Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8572
Revised: January 14, 2014
Accepted: April 2, 2014
Published online: July 14, 2014
Processing time: 242 Days and 18.4 Hours
AIM: To investigate the effect of zinc protoporphyrin IX on the response of hepatoma cells to cisplatin and the possible mechanism involved.
METHODS: Cytotoxicity was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Apoptosis was determined by a flow cytometric assay. Western blotting was used to measure protein expression. Heme oxygenase (HO)-1 activity was measured by determining the level of bilirubin generated in isolated microsomes. Reactive oxygen species (ROS) production was monitored by flow cytometry. Caspase-3 activity was measured with a colorimetric assay kit. Mice were inoculated with 1 × 107 tumor cells subcutaneously into the right flanks. All mice were sacrificed 6 wk after the first treatment and tumors were weighed and measured.
RESULTS: Overexpression of HO-1 in HepG2 cell line was associated with increased chemoresistance to cis-diaminedichloroplatinum (cisplatin; CDDP) compared to other cell lines in vitro. Inhibition of HO-1 expression or activity by zinc protoporphyrin IX (ZnPP IX) markedly augmented CDDP-mediated cytotoxicity towards all liver cancer cell lines in vitro and in vivo. In contrast, induction of HO-1 with hemin increased resistance of tumor cells to CDDP-mediated cytotoxicity in vitro and in vivo. Furthermore, cells treated with ZnPP IX plus CDDP exhibited marked production of intracellular ROS and caspase-3 activity, which paralleled the incidence of cell apoptosis, whereas hemin decreased cellular ROS and caspase-3 activity induced by CDDP.
CONCLUSION: ZnPP IX increases cellular sensitivity and susceptibility of liver cancer cell lines to CDDP and this may represent a mechanism of increasing ROS.
Core tip: Overexpression of heme oxygenase (HO)-1 in HepG2 cell line was associated with increased chemoresistance to cis-diaminedichloroplatinum (cisplatin; CDDP) compared to other cell lines. Inhibition of HO-1 expression by zinc protoporphyrin IX (ZnPP IX) markedly augmented CDDP-mediated cytotoxicity towards other hepatoma cells. Induction of HO-1 with hemin increased resistance of tumor cells to CDDP-mediated cytotoxicity. Furthermore, cells treated with ZnPP IX plus CDDP exhibited marked production of intracellular reactive oxygen species (ROS) and caspase-3 activity, whereas hemin decreased cellular ROS and caspase-3 activity induced by CDDP. Therefore, administration of HO-1 inhibitors may evolve into a new liver cancer treatment strategy.
- Citation: Liu YS, Li HS, Qi DF, Zhang J, Jiang XC, Shi K, Zhang XJ, Zhang XH. Zinc protoporphyrin IX enhances chemotherapeutic response of hepatoma cells to cisplatin. World J Gastroenterol 2014; 20(26): 8572-8582
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8572
Hepatocellular carcinoma (HCC) is the fourth most common cancer and the third leading cause of cancer-related death worldwide[1,2]. Surgical resection is the best treatment to reduce the growth of HCC and improve life expectancy of HCC patients. However, more than 80% of HCC patients are diagnosed with an advanced-stage or unresectable disease. In patients who undergo resection, the prognosis is poor with a recurrence rate as high as 50% at two years[3,4]. Current chemotherapy is also not effective and the development of chemoresistance is the major limitation. Therefore, a new understanding of the molecular mechanisms of drug resistance is urgently needed for treatment of this deadly disease.
Heme oxygenase (HO) is a microsomal initial and rate-limiting enzyme that catalyzes the degradation of heme to produce equimolar quantities of biliverdin, CO and free iron, which play crucial roles in the defense against oxidative and cellular stress[5]. To date, three distinct mammalian HO isoforms have been identified: HO-1, HO-2 and HO-3. HO-2 and HO-3 are constitutively expressed, whereas HO-1 is known to be highly induced by a vast array of stress-inducing stimuli such as H2O2, UV irradiation, hypoxia, and extracellular acidosis[6-9]. Induction of the HO-1 protein represents a cytoprotective defense mechanism in the adaptive response to cellular stress[10]. Moreover, new observations indicate that HO-1 and its products also exert anti-inflammatory effects and influence the growth and proliferation of tumor cells. Elevated expression and increased activity of HO-1 have been found in various malignant tumors such as human renal cell carcinoma, pancreatic cancer, hepatoma, prostate cancer, and Kaposi sarcoma[11-15]. Furthermore, overexpression of HO-1 in tumor cells can be further elevated by chemotherapy, radiotherapy or photodynamic therapy[16]. Its overexpression in human cancers may give cancer cells a growth advantage and enhance resistance to chemotherapy and other stressors[16-18]. Inhibition of HO-1 expression or activity suppresses cellular proliferation and increases responsiveness of tumor cells to some anticancer treatments in vitro and in vivo[18-20]. In contrast, induction of HO-1 expression or activity decreases cell sensitivity to antitumor drugs. Accumulating evidence suggests that HO-1 can be a therapeutic target for antitumor treatment.
In this study, we investigated whether constitutively overexpressed HO-1 in liver cancer cells was associated with resistance to apoptosis induction by cis-diaminedichloroplatinum (cisplatin; CDDP), and then explored the role of HO-1 in protecting tumor cells against chemotherapeutic agents in vitro and in vivo.
CDDP, zinc protoporphyrin IX (ZnPP IX), hemin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 2,7-dichlorodihydrofluo-recin diacetate (DCFH-DA) were from Sigma (St. Louis, MO, United States). Dulbecco’s Modified Eagle’s Medium, fetal bovine serum (FBS), and other cell culture reagents were obtained from Gibco BRL Life Technologies (Grand Island, NY, United States). Rabbit anti-human HO-1 monoclonal antibodies were from Cell Signaling Technology (Danvers, MA, United States). Goat anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) polyclonal antibody and horseradish peroxidase-conjugated goat anti-mouse antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, United States).
The human liver cancer cell lines HepG2, SMMC7721, and 97H were purchased from the Cell Bank, Chinese Academy of Sciences (Shanghai, China). The three cell lines were cultured in RPMI 1640 containing 100 U/mL penicillin, 0.1 mg/mL streptomycin and 10% heat-inactivated FBS at 37 °C in an atmosphere of 5% CO2 and 95% air. To induce or inhibit the activity of HO-1, hemin or ZnPP IX was added 1 h prior to the addition of CDDP.
In vitro, the cytostatic and/or cytotoxic effects of treatments were determined using the MTT assay. Tumor cells were seeded in a 96-well plate at a concentration of 6 × 103 cells per well and cultured overnight for cell attachment. The following day, cells were treated with the investigational agents for 48 h. After an appropriate time, 20 μL MTT [5 mg/mL in phosphate-buffered saline (PBS)] was added to each well for 4 h. The supernatant was discarded and 150 μL DMSO was added to each well. The plates were shaken until the crystals had dissolved. Absorbance was measured at a wavelength of 570 nm using an enzyme-linked immunosorbent assay reader with background subtraction at 690 nm. Cell viability was expressed as percentage of untreated controls. Experiments were completely randomized in design and repeated six times.
Induction of apoptosis in vitro was determined by a flow cytometric assay with an annexin V and propidium iodide apoptosis kit according to the manufacturer’s instructions (Invitrogen, United States). Cells were plated in six-well plates at 1 × 105 cells/well and treated for 48 h. After treatment, the cells were harvested from the plate using trypsin and washed twice with PBS, and then incubated with annexin V-fluorescein isothiocyanate and propidium iodide for 15 min. The number of apoptotic cells was analyzed by flow cytometry using a FACScan Analyzer. Experiments were completely randomized in design and repeated six times.
Western blotting was used to measure protein expression as follows. Cells were harvested after treatment, and washed twice with PBS. The suspension was resuspended in a buffer containing 1% Triton X-100 with PBS and Halt Protease Inhibitor Cocktail for 30 min on ice and then centrifuged at 14000 ×g for 20 min. Protein concentration was measured with the bicinchoninic acid protein assay reagent according to the manufacturer’s instructions (Thermo Scientific, United States). Equivalent amounts of total proteins (80 mg) from each sample were separated by 10% gradient SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. After blocking with 10% milk, the membranes were incubated with the primary antibody for 3 h at room temperature. The dilutions of the primary antibodies were as follows: 1:1000 for anti-hHO-1 antibody and 1:2000 for anti-GAPDH antibody. The membranes were washed four times with 0.1% Tween 20 in Tris-buffered saline and then incubated with a secondary antibody for 1 h. The membranes were washed extensively again and the protein bands were visualized with the ECL-Plus chemiluminescence system according to the manufacturer’s instructions (Applygen Technologies, Beijing, China). The relative optical density of each Western blotting band was measured using the Quantity One Quantification Software according to the manufacturer’s guidelines (Bio-Rad Laboratories).
HO-1 activity was measured by determining the level of bilirubin generated in isolated microsomes. After treatment, cells were collected and homogenized in a homogenization buffer [20 mmol/L potassium phosphate buffer (pH 7.4), 250 mmol/L sucrose, 2 mmol/L EDTA, 2 mmol/L phenylmethyl sulfonyl fluoride (PMSF) and 10 μg/mL leupeptin]. Homogenates were centrifuged at 10000 ×g for 30 min at 4 °C. The resulting supernatants were centrifuged at 100000 ×g for 1 h at 4 °C. The pellet was suspended in phosphate buffer (pH 7.0) and designated the microsome fraction. An aliquot of the microsomal fraction was then added to a reaction mixture containing cytosol of the cells (2 mg cytosolic protein), hemin (20 μmol/L), glucose-6-phosphate (2 mmol/L), glucose-6-phosphate-dehydrogenase (0.2 units), and NADPH (0.8 mmol/L). The reaction mixture was incubated for 60 min at 37 °C in the dark and terminated by the addition of 1 mL chloroform. The bilirubin concentration was calculated by measuring the difference in absorbance between 465 and 530 nm using a Shimadzu UV-160A spectrophotometer with a molar extinction coefficient of 40/mmol/L/cm. Experiments were completely randomized in design and repeated six times.
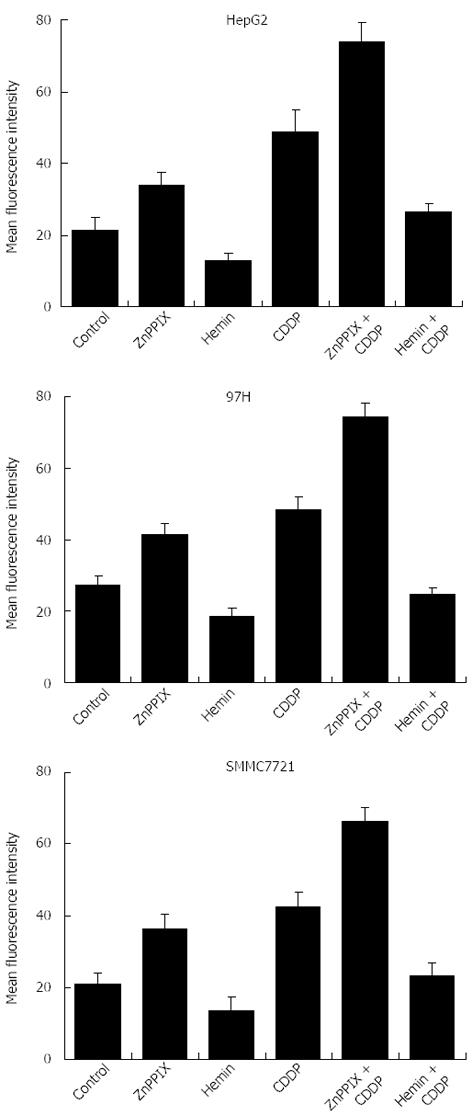
ROS production in each sample was monitored by flow cytometry using the DCFH-DA fluorescent probe. DCFH-DA is a stable compound that rapidly diffuses into cells and is activated by intracellular esterases to DCFH, which is converted by H2O2 and peroxidases to the DFC fluorescent derivate. Thus, the fluorescence intensity is proportional to the amount of peroxide produced by cells. After treatment, the cells were incubated with 10 μmol/L DCFH-DA for 30 min at 37 °C in the dark. Cells were then washed twice with PBS and resuspended again. The intracellular ROS was quantitated as a function of fluorescence intensity measured by flow cytometry.
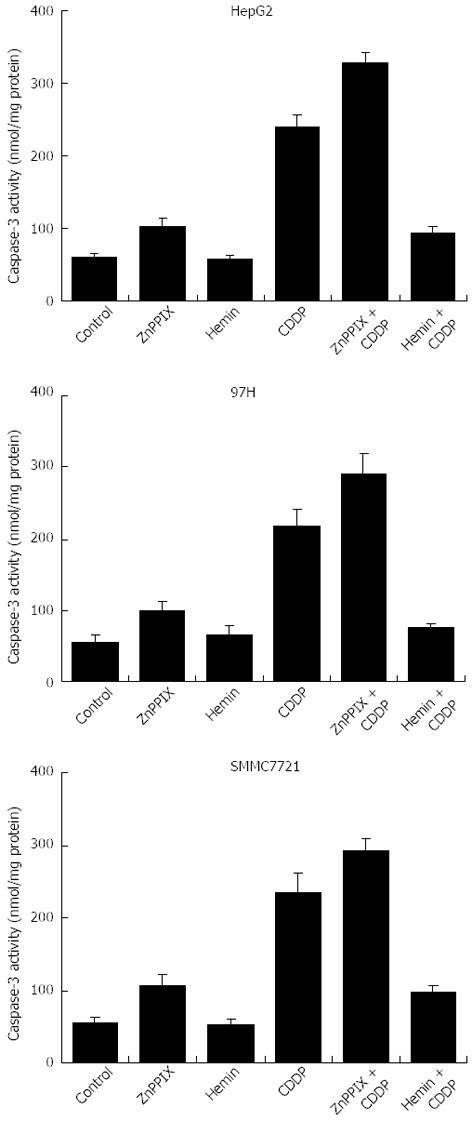
Caspase-3 activity was measured with a Colorimetric Assay Kit according to the manufacturer’s instructions (Roche). After treatment cells were collected and lysed with chilled lysis buffer [50 mmol/L HEPES (pH 7.5), 150 mmol/L NaCl, 20 mmol/L EDTA, 0.2% Triton X-100, 1 mmol/L PMSF, 10 μg/mL aprotinin, and 5 mmol/L dithiothreitol] for 10 min on ice. The supernatant containing 100 μg protein was incubated with 0.2 mmol/L AcDEVD-pNA, a specific substrate for caspase-3. Caspase-3 activity was measured at 405 nm with background subtraction at 570 nm and expressed in Ac-pNA cleavage or released absorbance.
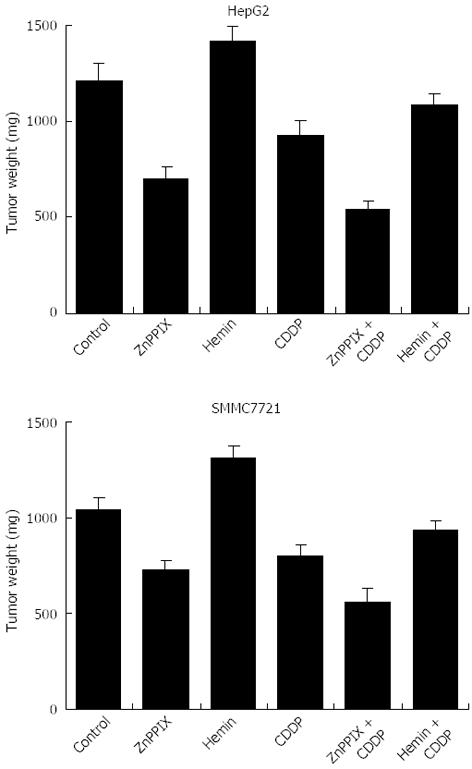
Female athymic nude mice (6 wk old) were purchased from the Central Animal Laboratory, Nanjing University, Nanjing, China. Mice were maintained according to Nanjing University of Traditional Chinese Medicine institutional policies. Mice were inoculated subcutaneously into the right flanks with 1 × 107 tumor cells, resuspended in 0.2 mL PBS. When tumors reached 100-300 mm3 in volume, mice were randomly divided into 6 groups of 8 mice each: control (drug vehicle), hemin (10 mg/kg), ZnPP IX (5 mg/kg), CDDP (5 mg/kg), combination of hemin (10 mg/kg) plus CDDP (5 mg/kg), and combination of ZnPP IX (5 mg/kg) plus CDDP (5 mg/kg). All the drugs were injected weekly into their peritoneal cavities four times. The control group was injected with PBS. All mice were sacrificed 6 wk after the first treatment. Tumors were weighed as previously described[21].
All data are expressed as mean ± standard deviation (SE). Statistical analysis was performed using one-way analysis of variance and independent-sample t test for each paired experiment with Windows version 15.0. In all assays, P < 0.05 was considered statistically significant.
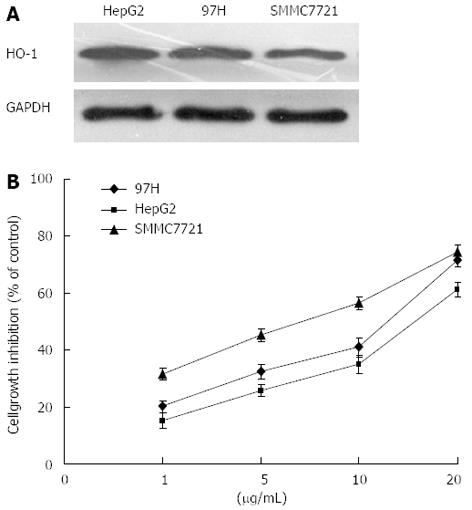
Liver cancer cell lines showed different expression levels of HO-1 (Figure 1A). Native HO-1 expression was higher in HepG2 than in other cells, without either hemin or antitumor drug treatment. Furthermore, we compared CDDP-induced growth inhibition in three kinds of human liver cancer cells. HepG2 cells with high native HO-1 expression showed significantly greater in vitro chemoresistance to CDDP than the other cell lines with low native HO-1 expression (Figure 1B).
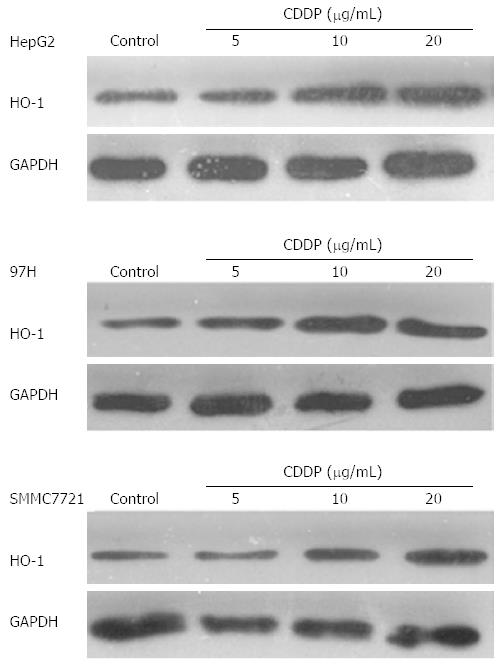
The level of HO-1 was significantly increased in all liver cancer cell lines when cells were treated with CDDP (Figure 2). The highest level of HO-1 expression in all cell lines was achieved with CDDP at concentrations of 5-10 μg/mL. The expression of HO-1 achieved a plateau when CDDP was used at a concentration of 20 μg/mL in all liver cancer cells.
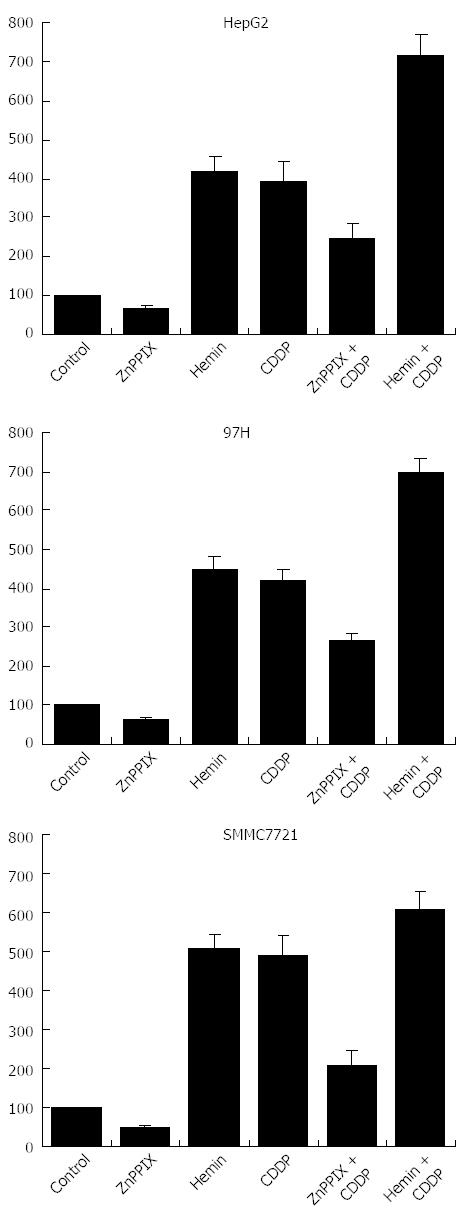
As shown in Figure 3, HO-1 activity was significantly elevated in all liver cancer cell lines after treatment with CDDP compared with untreated cells in vitro (P < 0.05). ZnPP IX decreased HO-1 activity as well as CDDP-induced HO-1 activity (P < 0.05). In contrast, administration of CDDP in combination with hemin significantly increased HO-1 activity in all liver cancer cell lines compared to CDDP treatment alone (P < 0.05).
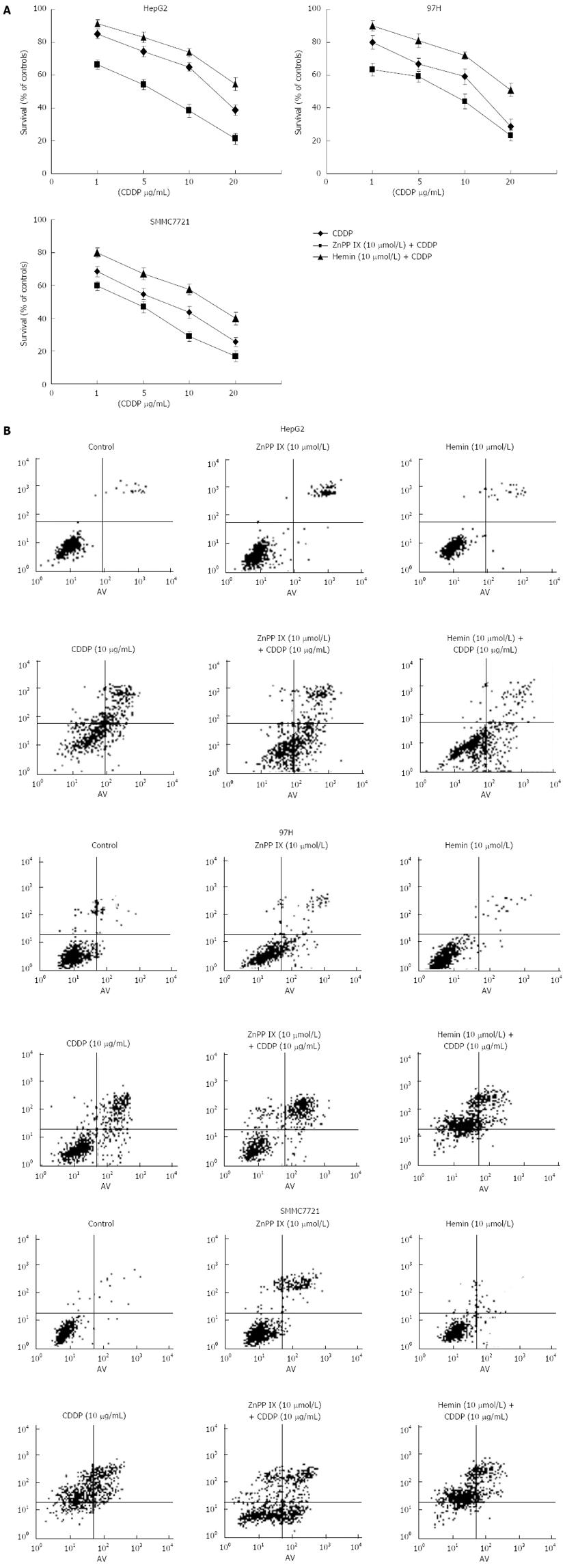
To demonstrate further the cytoprotective properties of HO-1, the HO-1 inductor hemin and ZnPP IX were added to tumor cells 1 h prior to the application of CDDP. The cytotoxic effect induced by CDDP was largely increased by addition of ZnPP IX, which suggests that inhibition of HO-1 activity by ZnPP IX boosted the anticancer effects of CDDP (Figure 4). In addition, hemin treatment significantly decreased apoptosis induced by CDDP. These findings suggest that augmentation of HO-1 activity induced by hemin is associated with reduced CDDP-induced apoptosis in all liver cancer cell lines. However, it seemed that apoptosis induced by CDDP plus ZnPP IX in the HepG2 cell line was higher than that in the other cell lines.
To investigate whether apoptosis induced by the addition of ZnPP IX was related to the increase in intracellular production of ROS in liver cancer cells, flow cytometry was performed using the oxidant-sensitive fluorescence probe DCDHF. As shown in Figure 5, ZnPP IX (10 μmol/L) increased the fluorescence intensity and CDDP (10 μg/mL)-induced ROS in liver cancer cell lines (P < 0.05). In contrast, hemin (10 μmol/L) decreased CDDP-induced ROS in all liver cancer cell lines (P < 0.05). These findings suggest that the ZnPP IX-induced increase in apoptosis after exposure to CDDP is related to increased intracellular ROS.
Caspase-3 activity of the cells paralleled cellular apoptotic vulnerability. Caspase-3 activity in cells was not significantly affected by hemin at the concentration used (10 μmol/L) compared with untreated cells (Figure 6). However, caspase-3 activity was significantly increased in all liver cancer cells after treatment with ZnPP IX (10 μmol/L) or CDDP (10 μg/mL) (P < 0.05). Furthermore, after treatment with CDDP, caspase-3 activity was re-elevated by the addition of ZnPP IX (P < 0.05). Caspase-3 activity induced by CDDP was markedly reduced in the presence of hemin (10 μmol/L) (P < 0.05).
Mice treated with ZnPP IX (5 mg/kg) or CDDP (5 mg/kg) showed significantly reduced tumor growth in comparison with untreated control mice (Figure 7). Inhibition of HO-1 activity by ZnPP IX (5 mg/kg) boosted the anticancer effects of CDDP (5 mg/kg), resulting in a significant reduction of tumor growth in CDDP-treated mice, compared to tumors treated with CDDP alone. In contrast, administration of hemin (10 mg/kg) in combination with CDDP tended to increase tumor weight compared to tumors treated with CDDP alone.
Accumulating evidence indicates that overexpression of HO-1 increases the proliferation of cancer cells or renders them resistant to apoptosis induced by chemicals, radiotherapy, photodynamic therapy, and other stressors[12,13,17]. Downregulation of HO-1 expression or activity can suppress cell proliferation or increase cellular sensitivity to some therapeutic regimens. For example, inhibition of HO-1 activity could suppress the proliferation of lung cancer, pancreatic cancer and leukemia cells, and sensitize cells to antitumor drugs in vitro[22-25]. Moreover, inhibition of HO-1 activity could also lead to increased pancreatic cancer cell susceptibility to chemotherapy in vivo[26]. However, little is known about the chemoresistant and poor prognostic function of HO-1 in liver tumors.
In this study, we found that constitutive overexpression of HO-1 was specific to HepG2 cells, and this overexpression of HO-1 was associated with higher resistance to CDDP in vitro (Figure 1). We also found that CDDP increased HO-1 activity in liver cancer cells, and inhibition of HO-1 activity by ZnPP IX boosted the anticancer effects of CDDP. These results suggest that the cellular sensitivity of liver cancer cells to CDDP is augmented by downregulation of HO-1 activity by ZnPP IX. In support of this conclusion, induction of HO-1 activity by the addition of hemin resulted in a decrease in CDDP-induced apoptosis in all liver cancer cells (Figure 1). These findings suggest that HO-1, as a stress response gene, plays an important role in the determination of the sensitivity of liver cancer cells to CDDP. Recent studies in other models support this view: induction of HO-1 expression made colon cancer cells and leukemia resistant to antitumor drugs such as merocyanine and pyrrolidine dithiocarbamate[27,28]. Furthermore, inhibition of HO-1 activity by polyethylene glycol-ZnPP IX augmented the cytotoxic effect of several chemotherapeutic agents in an experimental murine sarcoma model[17]. However, opposite effects were observed in breast cancer and B lymphoblasts, in which HO-1 did not protect the cells from chemotherapy-induced apoptosis[29]. This might be because HO-1 has different biological actions in different cancer cells.
The signaling pathways mediating these effects on cancer are poorly understood, although there is accumulating evidence showing that HO-1 is an antiapoptotic agent in several types of cells. Some previous studies have demonstrated that apoptosis of human oral and gastric cancer cells could be regulated by Nrf2 and p21[30,31]. Overexpression of HO-1 resulted in a significant increase in Nrf2 and p21 levels and decreased cellular sensitivity to chemotherapy. In contrast, inhibition of HO-1 expression or activity leads to the downregulation of Nrf2 and p21 and decreases cellular sensitivity to chemotherapy. However, we have also shown that the mechanism of resistance to apoptosis by upregulation of HO-1 may be related to increased ROS level and caspase-3 activity. The destructive mechanisms of chemotherapy on cancer cells are mainly based on the generation of oxidative stress and/or induction of apoptosis[32]. The accumulation of ROS results in the subsequent loss of mitochondrial membrane potential and activation of the caspase-9/3 pathway. The present study showed that the activity of caspase-3 and ROS level induced by CDDP were significantly inhibited by elevated hemin (Figures 5 and 6). In contrast, ROS level and caspase-3 activity were significantly re-elevated by the addition of ZnPP IX in liver cancer cells after treatment with CDDP. Therefore, our study suggests that the resistance to apoptosis by HO-1 may be through a pathway with elevated ROS level and caspase-3 activity in human liver cancer cells. In addition, the products of HO-1 enzymatic activity directly influence several other cell signaling pathways, such as the Ras-Raf-ERK pathway, which have crucial roles in human renal cancer cells[33]. However, the mechanisms involved in increased chemosensitivity need additional investigation.
A more important finding, consistent with in vitro results, is that inhibition of HO-1 activity by ZnPP IX boosts anti-cancer effects of CDDP, resulting in a significant reduction of tumor growth in mice, compared to those treated with CDDP alone (Figure 7). In contrast, administration of hemin in combination with CDDP tends to increase tumor weight. These findings suggest that inhibition of HO-1 expression leads to increased sensitivity of liver cancer cells to CDDP in vivo.
In conclusion, the findings in this study suggest that high HO-1 levels in HepG2 cells are, at least partially, responsible for resistance to chemotherapy and environmental stress. Inhibition of HO-1 activity by ZnPP IX results in sensitizing tumor cells to chemotherapy such as CDDP by increasing the cellular ROS level and caspase-3 activity in vitro and in vivo. The combined treatment with HO-1 inhibitors may intensify the antitumor activity of chemotherapeutics and may open up new perspectives in the treatment of liver cancer.
Inhibition of heme oxygenase (HO)-1 expression or activity was shown to suppress cellular proliferation and increase responsiveness of tumor cells to some anti-cancer treatments. In contrast, induction of HO-1 expression or activity decreases cell sensitivity to anti-tumor drugs. Accumulating evidence suggests that HO-1 could be a therapeutic target for anti-tumor treatment.
The cytoprotective enzyme HO-1 is significantly overexpressed in many tumors and seems to play an important role in cellular resistance to chemotherapy and radiotherapy. In this study, the authors examined whether constitutively overexpressed HO-1 in liver cancer cells was associated with resistance to apoptosis induction by cis-diaminedichloroplatinum (cisplatin; CDDP), and explored the role of HO-1 in protecting tumor cells against chemotherapeutic agents in vitro and in vivo.
The results suggested that overexpression of HO-1 in HepG2 cell lines was associated with increased chemoresistance to CDDP compared with other cell lines in vitro. The inhibition of HO-1 by zinc protoporphyrin IX (ZnPP IX) was associated with increased cellular sensitivity and susceptibility of liver cancer cell lines to CDDP in vivo or in vitro. Reactive oxygen species (ROS) and caspase-3 seem to be involved in HO-1-mediated resistance to anti-cancer treatment. Therefore, administration of HO-1 inhibitors may evolve into a new liver cancer treatment strategy.
The findings in this study suggest that high HO-1 levels in HepG2 cells may, at least partially, be responsible for their resistance to chemotherapy and environmental stress. The combined treatment with HO-1 inhibitors may intensify the anti-tumor activity of chemotherapeutic agents and open up new perspectives in the treatment of liver cancer.
High HO-1 levels in HepG2 cells may be responsible for their resistance to chemotherapy and environmental stress. Inhibition of HO-1 activity by ZnPP IX sensitizes tumor cells to chemotherapy (e.g., with CDDP) by increasing the cellular ROS level and caspase-3 activity in vitro and in vivo.
This manuscript reports the results of a study on the effect of ZnPP IX, an HO-1 inhibitor, on the cellular sensitivity and susceptibility of liver cancer cell lines to CDDP in vitro and in vivo. The results are interesting and may open up new perspectives in the treatment of liver cancer.
P- Reviewers: Lee HC, Mehdi I, Yamasaki T, Zhang YJ S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Bertino G, Di Carlo I, Ardiri A, Calvagno GS, Demma S, Malaguarnera G, Bertino N, Malaguarnera M, Toro A, Malaguarnera M. Systemic therapies in hepatocellular carcinoma: present and future. Future Oncol. 2013;9:1533-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Shin JW, Chung YH. Molecular targeted therapy for hepatocellular carcinoma: current and future. World J Gastroenterol. 2013;19:6144-6155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 3. | Tanaka H, Kubo S, Tsukamoto T, Shuto T, Takemura S, Yamamoto T, Okuda T, Kanazawa A, Hirohashi K. Recurrence rate and transplantability after liver resection in patients with hepatocellular carcinoma who initially met transplantation criteria. Transplant Proc. 2005;37:1254-1256. [PubMed] |
| 4. | Yamamoto S, Sato Y, Takeishi T, Hirano K, Kobayashi T, Watanabe T, Hatakeyama K. Successful surgical treatment for hepatocellular carcinoma and concomitant risky esophageal varices. Hepatogastroenterology. 2005;52:1083-1086. [PubMed] |
| 5. | Pittalà V, Salerno L, Romeo G, Modica MN, Siracusa MA. A focus on heme oxygenase-1 (HO-1) inhibitors. Curr Med Chem. 2013;20:3711-3732. [PubMed] |
| 6. | Lin HY, Shen SC, Lin CW, Yang LY, Chen YC. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim Biophys Acta. 2007;1773:1073-1086. [PubMed] |
| 7. | Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, Kuo YH, Wu CR, Chen SC, Yang HL. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Jain K, Suryakumar G, Prasad R, Ganju L. Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt Med Biol. 2013;14:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Guan J, Wu X, Arons E, Christou H. The p38 mitogen-activated protein kinase pathway is involved in the regulation of heme oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. J Cell Biochem. 2008;105:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Heasman SA, Zaitseva L, Bowles KM, Rushworth SA, Macewan DJ. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget. 2011;2:658-668. [PubMed] |
| 11. | Lin PH, Lan WM, Chau LY. TRC8 suppresses tumorigenesis through targeting heme oxygenase-1 for ubiquitination and degradation. Oncogene. 2013;32:2325-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Vashist YK, Uzungolu G, Kutup A, Gebauer F, Koenig A, Deutsch L, Zehler O, Busch P, Kalinin V, Izbicki JR. Heme oxygenase-1 germ line GTn promoter polymorphism is an independent prognosticator of tumor recurrence and survival in pancreatic cancer. J Surg Oncol. 2011;104:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Doi K, Akaike T, Fujii S, Tanaka S, Ikebe N, Beppu T, Shibahara S, Ogawa M, Maeda H. Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer. 1999;80:1945-1954. [PubMed] |
| 14. | Li Y, Su J, DingZhang X, Zhang J, Yoshimoto M, Liu S, Bijian K, Gupta A, Squire JA, Alaoui Jamali MA. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol. 2011;224:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | McAllister SC, Hansen SG, Ruhl RA, Raggo CM, DeFilippis VR, Greenspan D, Früh K, Moses AV. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood. 2004;103:3465-3473. [PubMed] |
| 16. | Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, Wilczynski GM, Głodkowska E, Mrówka P, Issat T, Dulak J. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25:3365-3374. [PubMed] |
| 17. | Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109:1-8. [PubMed] |
| 18. | Kim HJ, So HS, Lee JH, Lee JH, Park C, Park SY, Kim YH, Youn MJ, Kim SJ, Chung SY. Heme oxygenase-1 attenuates the cisplatin-induced apoptosis of auditory cells via down-regulation of reactive oxygen species generation. Free Radic Biol Med. 2006;40:1810-1819. [PubMed] |
| 19. | Sass G, Leukel P, Schmitz V, Raskopf E, Ocker M, Neureiter D, Meissnitzer M, Tasika E, Tannapfel A, Tiegs G. Inhibition of heme oxygenase 1 expression by small interfering RNA decreases orthotopic tumor growth in livers of mice. Int J Cancer. 2008;123:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Was H, Cichon T, Smolarczyk R, Rudnicka D, Stopa M, Chevalier C, Leger JJ, Lackowska B, Grochot A, Bojkowska K. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181-2198. [PubMed] |
| 21. | Hennig R, Ventura J, Segersvard R, Ward E, Ding XZ, Rao SM, Jovanovic BD, Iwamura T, Talamonti MS, Bell RH. LY293111 improves efficacy of gemcitabine therapy on pancreatic cancer in a fluorescent orthotopic model in athymic mice. Neoplasia. 2005;7:417-425. [PubMed] |
| 22. | Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47-56. [PubMed] |
| 23. | Do MT, Kim HG, Khanal T, Choi JH, Kim DH, Jeong TC, Jeong HG. Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol. 2013;271:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Mayerhofer M, Gleixner KV, Mayerhofer J, Hoermann G, Jaeger E, Aichberger KJ, Ott RG, Greish K, Nakamura H, Derdak S. Targeting of heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: a novel approach to overcome resistance against imatinib. Blood. 2008;111:2200-2210. [PubMed] |
| 25. | Tibullo D, Barbagallo I, Giallongo C, La Cava P, Parrinello N, Vanella L, Stagno F, Palumbo GA, Li Volti G, Di Raimondo F. Nuclear translocation of heme oxygenase-1 confers resistance to imatinib in chronic myeloid leukemia cells. Curr Pharm Des. 2013;19:2765-2770. [PubMed] |
| 26. | Nuhn P, Künzli BM, Hennig R, Mitkus T, Ramanauskas T, Nobiling R, Meuer SC, Friess H, Berberat PO. Heme oxygenase-1 and its metabolites affect pancreatic tumor growth in vivo. Mol Cancer. 2009;8:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Lin F, Girotti AW. Hyperresistance of leukemia cells to photodynamic inactivation after long-term exposure to hemin. Cancer Res. 1996;56:4636-4643. [PubMed] |
| 28. | Hellmuth M, Wetzler C, Nold M, Chang JH, Frank S, Pfeilschifter J, Mühl H. Expression of interleukin-8, heme oxygenase-1 and vascular endothelial growth factor in DLD-1 colon carcinoma cells exposed to pyrrolidine dithiocarbamate. Carcinogenesis. 2002;23:1273-1279. [PubMed] |
| 29. | Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033-1040. [PubMed] |
| 30. | Lee YM, Auh QS, Lee DW, Kim JY, Jung HJ, Lee SH, Kim EC. Involvement of Nrf2-mediated upregulation of heme oxygenase-1 in mollugin-induced growth inhibition and apoptosis in human oral cancer cells. Biomed Res Int. 2013;2013:210604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Liu ZM, Chen GG, Ng EK, Leung WK, Sung JJ, Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004;23:503-513. [PubMed] |
| 32. | Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900-26907. [PubMed] |
| 33. | Banerjee P, Basu A, Datta D, Gasser M, Waaga-Gasser AM, Pal S. The heme oxygenase-1 protein is overexpressed in human renal cancer cells following activation of the Ras-Raf-ERK pathway and mediates anti-apoptotic signal. J Biol Chem. 2011;286:33580-33590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |