Published online Aug 7, 2014. doi: 10.3748/wjg.v20.i29.10202
Revised: March 18, 2014
Accepted: April 21, 2014
Published online: August 7, 2014
Processing time: 206 Days and 13.1 Hours
Primary gastric plasmacytoma (GP) is a rare extramedullary plasmacytoma with clinical and imaging features that are common among other gastric tumors, such as gastric adenocarcinomas, gastric stromal tumors, and lymphomas. Here, we present a histologically confirmed case of primary GP examined with biphasic computed tomography (CT), magnetic resonance imaging (MRI), and endosonography. A well-circumscribed extraluminal mass appearing as homogeneous attenuation/intensity with gradual enhancement was identified on biphasic enhancement CT and MRI. This mass was hyperintense on diffusion-weighted imaging and hypointense on the apparent diffusion coefficient map, implying that water diffusion in the mass was restricted. In addition, endosonography indicated a low echogenic mass in the gastric wall. These imaging findings increase the available knowledge about imaging of this disease and provide valuable information for differentiating primary GP from common gastric tumors.
Core tip: Current knowledge regarding the imaging of primary gastric plasmacytoma is limited. Here, we report findings from biphasic enhancement computed tomography, magnetic resonance imaging, and endosonography for this disease, thereby increasing imaging knowledge about primary gastric plasmacytoma and providing useful information for differentiating these tumors from common gastric tumors.
- Citation: Zhao ZH, Yang JF, Wang JD, Wei JG, Liu F, Wang BY. Imaging findings of primary gastric plasmacytoma: A case report. World J Gastroenterol 2014; 20(29): 10202-10207
- URL: https://www.wjgnet.com/1007-9327/full/v20/i29/10202.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i29.10202
Primary gastric plasmacytoma (GP) constitutes approximately 2% of all cases of extramedullary plasmacytoma[1]. However, knowledge regarding the imaging of this disease is limited and there is only one report on imaging, which describes computed tomography (CT) findings showing homogeneous concentric gastric wall thickening but with poor contrast enhancement[2]. To the best of our knowledge, findings from biphasic enhancement CT, magnetic resonance imaging (MRI), and endosonography have not been described for this disease. Here, we report a new case of primary GP and present the imaging findings.
A 79-year-old male was admitted to our hospital because of epigastric pain and a choking feeling after eating, which had lasted approximately one month. The patient reported no significant medical history or medication use. The physical examination showed only mild epigastric tenderness on deep palpation without rebound, and the laboratory results were within normal limits.
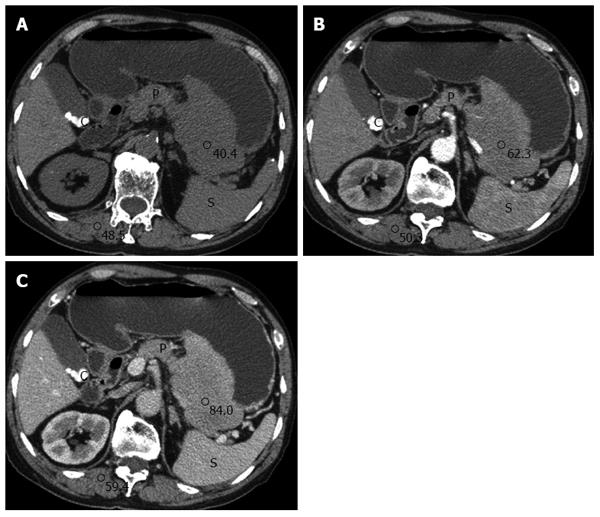
An abdominal CT (Philips 64, Philips Medical Systems, Cleveland, OH, United States) demonstrated a well-circumscribed extraluminal mass measuring 8.4 cm × 13.2 cm × 15.7 cm on the posterior side of the gastric midbody. The mass was a homogeneous iso-attenuation lesion similar to the paraspinal muscle and without any area of calcification or necrosis on a plain scan (Figure 1A). Following the administration of contrast agent (iopromide, 300 mg iodine/mL; Ultravist 300, Schering, Berlin, Germany), the lesion was revealed as a mild enhancement on the arterial phase with moderate enhancement on the portal phase. The CT values of the lesion on the plain scan, arterial, and portal phase were 40.4 hounsfield units (HU), 62.3 HU, and 84.0 HU, respectively (Figure 1B and C). The adjacent pancreas was compressed and displaced, and there was evidence of cholelithiasis in the gallbladder.
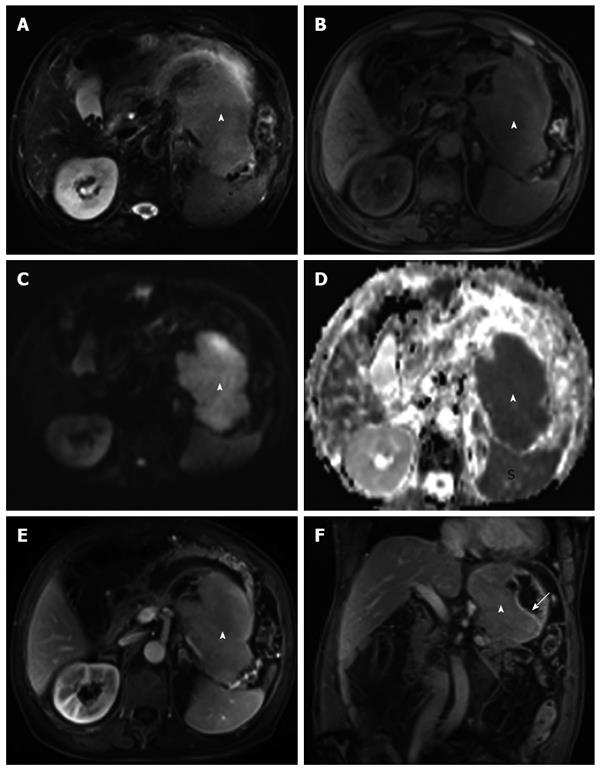
Regarding the MRI (3T Siemens Magnetom Verio, Erlangen, Germany) findings, the lesion was homogeneous and iso-hyperintense on T2-weighted images (T2WI) and iso-hypointense on T1-weighted images (T1WI) (Figure 2A and B). In particular, the mass was hyperintense on diffusion-weighted imaging (DWI) (b value = 800), in contrast to its hypointense presentation on the apparent diffusion coefficient (ADC) map (ADC value = 0.863 × 10-3 mm2/s) (Figure 2C and D). This finding implied that water diffusion in the mass was restricted. After intravenous administration of gadolinium contrast agent, (gadopentetate dimeglumine, Bayer Schering Pharma AG, Berlin, Germany), a submucosal mass with gradual and homogeneous enhancement was revealed (Figure 2E amd F). The lesion infiltrated the adjacent pancreas and spleen, although lymphadenopathy was not present in the abdomen.
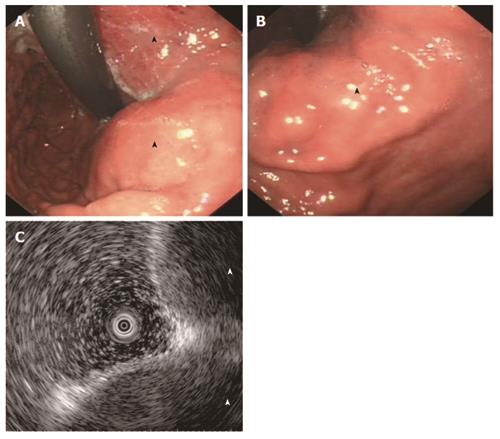
An upper endoscopy revealed irregular bulging from the fundus to the posterior side of the midbody, as well as several superficial mucosal erosions overlying the mass (Figure 3A and B), whereas endosonography indicated effacement of normal layers and a low echogenic mass in the gastric wall (Figure 3C).
Analysis of the biopsy specimen obtained from endoscopy indicated primary GP, and Helicobacter pylori (H. pylori) was not detected. The skeletal survey and bone marrow examination showed no abnormalities.
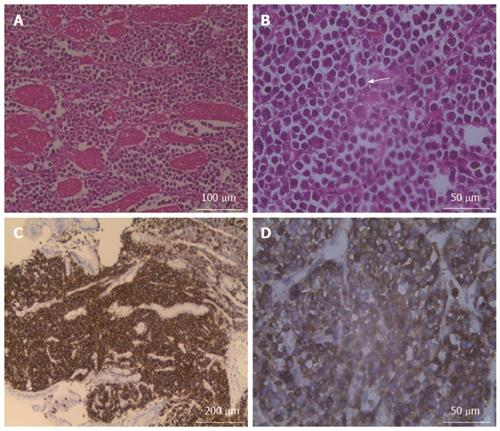
The patient received surgery that consisted of total gastrectomy with a subtotal pancreatectomy, splenectomy, and oesophageal and jejunum Roux-en-Y anastomosis. One 10.1 cm × 15.0 cm × 16.0 cm mass was identified in the gastric fundus and midbody. The mass had infiltrated the lamina propria, pancreas, and spleen and was classified at an advanced stage. Haematoxylin-eosin (HE) staining showed diffuse plasma cell infiltration into the muscular layers of the stomach; some of these cells were binuclear with large nuclei and very prominent centrally located nucleoli, and a few mitotic cells were also observed, which indicated histological grade 3 (Figure 4A and B). Immunohistochemistry examination revealed that the tumor cells were positive for CD38 and CD138 and negative for CD3, CD20, CD45, and k-chain expression (Figure 4C and D). Based on these pathological features, the tumor was diagnosed as a primary GP.
Abdominal and pelvic CT analysis performed eight months after the operation indicated no recurrence or metastasis in any abdominal organs, and the laboratory results were within normal limits.
Primary GP is a rare disease, and the final diagnosis of GP requires the demonstration of a single lesion showing monoclonal plasma cell infiltration in the stomach, a negative skeletal survey, and no evidence of tumor cells in the bone marrow[3]. Stasi et al[4] proposed that H. pylori infection may play an important role in the pathogenesis of GP, although this organism was not detected in several published cases[5,6]. Pathologically, primary GP has been hypothesized to originate from lymphoid follicles in the submucosa or from plasma cells in the lamina propria; accordingly, primary GP is classified into the following four types: nodular, infiltrative, ulcerative, and polypoid[2]. According to whether tumor cells infiltrate the lamina propria, GP is classified as early or advanced-stage disease. Most primary GP patients are elderly and show nonspecific symptoms, including anorexia, weight loss, epigastric discomfort, and gastrointestinal bleeding[3]. Several imaging methods are used to evaluate GP, including upper gastrointestinal series, CT, MRI, positron emission tomography (PET), and endoscopy or endosonography.
Abdominal CT plays an important role in diagnosing and staging gastric tumors. Yoon et al[2] reported two cases of primary GP that showed homogeneous concentric gastric wall thickening with poor contrast enhancement similar to the degree of enhancement of the paraspinal muscle. However, our case differed from previous cases in several respects. First, our case demonstrated a well-circumscribed extraluminal mass that may have been related to its origin within lymphoid follicles of the submucosa or plasma cells in the lamina propria. Second, a gradual enhancement pattern was observed. Biphasic enhancement CT may provide more information on the enhancement characteristics of primary GP, and we hypothesize that the observed gradual enhancement is consistent with the dense cellularity of plasma cells in the mass. MRI is a more sensitive technique for detecting the inner components of gastric tumors, and DWI is helpful in evaluating the cellularity of the lesions by measuring water molecule diffusion. In our case, the lesion showed homogeneous iso-hyperintensity on T2WI and iso-hypointensity on T1WI imaging. After administration of the contrast agent, the MRI clearly indicated a submucosal mass with homogeneous enhancement. In particular, the mass showed evident hyperintensity on DWI images and hypointensity on the ADC map, with a 0.863 × 10-3 mm2/s ADC value. The ADC value is estimated to be lower in viable tumor tissues with densely packed diffusion-hindering obstacles than in tissues with less densely packed obstacles, such as necrotic and benign tissues[7]. The ADC value in our case further confirmed the dense cellularity of the plasma cells in the mass and was consistent with our pathological findings.
Because primary GP originates from the submucosa or the lamina propria of the mucosa[2], endoscopy occasionally shows only bulging in the gastric wall; however, the biopsy specimen obtained by endoscopy may suggest GP.
Tan et al[5] reported two cases of gastric plasmacytoma following definitive radiotherapy and suggested that PET and CT imaging provided little or no value for estimating the extent of disease in the stomach but was valuable for excluding dissemination beyond the stomach.
Primary GP tumors differentiate from common gastric tumors, including gastric adenocarcinomas, gastric stromal tumors, and lymphomas. In particular, adenocarcinomas arising from the gastric epithelium are usually confirmed by endoscopy. On CT, the common appearance of nonmucinous and mucinous gastric carcinomas include focal wall thickening with ulceration, a diffusely infiltrative wall with evident homogeneous enhancement, and a layered enhancement pattern[8]. These CT features are different from those observed in our case of primary GP, which manifested as a well-circumscribed extraluminal mass with mild to moderate homogeneous enhancement. Large gastric stromal tumors greater than 3 cm in diameter typically appear with lobulated margins, mucosal ulceration, central necrosis, cavitation, and heterogeneous enhancement[9]. Whether in our case or in other reported cases, these tumors consistently demonstrate homogeneous attenuation without necrosis and mild or moderate enhancement. Gastric lymphoma is considered a “soft” tumor that typically appears as segmental or diffuse wall thickening or nonenhancing extramucosal masses with homogeneous low attenuation[10,11]. The present case of primary GP appeared as an extraluminal mass with gradual enhancement. Avcu et al[12] evaluated gastric tumors according to the ADC measurement and found that the ADC value in lymphoma cases was (1.09 ± 0.08) × 10-3 mm2/s; as a result, these authors hypothesized that ADC measurements may help in the diagnosis of gastric lymphoma. In our case, the ADC value of the primary GP was 0.863 × 10-3 mm2/s, which indicates that this measurement may be helpful for differentiating primary GP from gastric lymphoma. However, additional cases are needed to analyse the ability of CT and MRI findings to differentiate primary GP from lymphoma.
In addition to homogeneous concentric gastric wall thickening with poor contrast enhancement, primary GP may appear as a well-circumscribed extraluminal mass with homogeneous attenuation/intensity and a gradual enhancement pattern on both biphasic enhancement CT and MRI. The mass in the present case was hyperintense on DWI, hypointense on the ADC map, and demonstrated a low echo on endosonography. We presented the imaging findings of this new case of primary GP to enrich knowledge of the imaging of this disease among radiologists and clinicians, with the goal of providing useful information for differentiating primary GPs from common gastric tumors. However, further research is needed to explore the imaging characteristics of primary GP.
The authors thank Wei Wang for reviewing the histopathological images.
A 79-year-old male presented with epigastric pain and a choking feeling after eating, which had been ongoing for approximately one month.
The physical examination showed only mild epigastric tenderness on deep palpation without rebound.
This included gastric adenocarcinoma, gastric stromal tumor, and lymphoma.
The laboratory data were within normal limits.
Both computed tomography and magnetic resonance imaging (MRI) demonstrated a well-circumscribed extraluminal mass with a gradual enhancement pattern. In particular, the mass showed evident hyperintensity on diffusion-weighted imaging (DWI) and hypointensity on the apparent diffusion coefficient map, which implied that water diffusion in the mass was restricted.
Histopathological and immunohistochemical examinations revealed that plasma cells had diffusely infiltrated the muscular layers of the stomach and that the tumor cells were positive for CD38 and CD138 and negative for CD3, CD20, CD45, and k-chain expression.
The patient underwent total gastrectomy associated with subtotal pancreatectomy as well as splenectomy and oesophageal and jejunum Roux-en-Y anastomosis.
The imaging features of primary gastric plasmacytoma are not very well understood. Here, we present a new case in detail and with imaging findings from different modalities to increase the knowledge of this disease.
DWI is one of the functional imaging methods of MRI and is used to evaluate cellularity in lesions by observing water molecule diffusion.
This case report not only presents additional imaging findings for primary gastric plasmacytoma compared to previous cases but also encourages these new findings to aid in diagnosis and differentiatial diagnosis.
This case report describes the imaging findings for a rare case of primary gastric plasmacytoma, as few previous reports have described imaging findings for this disease. It is a good report.
P- Reviewer: Ciledag N, Gao BL, Lee WK S- Editor: Gou SX L- Editor: Ma JY E- Editor: Ma S
| 1. | Alexiou C, Kau RJ, Dietzfelbinger H, Kremer M, Spiess JC, Schratzenstaller B, Arnold W. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305-2314. [PubMed] |
| 2. | Yoon SE, Ha HK, Lee YS, Kim PN, Lee MG, Yu E, Auh YH. Upper gastrointestinal series and CT findings of primary gastric plasmacytoma: report of two cases. AJR Am J Roentgenol. 1999;173:1266-1268. [PubMed] |
| 3. | Krishnamoorthy N, Bal MM, Ramadwar M, Deodhar K, Mohandas KM. A rare case of primary gastric plasmacytoma: an unforeseen surprise. J Cancer Res Ther. 2010;6:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Stasi R, Evangelista ML, Brunetti M, Bussa S, Maritati R, Gallo A, Turrini L, Taccogna S, Crescenzi A, Angelini F. Primary gastric plasmacytoma and Helicobacter pylori infection. J Clin Oncol. 2009;27:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Tan J, Lade S, Harrison S, Opat S, Mac Manus MP. Complete remission of localised gastric plasmacytomas following definitive radiotherapy. J Med Imaging Radiat Oncol. 2012;56:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Kanzawa M, Hirai C, Morinaga Y, Kawakami F, Hara S, Matsuoka H, Itoh T. Primary submucosal nodular plasmacytoma of the stomach: a poorly recognized variant of gastric lymphoma. Diagn Pathol. 2013;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45:208-213. [PubMed] |
| 8. | Park MS, Yu JS, Kim MJ, Yoon SW, Kim SH, Noh TW, Lee KH, Lee JT, Yoo HS, Kim KW. Mucinous versus nonmucinous gastric carcinoma: differentiation with helical CT. Radiology. 2002;223:540-546. [PubMed] |
| 9. | Lee NK, Kim S, Kim GH, Jeon TY, Kim DH, Jang HJ, Park do Y. Hypervascular subepithelial gastrointestinal masses: CT-pathologic correlation. Radiographics. 2010;30:1915-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75-87. [PubMed] |
| 11. | Park SH, Han JK, Kim TK, Lee JW, Kim SH, Kim YI, Choi BI, Yeon KM, Han MC. Unusual gastric tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1435-1446. [PubMed] |