[18]. It has been shown that preventing oxidative stress in patients with severe conditions or multiple traumas or undergoing major surgery is useful as a treatment adjunct. In this setting, antioxidant therapy improves patient prognosis and decreases the overall rate of complications[19].
Clinical observations have shown that patients receiving dietary glutamine supplementation had a better tolerance to colitis resulting from radiation therapy for prostate and cervical neoplasms[16]. The same substance was then used in patients with Crohn’s disease (granulomatous enterocolitis) and ulcerative rectocolitis. A clinical improvement was observed in these patients, namely decreased diarrhea, increased fistulae healing rates and decreased use of medications. Because of the importance of active oxygen species in the genesis of colitis, the relationship between oxidative stress and the supposed beneficial clinical effect of glutamine in colitis has become a subject of research. The mechanism by which glutamine exerts beneficial effects appears to be associated with the biosynthesis of glutathione, which causes a consequent reduction in lipid peroxidation of the intestinal membrane during mesenteric I/R[20].
The aim of our study was to investigate the effects of glutamine treatment in an animal model of mesenteric I/R analyzing parameters such as lipid peroxidation, SOD activity, and immunohistochemical expression of IL-6 and NF-κB.
Animal care was in compliance with the normative resolution 04/97 of the Research and Ethics Committee of the Health Research Group and Graduate Teaching Hospital of Porto Alegre (Hospital de Clinicas de Porto Alegre-HCPA)[21].
Male Wistar rats [250-300 g; State Foundation for Production and Health Research (Fundação Estadual de Produção e Pesquisa em Saúde-FEPPS)] were housed in a controlled environment and allowed access to food and water ad libitum.
After trichotomy, rats were anesthetized with ketamine and xylazine solution [45 mg/kg intraperitoneally (ip)]. After midline laparotomy, the celiac and superior mesenteric arteries were isolated near their aortic origins. During this procedure, the intestinal tract was placed between gauze pads soaked with warm 0.9% NaCl solution. The superior mesenteric artery and the celiac trunk were clamped, resulting in total occlusion of these arteries for 30 min to induce splanchnic artery occlusion injury. After occlusion, the clamps were removed, and after 15 min of reperfusion, intestinal segments (10 cm) and pieces of the lung were removed for histological examination and biochemical studies.
Rats were randomly allocated into the following groups: (1) ischemia/reperfusion (I/R): rats were subjected to splanchnic artery occlusion injury (30 min) followed by reperfusion (15 min) (n = 5); (2) ischemia/reperfusion + glutamine group (G + I/R): identical to the ischemia/reperfusion group but were treated with glutamine (25 mg/kg ip) 24 and 48 h before I/R (n = 5); (3) control group (control): rats were subjected to identical surgical procedures as the above groups, except the blood vessels were not occluded and the rats were maintained under anesthesia for the duration of the experiment (n = 5); and (4) control + glutamine group (control-GLU): identical to the Control group except for the administration of glutamine (25 mg/kg ip) 24 and 48 h before identical surgical procedures (n = 5). The glutamine treatment dose of 25mg/kg ip was chosen based on previous studies[22].
Thiobarbituric acid reactive substances: Tissue samples were placed in test tubes; solutions were added in the following order: 0.75 mL of 10% trichloroacetic acid (TCA), 0.25 mL of homogenate, 0.5 mL of 0.67% thiobarbituric acid (TBA), and 0.25 mL of distilled water.
Thiobarbituric acid reactive substances (TBARS) consists of heating the homogenate with thiobarbituric acid and measuring the consequent formation of a colored product in a spectrophotometer at 535 nm. The coloration is due to the presence of malondialdehyde and other substances from biological lipid peroxidation[23].
SOD was measured according to Misra and Fridovich. The rate of auto-oxidation of epinephrine, which is inhibited by SOD, is measured in the presence of progressively increasing doses of SOD with a spectrophotometer at 560 nm. The amount of enzyme that inhibits auto-oxidation of epinephrine at 50% of the maximum dose is defined as 1 U SOD[24].
To prepare slides for subsequent immunohistochemical analysis, tissue was sectioned at 3-μm thickness using a microtome (Leica SM 2000R, Germany). The sections were placed on slides pretreated with HistoGrip (Zymed, United States) and incubated in an oven at 60 °C for 24 h.
Sections were deparaffinized by incubating in xylene three times for 10 min, followed by rehydration of the sections using decreasing concentrations of ethanol. Antigen exposure was performed using the pTLINK platform (DAKO) for 40 min at 98 °C with the Envision Flex antigen retrieval solution, high pH (DAKO). The slides were then immediately washed in phosphate-buffered saline (PBS), pH 7.2. The blocking of endogenous peroxidases was performed with two 15-min incubations in a 3% solution of H2O2 in methyl alcohol, which were followed by three washes with PBS, pH 7.2. Non-specific binding was blocked using the commercial solution Serum-Free Protein Block (Dako, United States) for 30 min at room temperature.
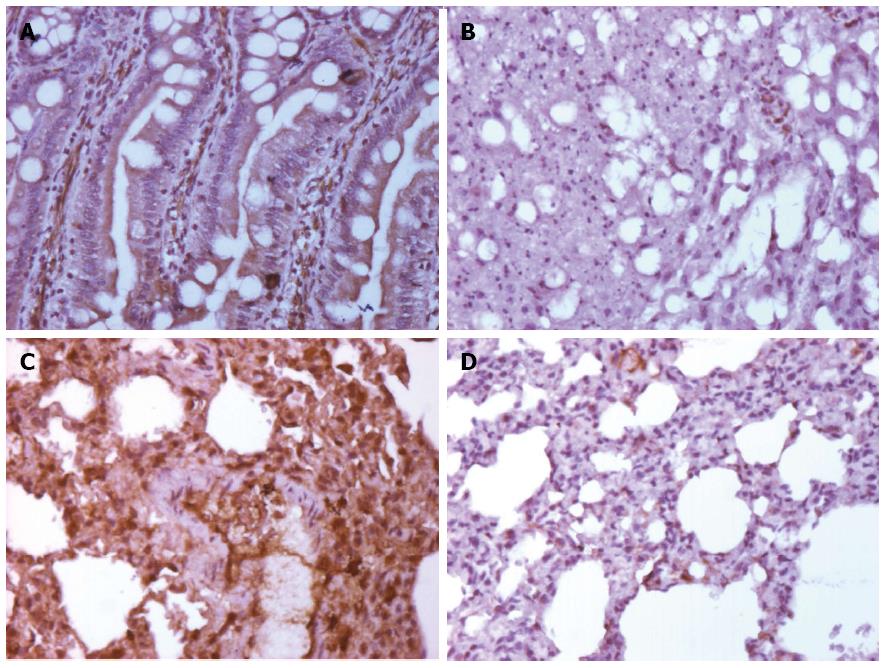
The sections were incubated using the immunostaining Sequenza station (Thermo Shandon, United States) overnight at 2 °C and 6 °C and with the following primary antibodies diluted in Antibody Diluent with Background Reducing Components (Dako, United States): anti-NF-κB (Santa Cruz Biotechnology, United States) at 1:100 and anti-IL-6 (Santa Cruz Biotechnology, United States) at 1:100. After incubation with the primary antibody, sections were washed three times in PBS, pH 7.2. To amplify the antigen-antibody reaction, the Advance system HRP was used for IL-6 (Dako, United States) according to the manufacturer’s recommendations, and for NF-κB, goat anti-rabbit IgG-HRP secondary antibody was used at 1:300 in PBS for 30 min at room temperature. Next, the slides were washed with PBS and incubated with diaminobenzidine (Dako Liquid DAB Substrate Chromogen System, United States) for 5 min. After washing with distilled water, slides were counterstained with Harris hematoxylin for 1 min, washed with water until complete removal of the dye and incubated in a 37 mmol/L ammonia solution for 15 s. Finally, the slides were dehydrated in absolute ethanol (four incubations of 2 min) and two treatments with xylene for 5 min. The slides were mounted with Entellan synthetic medium (Merck, Germany)[25].
We used a digital analysis system composed of a Zeiss Axioskop 40 microscope (Oberkochen, Germany) with Neofluar lenses connected by a Roper Scientific video camera (Media Cybernetics, Rockville, United States) to a computer with an Image Capture Pro kit (Media Cybernetics, Rockville, MD, United States) capture card. Image Pro Plus version 4.5 (Media Cybernetics, Rockville, United States) was used to analyze digital images. The images were captured in TIFF (True Image File Format) format without compression by the same examiner with a light intensity pattern for all photos. Images were captured of at least fifteen random, non overlapping fields for each histological slide at 200 × magnification (44 pixel = 1 μm). The hot spot method was used to select fields on slides with focal positivity for the markers. Color selection was performed interactively by three trained observers and was then applied to all samples by the automated digital image analysis system. The initial area considered was 0.01 cm.
Quantitative data were initially described by mean and standard deviation. To compare groups, we used analysis of variance. For categorical data, we used scores and comparisons based on Fisher’s exact test.
Analysis of variance with robust standard errors (Welch) was used to verify NF-κB and IL-6 results between groups.
The significance level for the experiments was P < 0.05. Data were analyzed with SPSS version 21.0.
Lipid peroxidation of the membrane was increased in both the gut and the lung in the animals subjected to I/R (P < 0.05). However, the group that received glutamine 24 and 48 h before the I/R procedure showed levels of lipid peroxidation similar to the control groups (animals not subjected to I/R and also the group receiving glutamine without I/R) that were significantly different from animals that only received I/R (P < 0.05). These results are shown in Figure 1.
Figure 2 shows that the activity of the antioxidant enzyme SOD was decreased in the gut of animals subjected to I/R. These findings were statistically significant (P < 0.05) when compared with the control group of animals not subjected to I/R. However, the group that received glutamine 24 and 48 h before I/R showed similar SOD activity to both control groups not subjected to I/R. There was a significant difference between the group of animals subjected to I/R and the group that received glutamine before I/R, suggesting that glutamine is a protective factor for mesenteric I/R.
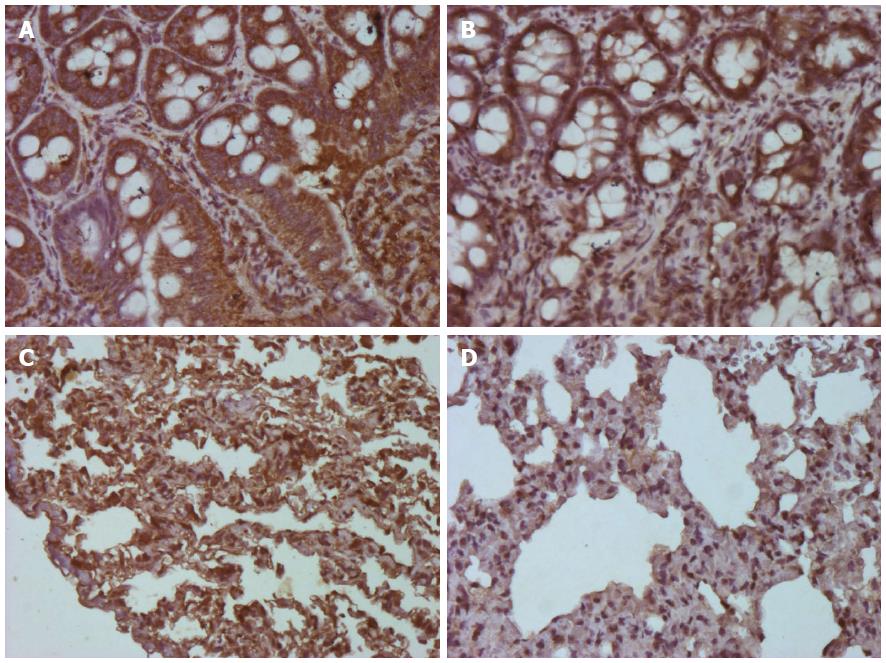
We calculated the mean area of NF-κB staining for each of the groups. As shown in Figures 3 and 4, the control and control-GLU groups presented similar mean areas. The I/R group showed the largest area of staining. The G + I/R group had the second highest amount of staining, but the mean value was much lower than that of the I/R group. The same differences were observed among groups in the large intestine and the lung. These findings were statistically significant (P < 0.05).
Images of IL-6 staining were analyzed in the same method as those stained for NF-κB. As shown in Figures 5 and 6, the control and control-GLU groups showed similar areas of staining. The I/R group contained the largest area of staining, followed by the G + I/R animals; however, this area was significantly lower than that of the group that underwent I/R without glutamine (P < 0.05).
As glutamine is glutathione precursor, and glutathione is the main non-enzymatic cellular antioxidant, is vital in the regulation of the intracellular oxidative balance[26].
This study demonstrates that glutamine treatment exerts important protective effects against splanchnic artery occlusion injury in a murine model. Our data provide evidence that glutamine attenuates: (1) the lipid peroxidation of gut mucosa; (2) the decrease in SOD activity; (3) the increases in NF-κB expression; and (4) IL-6 expression that occur after I/R.
In our study, the amount of lipid peroxidation was greater in the group of animals subjected to I/R. The addition of glutamine significantly decreased lipid peroxidation compared with animals that underwent I/R without glutamine treatment. Other authors, such as Mondello et al[27] and He et al[13] observed similar beneficial results of glutamine for I/R with different methodologies. Mondello et al[27] have induced intestinal ischemia in rats by clamping the superior mesenteric artery and the celiac trunk for 30 min, then releasing it and promoting reperfusion during 1 h. Glutamine was administered 15 min before reperfusion at the dose of 1.5 mg/kg, iv Their findings showed a reduction in: (1) the infiltration of neutrophils in the ileum; (2) the formation of the proinflammatory cytokines; (3) the expression of the adhesion molecules ICAM-1 and P-selectin; (4) the IKB-α degradation and the nuclear translocation of NF-κB; and (5) the nitrotyrosine formation and PARP activation. He et al[13] utilized a rat model of I/R, but administering glutamine enterically before and after a 60 min ischemia; additionally, in one subgroup the lymphatic mesenteric duct was also ligated before the production of intestinal ischemia. They concluded that both the enteral administration of glutamine and the ligature of the lymphatic mesenteric duct prevented intestinal permeability, attenuating systemic inflammatory reactions and ALI. In contrast, Fukatsu et al[28] have shown that in a murine model of gut I/R, an iv glutamine was detrimental in terms of survival and organ injury due to the increased priming of circulating myeloid cells.
In our study, SOD activity was decreased in animals submitted to I/R. In the Control and Control-GLU groups, the decrease in SOD activity was much lower and similar between the two groups. The addition of glutamine to animals submitted to I/R produced a decrease that was not as significant but that was lower than that found in the I/R only group. The first authors to describe the role of SOD in oxidative stress were Misra and Fridovich[24]. In their pivotal study, SOD was prepared from bovine erythrocytes, being able to inhibit the autoxidation of epinephrine at a pH 10.2. Recently, Salman et al[29] administered glutamine by gavage to Sprague-Dawley rats, at a dose of 1 g/kg for 10 d prior to intestinal I/R, studying tissue damage in the intestines and lungs. These authors measured the intestinal and pulmonary levels of SOD, in addition to serum levels of TNF-α and IL-6, concluding that pre-treatment with a bolus dose of enteral glutamine was able to minimize the extent of ALI in rats.
Tissue I/R activates families of protein kinases that converge on specific transcription factors (protein activator-1 (PA-1) and NF-κB) that regulate the expression of pro-inflammatory genes. In our study, the activity of NF-κB was higher in both the intestines and the lungs in the group subjected to I/R. However, in the group of animals that received prophylactic glutamine ip 24 and 48 h before I/R, the levels of NF-κB were lower. This difference between groups was statistically significant. Sen and Baltimore[9] published the first report on NF-κB, suggesting its important role in cellular inflammatory response to injury. However, the exact role of this transcription factor remains controversial. According to Haddad[10], NF-κB appears to perform an important function in the generation and resolution of intestinal I/R lesions, as a transcription factor that is directly influenced by reactive species and pro-inflammatory signs. Bowie et al[30], in a review article about oxidative stress and Nf-kB activation, determined that in most cases, the role of oxidative stress in NF-κB activation is at best facilitatory rather than causal, if a there exists a role at all. Ypsilantis et al[31] tested the hypothesis that the action of 2-mercaptoethane-sulfonate (mesna) is mediated by the inhibition of NF-κB, studying the oxidative stress on a rat model of I/R, analyzing glutathione, malondialdehyde concentration, SOD and NF-κB. These authors concluded that prophylaxis with mesna prevents oxidative stress induced by I/R in the intestine via inhibition of NF-κB activation.
ROS-mediated oxidative injury as a consequence of increased production of inflammatory cytokines such as IL-6 and TNF-α and the neutrophil activation play critical roles in the pathogenesis of I/R. IL-6 and TNF-α not only directly induce tissue damage but are also potent activators of neutrophils. The neutrophils and their enzymatic products cause increased microvascular permeability, perivascular and interstitial edema, and even promote distant organ injury such as pulmonary edema when sequestrated in intestinal tissue. Cuzzocrea et al[11] studied the inflammatory process secondary to I/R in a knock-out mice model, verifying by immunohistochemistry that IL-6 plays an important role in I/R injury, suggesting that the inhibition of IL-6 may actually represent a novel and possible strategy in the prevention of I/R injuries.
In our study, similarly to NF-κB, the immunohistochemical expression of IL-6 was found to be high in animals that underwent I/R in both the intestines and the lungs. The control and control-GLU groups showed similar results for IL-6, with observed levels well below those of the I/R group. However, the group that received a potentially protective factor, glutamine, before I/R showed a higher expression of IL-6 than the control and control-GLU groups but at levels that were statistically inferior to the I/R group.
In conclusion, this study demonstrates that ip administration of glutamine at a dose of 25 mg/kg 24 and 48 h before animals are subjected to 30 min of mesenteric ischemia and 15 min of reperfusion effectively protected against lipid peroxidation and preserved SOD activity. The activity of NF-κB and IL-6 were also reduced upon ip administration of glutamine at 24 and 48 h prior to I/R in rats. This adds to previously published data on glutamine as a protective factor in mesenteric I/R states in rats. Further studies are necessary to test the role of glutamine as a potential protective agent against I/R lesions in humans.
Ischemia-reperfusion (I/R) leads to oxidative stress, with local and systemic consequences. Many enzymes and interleukins have been implicated in this process, among them interleukin-6 (IL-6) and nuclear factor kappa beta (NF-κB). The exact role of these enzymes is still not clear. Substances that inhibit or minimize the inflammatory process caused by aggressive agents, such as glutamine, have been used for prevention purposes.
Glutamine is the most abundant amino acid in peripheral blood. That amino acid acts on macrophage activity, interfering with phagocytosis at inflammatory sites. Plays an important role in intestinal inflammatory processes by acting on reactive oxygen species (ROS). Glutamine is a multifunctional amino acid used for the synthesis of urea in the liver, renal aminogenesis, gluconeogenesis, and as the main respiratory fuel for many cells.
This substance was initially used prophylactically in patients undergoing radiation therapy, leading to a reduction in the incidence and severity of actinic enteritis. It is thus vital in the regulation of the intracellular oxidative balance. Glutamine has been used as a nutritional supplement in severely debilitated patients to reduce the deleterious effects of oxidative stress. The present study demonstrated that the pretreatment with glutamine prevents mucosal injury and improves gut and lung recovery after I/R injury in a rat model.
The study results suggest that the glutamine protected against lipid peroxidation and preserved superoxide dismutase (SOD) activity. The activity of NF-κB and IL-6 were also reduced upon ip administration of glutamine at 24 and 48 h prior to I/R in rats. This adds to previously published data on glutamine as a protective factor in mesenteric I/R states in rats.
Ischemia/reperfusion (I/R): gut ischemia usually results from occlusion of the celiac trunk and/or the superior mesenteric artery by thrombi or emboli and, more frequently, from non-occlusive processes, such as in the case of decreased mesenteric blood flow that occurs in heart failure and sepsis. In the gut, ischemia followed by reperfusion frequently results in multiple organ failure (MOF), with the gut being the organ that triggers the injury process in distant organs; SOD: is an antioxidant enzyme highly specific for superoxide elimination, thus reducing gastrointestinal lesions caused by I/R; NF-κB: nuclear factor of kappa light polypeptide gene enhancer in B-cells is a transcription factor that plays a crucial role not only in normal states but also in the coordination of adaptive immune responses by regulating the expression of many cell mediators; IL-6: levels are elevated in I/R as well as in MOF; Glutamine: is a polar amino acid that is non-essential or occasionally essential, hydrophilic, and found on the surface of proteins where it interacts with water.
This is an interesting article studying how the pretreatment with glutamine prevents mucosal injury and improves gut and lung recovery after I/R injury in a rat model. The manuscript includes six clear figures. This research is easy to follow and finds some valuable information for scientific community interested in both glutamine and ischemia/reperfusion, as well as in oxidative damage and ROS. To date, this is the first investigation to study glutamine effect on NF-κB and IL-6, as well as in SOD and TBARS in a model of mesenteric ischemia/reperfusion.
P- Reviewer: Matés JM S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Cerqueira NF, Hussni CA, Yoshida WB. Pathophysiology of mesenteric ischemia/reperfusion: a review. Acta Cir Bras. 2005;20:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Ding Y, Zhang Y, Peng T, Lu Y, Jin D, Ren Q, Liu Y, Han J, Xi P. Observation of mesenteric microcirculatory disturbance in rat by laser oblique scanning optical microscopy. Sci Rep. 2013;3:1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Moore EE, Moore FA, Franciose RJ, Kim FJ, Biffl WL, Banerjee A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 223] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Sözen S, Kisakürek M, Yildiz F, Gönültaş M, Dinçel AS. The effects of glutamine on hepatic ischemia reperfusion injury in rats. Hippokratia. 2011;15:161-166. [PubMed] |
| 5. | Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749-G753. [PubMed] |
| 6. | Matés JM, Segura JA, Alonso FJ, Márquez J. Natural antioxidants: therapeutic prospects for cancer and neurological diseases. Mini Rev Med Chem. 2009;9:1202-1214. [PubMed] |
| 7. | Gutiérrez MB, Miguel BS, Villares C, Gallego JG, Tuñón MJ. Oxidative stress induced by Cremophor EL is not accompanied by changes in NF-kappaB activation or iNOS expression. Toxicology. 2006;222:125-131. [PubMed] |
| 8. | Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921-928. [PubMed] |
| 10. | Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Cuzzocrea S, De Sarro G, Costantino G, Ciliberto G, Mazzon E, De Sarro A, Caputi AP. IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J Leukoc Biol. 1999;66:471-480. [PubMed] |
| 12. | Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | He GZ, Dong LG, Chen XF, Zhou KG, Shu H. Lymph duct ligation during ischemia/reperfusion prevents pulmonary dysfunction in a rat model with ω-3 polyunsaturated fatty acid and glutamine. Nutrition. 2011;27:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Márquez J, Tosina M, de la Rosa V, Segura JA, Alonso FJ, Matés JM, Campos-Sandoval JA. New insights into brain glutaminases: beyond their role on glutamatergic transmission. Neurochem Int. 2009;55:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, Mendenhall WM, Bova FJ, Khan SR, Hackett RL. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62-68. [PubMed] |
| 17. | Gianotti L, Alexander JW, Gennari R, Pyles T, Babcock GF. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. JPEN J Parenter Enteral Nutr. 1995;19:69-74. [PubMed] |
| 18. | Esposito E, Mondello S, Di Paola R, Mazzon E, Italiano D, Paterniti I, Mondello P, Aloisi C, Cuzzocrea S. Glutamine contributes to ameliorate inflammation after renal ischemia/reperfusion injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Demirkan A, Savaş B, Melli M. Endotoxin level in ischemia-reperfusion injury in rats: effect of glutamine pretreatment on endotoxin levels and gut morphology. Nutrition. 2010;26:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371-2384. [PubMed] |
| 21. | Goldim JR, Raymundo MM. Research in health and animal rights. Porto Alegre: Hospital da Clínicas de Porto Alegre 1995; . |
| 22. | Marques C, Mauriz JL, Simonetto D, Marroni CA, Tuñon MJ, González-Gallego J, Marrón NP. Glutamine prevents gastric oxidative stress in an animal model of portal hypertension gastropathy. Ann Hepatol. 2011;10:531-539. [PubMed] |
| 23. | Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114-121. [PubMed] |
| 24. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 25. | Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283-2291. [PubMed] |
| 26. | Yamamoto S, Watanabe B, Hiratake J, Tanaka R, Ohkita M, Matsumura Y. Preventive effect of GGsTop, a novel and selective γ-glutamyl transpeptidase inhibitor, on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2011;339:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Mondello S, Galuppo M, Mazzon E, Domenico I, Mondello P, Carmela A, Cuzzocrea S. Glutamine treatment attenuates the development of ischaemia/reperfusion injury of the gut. Eur J Pharmacol. 2010;643:304-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Fukatsu K, Ueno C, Hashiguchi Y, Hara E, Kinoshita M, Mochizuki H, Hiraide H. Glutamine infusion during ischemia is detrimental in a murine gut ischemia/reperfusion model. JPEN J Parenter Enteral Nutr. 2003;27:187-92; discussion 192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Salman AE, Yetişir F, Kılıç M, Onal O, Dostbil A, Zeybek D, Aksoy M, Kaymak F, Celik T, Unver S. The impact of pretreatment with bolus dose of enteral glutamine on acute lung injury induced by oleic acid in rats. J Anesth. 2014;28:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 689] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Ypsilantis P, Tentes I, Lambropoulou M, Anagnostopoulos K, Papadopoulos N, Kortsaris A, Simopoulos C. Prophylaxis with mesna prevents oxidative stress induced by ischemia reperfusion in the intestine via inhibition of nuclear factor-kappaB activation. J Gastroenterol Hepatol. 2008;23:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |