Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8525
Revised: April 11, 2013
Accepted: June 1, 2013
Published online: July 14, 2014
AIM: To develop an animal model that encompasses the different facets of non-alcoholic steatohepatitis (NASH), which has been a challenge.
METHODS: In this study, we used a high fat diet (HFD) feeding supplemented with fructose and sucrose in the water mimicking the high-fructose corn syrup that is abundant in the diet in the United States. We used C57Bl/6 wild-type mice for short and long-term feedings of 6 and 16 wk respectively, and evaluated the extent of liver damage, steatosis, and inflammasome activation. Our methods included histopathological analysis to assess liver damage and steatosis, which involved H and E and oil-red-o staining; biochemical studies to look at ALT and triglyceride levels; RNA analysis using quantitative polymerase chain reaction; and cytokine analysis, which included the enzyme-linked immunosorbent assay method to look at interleukin (IL)-1β and tumor necrosis factor-α (TNFα) levels. Furthermore, at each length of feeding we also looked at insulin resistance and glucose tolerance using insulin tolerance tests (ITT) and glucose tolerance tests.
RESULTS: There was no insulin resistance, steatosis, or inflammasome activation at 6 wk. In contrast, at 16 wk we found significant insulin resistance demonstrated by impaired glucose and ITT in male, but not female mice. In males, elevated alanine aminotransferase and triglyceride levels, indicated liver damage and steatosis, respectively. Increased liver TNFα and monocyte chemoattractant protein-1 mRNA and protein, correlated with steatohepatitis. The inflammasome components, adaptor molecule, Aim2, and NOD-like receptor 4, increased at the mRNA level, and functional inflammasome activation was indicated by increased caspase-1 activity and IL-1β protein levels in male mice fed a long-term HFD. Male mice on HFD had increased α-smooth muscle actin and pro-collagen-1 mRNA indicating evolving fibrosis. In contrast, female mice displayed only elevated triglyceride levels, steatosis, and no fibrosis.
CONCLUSION: Our data indicate gender differences in NASH. Male mice fed a long-term HFD display steatohepatitis and inflammasome activation, whereas female mice have steatosis without inflammation.
Core tip: Our work shows that there are gender differences in the development of non-alcoholic steatohepatitis. We used a high fat diet feeding supplemented with fructose and sucrose in mice, to mimic the high-fructose corn syrup that is abundant in the western diet. There is preferential steatohepatitis and inflammasome activation in male mice, whereas female mice display steatosis without inflammation.
- Citation: Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol 2014; 20(26): 8525-8534
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8525.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8525
Non-alcoholic fatty liver disease (NAFLD) affects approximately one third of the Western population and 75% of obese individuals[1,2]. It has become the most common cause of elevated liver enzymes in the Western world and will continue to be on the rise with the obesity epidemic[3-6]. The spectrum of NAFLD ranges from simple steatosis, which consists of intracellular lipid accumulation resulting in a fatty liver, to non-alcoholic steatohepatitis (NASH), which is characterized by the presence of inflammatory cells and necroinflammation, and fibrosis that develops in 20% of patients with NASH. These patients can progress to cirrhosis and hepatocellular carcinoma[1,7-9]. Factors determining the progression of NAFLD and strategies for effective treatment remain elusive. NASH is two to three times more common in men than women until after the age of 60, after which the prevalence is higher in women[10]. In animal models, there has been a lack of consensus as to the role of gender on the development of NAFLD[11-13].
The progression of NAFLD is centered around inflammation, and inflammasomes are major contributors to this process. Inflammasomes are intracellular multiprotein complexes that sense endogenous and exogenous danger signals through NOD-like receptors (NLRs)[14-16]. Components of intracellular inflammasome complexes are the sensors (Aim2, NLRP3 or NLRC4), adaptor molecule (ASC), and pro-caspase-1[14,17]. Inflammasome activation results in cleavage of pro-caspase-1 to activated caspase-1, which promotes cleavage and activation of pro-inflammatory cytokines [pro-interleukin-1β (pro-IL-1β) and pro-IL-18] resulting in a sustained inflammatory response[18,19]. It has been shown that the methionine-choline deficient (MCD) diet, induces significant upregulation of the NACHT, LRR, and PYD domains-containing protein 3 (NALP3) inflammasome, and that a 9-mo HFD feeding can also cause NALP3 activation[20].
In this study, we performed a HFD feeding for a short-term (6-wk) and long-term (16-wk) duration that included both male and female mice. Our data demonstrates that steatohepatitis and inflammasome upregulation and activation are observed in male mice, but not female mice, after a 16-wk HFD feeding.
This study was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Principles of laboratory animal care were followed. Male and Female C57Bl/6 wild-type mice that were 8-10 wk old (n = 10 per group) (Jackson Laboratory) were fed either a control chow diet or a high-fat, high-carbohydrate diet [Surwit diet (58% kcal % fat); Research Diets, New Brunswick, NJ, United States] with drinking water supplemented with a high-fructose corn syrup equivalent consisting of a total of 42 g/L of carbohydrates at a ratio of 55% fructose (Acros Organics, Morris Plains, NJ, United States) and 45% sucrose (Sigma-Aldrich, St. Louis, MO, United States) by weight, based on the study by Kohli et al[21]. The mice were given ad libitum access to the food for 6 or 16 wk.
Serum alanine aminotransferase levels were measured using a kinetic method (D-TEK, Bensalem, PA, United States), and liver triglyceride levels were determined using an L-type triglyceride H kit (Wako Chemicals United States, Inc., Richmond, VA, United States). Mouse IL-1β enzyme-linked immunosorbent assay (ELISA) kit was purchased from R and D Systems (Minneapolis, MN). Mouse TNF-α kit was purchased from BD Bioscience (San Jose, CA, United States), monocyte chemoattractant protein 1 (MCP-1) ELISA kit was purchased from Biolegend (San Diego, CA, United States).
Sections of formalin-fixed livers were stained with hematoxylin-eosin. Frozen samples were also stained with Oil-Red-O that were made at the optimal cutting temperature. Slides were analyzed using microscopy using Image J and Microsuite (Olympus Soft Imaging Solutions GmbH, Munster, Germany).
Glucose levels were measured after 14 wk feeding following an overnight fast. Mice were injected IP with 40% glucose (Sigma, St. Louis, MO, United States) at 2 mg/kg body weight and glucose levels were obtained at 0, 30, 75, 120 and 180 min. Blood samples were obtained by tail nick and glucose levels were measured using a GlucCell glucose monitor and strips. A subset of mice had blood collected by facial vein puncture at time point 0 and 30 min for serum insulin measurement using a mouse enzyme-linked immunosorbent assay kit (ALPCO, Salem, NH, United States).
After 15 wk of feeding and after an overnight fast, mice were injected IP with 0.75 U/kg body weight of recombinant human insulin (Novolin, Novo Nordisk, Bagsvaerd, Denmark). Glucose levels were measured in the same way as in the glucose tolerance tests (GTT) at time points 0, 30, 75 and 120 min following insulin injection.
RNA was purified using a RNeasy kit (Qiagen Sciences, Germantown, MD, United States) with the on-column DNA digestion (Qiagen Sciences, Germantown, MD, United States). Complementary DNA was transcribed using a reverse-transcription system (Promega Corp., Madison, WI, United States). Real-time quantitative polymerase chain reaction (qPCR) was performed with an iCycler (Bio-Rad Laboratories, Inc., Hercules, CA, United States) using SYBR Green as the detector of double-started DNA, as previously described[22].
Caspase-1 activity was determined using freshly prepared whole liver lysates with a colorimetric assay. The analysis was based on the cleavage of the WEHD-pNA (Trp-Glu-His-Asp-p-nitroanilide) substrate (R and D Systems, Minneapolis, MN, United States).
Statistical significance was determined using the nonparametric Kruskal-Wallis test and the Mann-Whitney test when appropriate. Data are presented as means and standard errors and are considered statistically significant for P≤ 0.05.
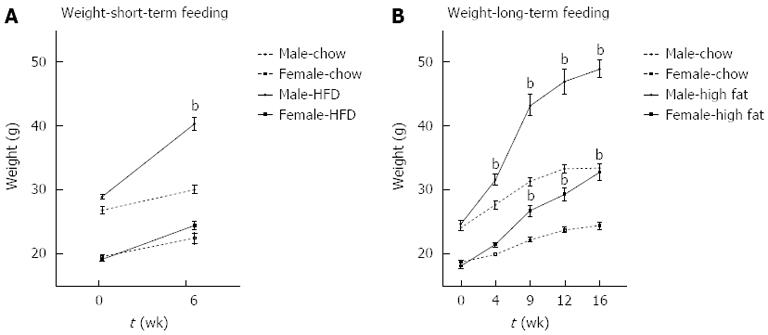
Male mice fed a short-term 6-wk HFD gained significantly more weight than chow-fed controls. Female mice did not gain significant weight on the HFD compared to chow fed mice at 6 wk (Figure 1A). In contrast, at 16 wk, both male and female mice gained significantly more weight on a HFD than chow fed mice (Figure 1B).
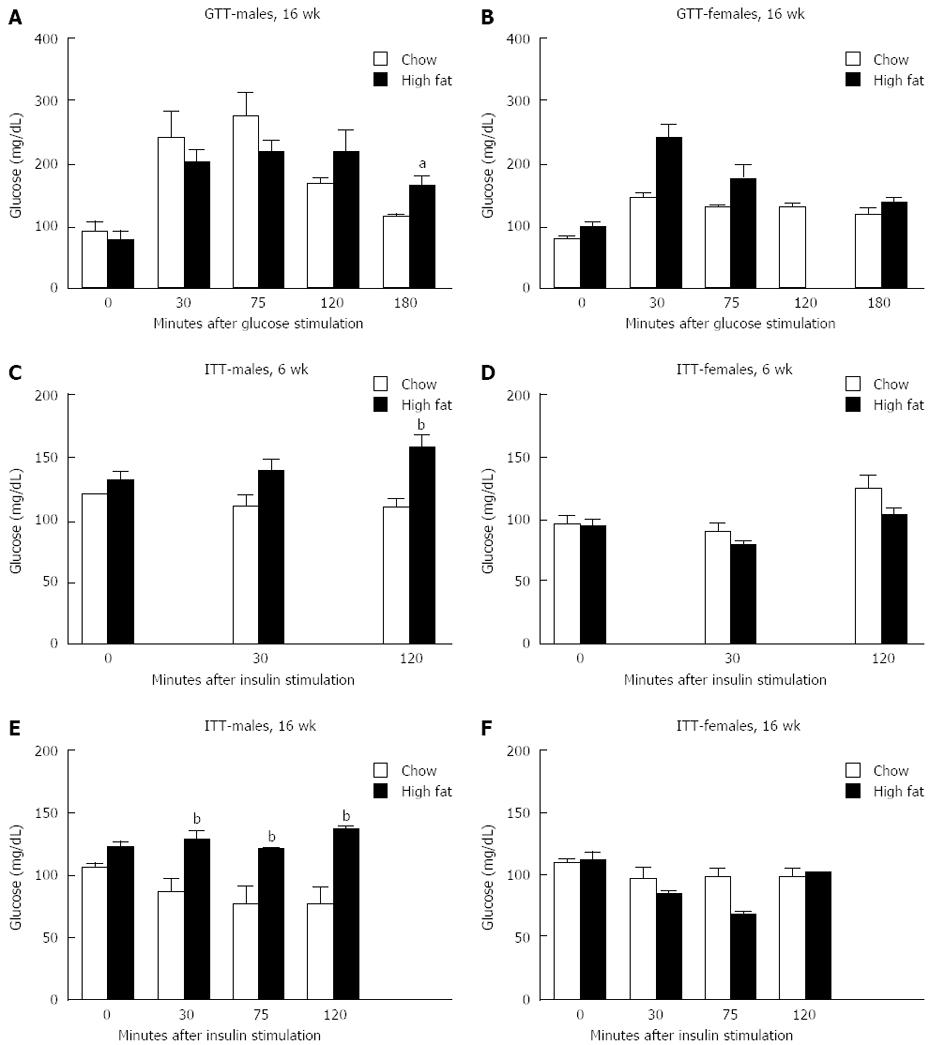
GTT were performed on a subset of mice one week before being euthanized. There was no difference in glucose levels following glucose injection at any time point in male or female mice after a short-term HFD (data not shown). In mice fed a long-term HFD, males showed significantly higher glucose levels 180 min after glucose injection than those on a chow diet (Figure 2A). Female mice on a long-term HFD diet showed no significant difference in glucose levels at any time point following glucose injection compared to controls (Figure 2B).
Insulin tolerance test was also performed one week prior to euthanasia. In the short-term feedings, male mice had significantly increased glucose levels compared to chow fed mice at the 120-min time point (Figure 2C), whereas female mice had no difference in glucose levels between the groups at any time point (Figure 2D). In the group that received a long-term HFD, males had significantly higher glucose levels at all time points following insulin injection compared to chow fed males (Figure 2E), whereas females had no difference in glucose levels compared to chow fed controls (Figure 2F).
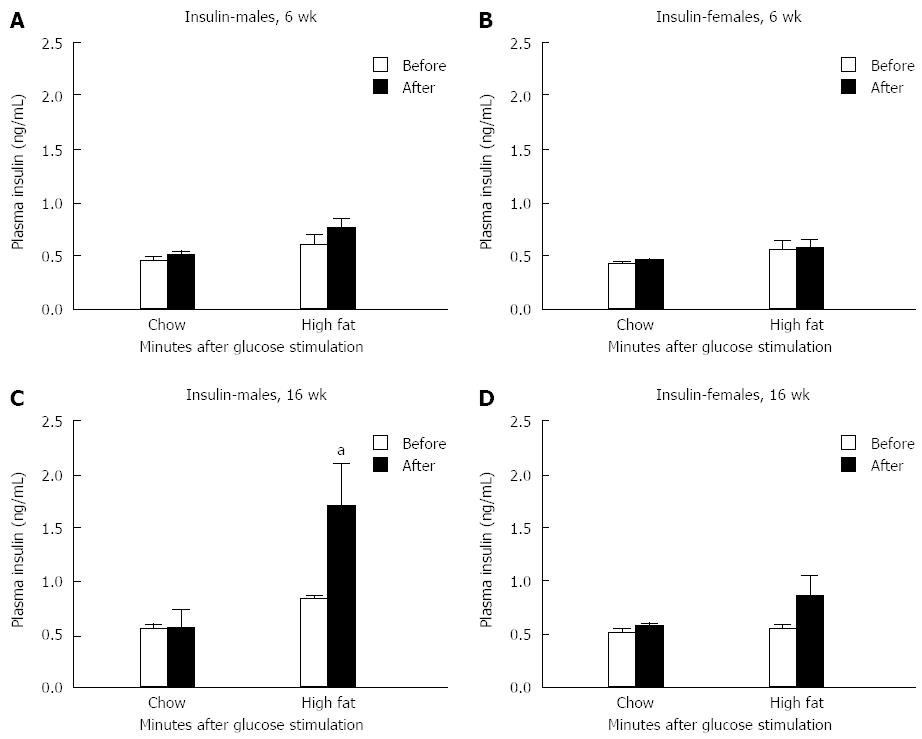
Serum insulin levels before and 30 min after glucose injection showed no significant difference in either gender after a 6-wk HFD feeding compared to chow fed controls (Figure 3A and B). In contrast, insulin levels were elevated in long-term HFD male mice 30 min after glucose injection compared to chow fed controls (Figure 3C). No change in insulin levels were detected in female mice fed the long-term HFD (Figure 3D).
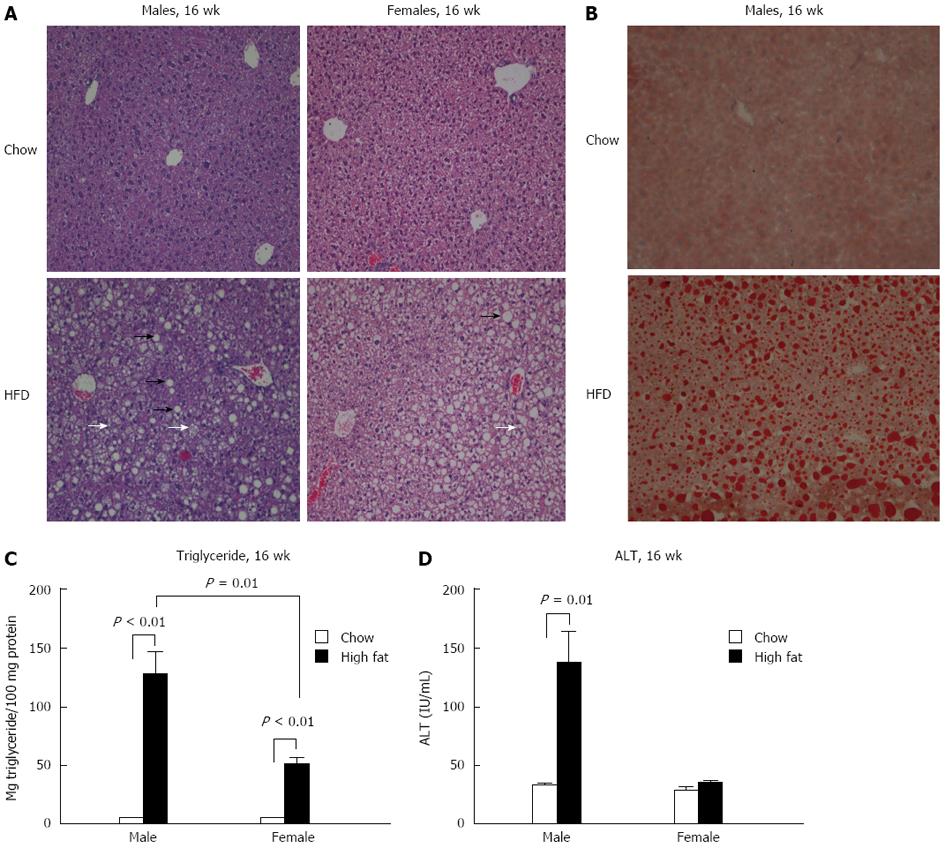
To assess liver steatosis and inflammation, liver sections were evaluated histologically using hematoxylin and eosin and Oil-Red-O staining. Neither gender showed changes in histology after a short-term (6-wk) HFD feeding compared to chow diet (data not shown). However, both male and female mice undergoing a long-term HFD feeding had significantly more micro and macrovesicular steatosis than chow fed controls (Figure 4A and B). Steatosis was further evaluated by triglyceride levels in the liver. Both male and female mice had significantly elevated liver triglyceride levels compared to chow fed mice at 16 wk (Figure 4C). However, male mice on a long-term HFD had higher triglyceride levels than female mice on the same diet (Figure 4C).
Liver damage was assessed by alanine aminotransferase (ALT) levels. No change in ALT levels in either gender was noted after a short-term HFD feeding (data not shown). Following a 16-wk HFD, male mice showed significantly higher levels of ALT than chow fed controls, whereas no significant change in ALT was noted in the long-term female HFD group (Figure 4D).
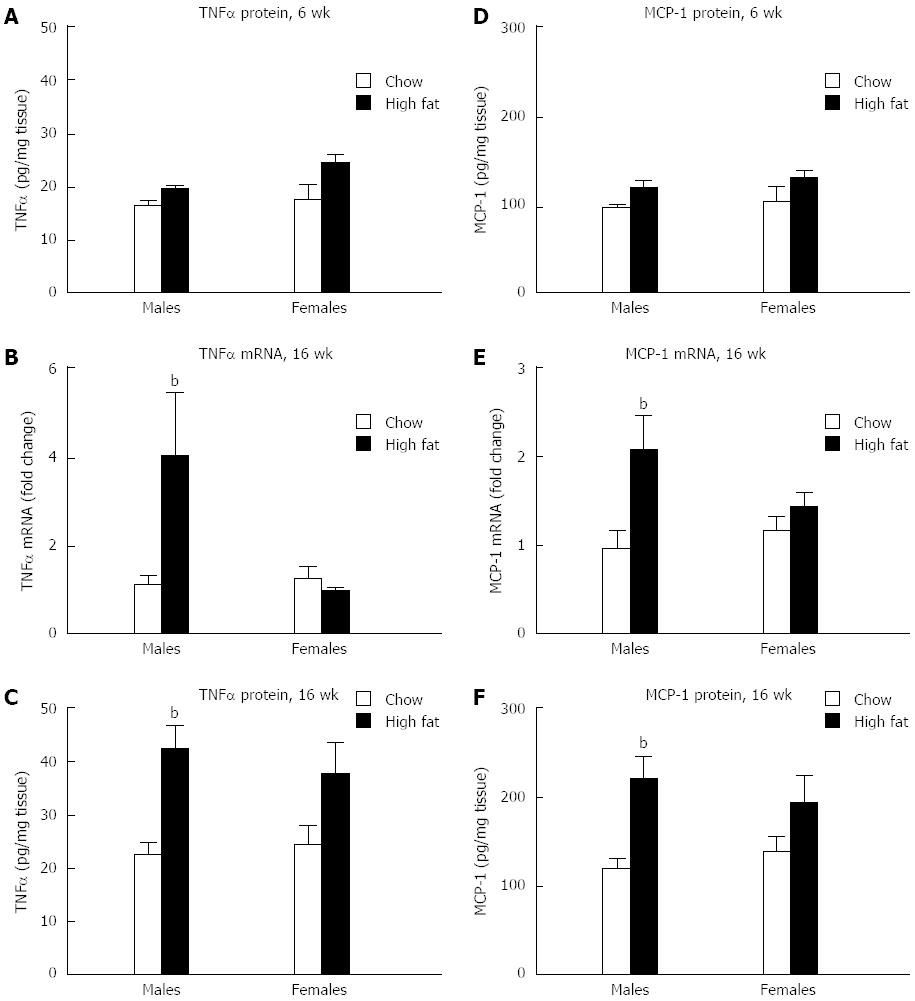
Next, we evaluated the presence of inflammation in the liver. There was no change in protein levels of tumor necrosis factor-α (TNFα) in the livers of either male or female mice in the short-term HFD group (Figure 5A). In the livers of long-term HFD males, TNFα mRNA levels were increased approximately 4-fold, whereas no significant change was noted in the females (Figure 5B). A similar trend was seen in TNFα protein levels in the liver, with a 2-fold increase in the long-term HFD males, and no increase in the long-term HFD females (Figure 5C). MCP-1 is a chemokine that contributes to steatosis and inflammatory cell infiltration. In a pattern similar to TNFα, no change in levels of MCP-1 protein was seen in mice fed a short-term HFD compared to chow-fed controls (Figure 5D). MCP-1 mRNA levels in the liver were increased in the long-term HFD males compared to chow fed mice, whereas no change was noted in the long-term HFD females (Figure 5E). Long-term HFD male mice showed a 2-fold increase in MCP-1 protein in the liver compared to chow-fed mice, whereas no change in MCP-1 protein was seen in female mice (Figure 5F).
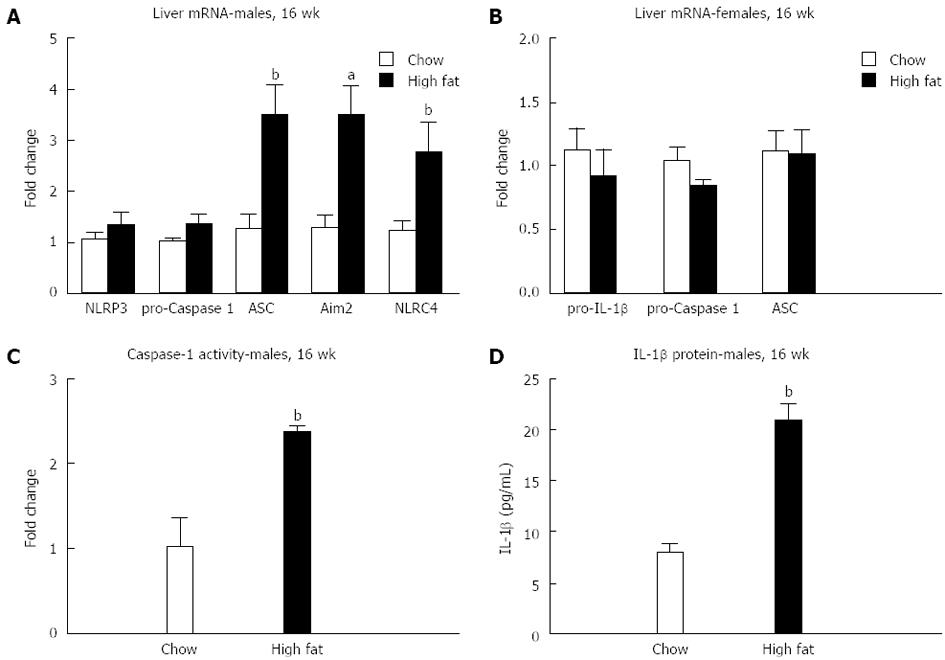
We evaluated inflammasome activation only in the long-term 16-wk HFD feeding based on previous studies that a longer feeding is needed for inflammasome activation[20]. We found an increase in mRNA in components of the inflammasome only in male mice fed a long-term HFD, including ASC, Aim2, and NLRC4 (Figure 6A and B). Inflammasome activation was indicated by increased caspase-1 activity (Figure 6C) and increased total IL-1β protein levels (Figure 6D) in the liver of male mice fed a long-term HFD.
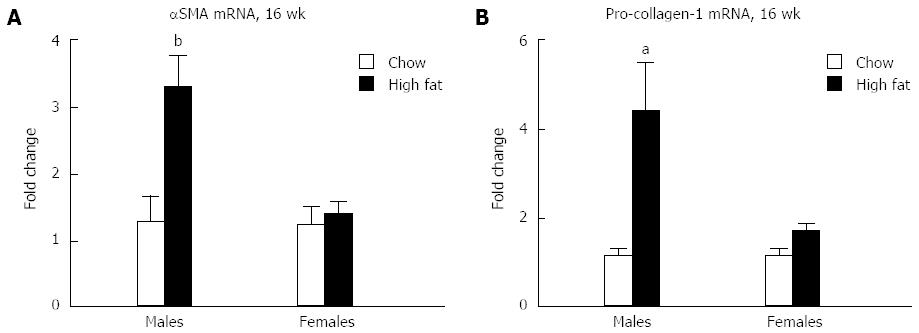
Because liver inflammation can trigger fibrosis, fibrotic markers were evaluated in mice fed a long-term HFD. Elevation in mRNA levels of both α-smooth muscle actin (αSMA) (Figure 7A) and pro-collagen-1 (Figure 7B) are seen in male mice who received long-term HFD feeding, whereas no significant change was seen in females compared to chow fed controls. Because of the early stage of fibrosis, we found no increase in αSMA protein levels (data not shown).
NASH has become a global epidemic and continued research into the pathogenesis resulting in inflammation and steatosis requires a robust animal model, which has remained a challenge[23]. Here we show that mice fed a HFD supplemented with fructose and sucrose in the drinking water for 16 wk, results in steatohepatitis and moderate activation of the inflammasome pathway in male, but not female, mice.
Our study evaluated gender differences on the degree of inflammation, steatosis, and fibrosis between male and female mice fed a high fat diet enriched in fructose and sucrose for 6 or 16 wk. We found evidence of steatohepatitis in male mice fed a long-term HFD as demonstrated by an increase in ALT, triglycerides, TNFα, and MCP-1, whereas in females only an increase in triglycerides was seen, consistent with the presence of steatosis, but no steatohepatitis. Several studies have investigated gender on the role of NAFLD progression with no consistent outcome. Kamada et al[13] show that estrogen plays a role in protection of liver injury following high fat diet, whereas another group showed that there was no protection in MCD diet induced steatohepatitis in female mice that received an ovariectomy or antiestrogen treatment[11]. Yet another study showed that female mice fed a chow diet supplemented with 30% fructose in the drinking water, displayed more pronounced inflammation than male mice[12]. In terms of other liver diseases in humans, men are about three to five times more likely than women to develop hepatocellular carcinoma (HCC)[24]. The underlying mechanism leading to a decreased incidence in women is thought to be due to estrogen suppression of IL-6 in Kupffer cells[25] and due to increased androgen receptor signaling by testosterone in men, resulting in increased hepatocyte proliferation[26]. Furthermore, studies have shown that the male gender is an independent risk factor in the progression of cirrhosis[27,28], and that young women have the lowest rate of developing cirrhosis from chronic hepatitis C[29]. Further studies need to be done looking into the differences of liver injury in males and females. Our study in mice indicates gender differences that were dependent on the length of HFD feeding and/or the estrogen status of female mice.
Previous work has shown that various high fat diet feedings induce the inflammasome and that knock-outs of parts of the inflammasome pathway can result in protection from HFD induced obesity[20,30]. Both upregulation of inflammasome expression and activation occurred in male mice after 16 wk. We show that there is an increase in mRNA in parts of the inflammasome pathway, including ASC, Aim2, and NLRC4, as well as an increase in caspase-1 activity and IL-1β protein in the liver, after a 16-wk HFD feeding in male mice, whereas mice fed a HFD for 9 mo, show an increase in mRNA in all parts of the NALP3 inflammasome (NALP3, ASC, Caspase-1, Pannexin, and IL-1β)[20]. This finding suggests that longer access to a HFD is required to obtain complete inflammasome activation and thus more inflammation and liver damage. Another possibility is that changing the composition of the diet could result in more extensive liver damage and fibrosis, such as the combination of dietary fat and dietary cholesterol[31]. It is conceivable that changes in fatty acid composition due to the diet affects inflammation, for example saturated fatty acids result in inflammasome activation[20].
The limitation of existing animal models of NASH that are based on genetic modifications and no single model captures the different aspects of human fatty liver disease[23,32,33]. MCD extensively used in our previous studies results in severe steatohepatitis in a short period of time[20,34], however in contrast to human fatty liver disease, mice lose weight and lack insulin resistance[23]. To use a model that results in steatohepatitis, in addition to weight gain and insulin resistance, we chose HFD model consisting mainly of medium chain hydrogenated saturated trans fatty acids supplemented with fructose and sucrose in the drinking water, similar to the high-fructose corn syrup that is so widespread in the United States[21]. Of note, there has been a wide variety in the extent of inflammation and steatosis seen in high fat diet feedings. Some groups have shown fibrosis after only 6 wk of feeding[35], whereas others have needed 24 wk or longer to see fibrosis[36,37]. This variation could be due to variances in the composition of the diet and in differences in gut microbiota in the mice in each individual study.
In summary, our data shows that gender differences exist in the development of steatohepatitis and inflammasome activation following a long-term high fat diet feeding. Male mice display steatohepatitis in addition to inflammasome upregulation and activation, whereas female mice develop steatosis and do not show activation of the inflammasome pathway.
The authors would like to thank Jan Petrasek for his help with editing the manuscript, and Dora Lippai for assistance with the glucose and insulin tolerance tests.
Non-alcoholic fatty liver disease is one of the most common causes of liver disease in the Western world and continues to be on the rise. Developing an animal model that closely resembles human non-alcoholic fatty liver disease has remained a challenge. Furthermore, it is known that males are more likely than females to develop cirrhosis.
Developing an animal model that resembles non-alcoholic fatty liver disease in humans is an important area. Further knowledge into non-alcoholic fatty liver disease can help further knowledge in the field.
Previous studies have shown that feeding mice a high fat diet with added sucrose and fructose results in steatohepatitis and fibrosis. A number of studies have looked at gender differences in non-alcoholic fatty liver disease with no consistent outcome. In the present study, the authors looked at the effect of both a short-term and long-term high fat diet feeding supplemented with sucrose and fructose in both male and female wild-type mice. This paper showed that male mice fed a long-term high fat diet display steatohepatitis and inflammasome activation, whereas female mice have steatosis without inflammation.
Further studies looking into why there are gender differences in developing non-alcoholic fatty liver disease are warranted. These studies could lead to potential treatments for non-alcoholic fatty liver disease.
The manuscript presents some animal data on the gender differences regarding the consequences of high fat feeding. The manuscript is in a very good condition. Although the animal model for gender difference may be questioned and translation of the data to the clinical area is difficult, the data may be attractive for some of the readers.
P- Reviewer: Tasci I S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Sheth SG, Gordon FD, Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;126:137-145. [PubMed] [Cited in This Article: ] |
| 2. | Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 605] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 939] [Cited by in F6Publishing: 918] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 4. | Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond). 2008;115:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Ismail MH. Nonalcoholic fatty liver disease and type 2 diabetes mellitus: the hidden epidemic. Am J Med Sci. 2011;341:485-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Rahimi RS, Landaverde C. Nonalcoholic fatty liver disease and the metabolic syndrome: clinical implications and treatment. Nutr Clin Pract. 2013;28:40-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154-160. [PubMed] [Cited in This Article: ] |
| 8. | Sugimoto K, Takei Y. Clinicopathological features of non-alcoholic fatty liver disease. Hepatol Res. 2011;41:911-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Tuyama AC, Chang CY. Non-alcoholic fatty liver disease. J Diabetes. 2012;4:266-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol. 2011;46 Suppl 1:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Kashireddy PR, Rao MS. Sex differences in choline-deficient diet-induced steatohepatitis in mice. Exp Biol Med (Maywood). 2004;229:158-162. [PubMed] [Cited in This Article: ] |
| 12. | Spruss A, Henkel J, Kanuri G, Blank D, Püschel GP, Bischoff SC, Bergheim I. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med. 2012;18:1346-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, Tsubakio M, Takemura T, Ezaki H, Hayashi N. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011;301:G1031-G1043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 638] [Cited by in F6Publishing: 664] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 15. | Ciraci C, Janczy JR, Sutterwala FS, Cassel SL. Control of innate and adaptive immunity by the inflammasome. Microbes Infect. 2012;14:1263-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 17. | Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 709] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 19. | Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1769] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 20. | Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 21. | Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Velayudham A, Hritz I, Dolganiuc A, Mandrekar P, Kurt-Jones E, Szabo G. Critical role of toll-like receptors and the common TLR adaptor, MyD88, in induction of granulomas and liver injury. J Hepatol. 2006;45:813-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig Dis. 2010;28:247-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] [Cited in This Article: ] |
| 25. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1362] [Cited by in F6Publishing: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 26. | Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993;53:790-794. [PubMed] [Cited in This Article: ] |
| 27. | Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730-739. [PubMed] [Cited in This Article: ] |
| 28. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2881] [Cited by in F6Publishing: 2983] [Article Influence: 229.5] [Reference Citation Analysis (0)] |
| 29. | Kim WR, Poterucha JJ, Benson JT, Therneau TM. The impact of competing risks on the observed rate of chronic hepatitis C progression. Gastroenterology. 2004;127:749-755. [PubMed] [Cited in This Article: ] |
| 30. | Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324-15329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 533] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 31. | Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, Chait A, Yeh MM, Quinn LS, Ioannou GN. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57:81-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 32. | Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300-2308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 379] [Cited by in F6Publishing: 378] [Article Influence: 31.5] [Reference Citation Analysis (2)] |
| 33. | Almonacid-Urrego CC, Sánchez-Campos S, Tuñón MJ, González-Gallego J. Non-alcoholic steatohepatitis: what can we learn from animal models? Curr Med Chem. 2012;19:1389-1404. [PubMed] [Cited in This Article: ] |
| 34. | Csak T, Dolganiuc A, Kodys K, Nath B, Petrasek J, Bala S, Lippai D, Szabo G. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Gäbele E, Dostert K, Dorn C, Patsenker E, Stickel F, Hellerbrand C. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcohol Clin Exp Res. 2011;35:1361-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Xu ZJ, Fan JG, Ding XD, Qiao L, Wang GL. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Ito M, Suzuki J, Tsujioka S, Sasaki M, Gomori A, Shirakura T, Hirose H, Ito M, Ishihara A, Iwaasa H. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |