Published online Jul 14, 2014. doi: 10.3748/wjg.v20.i26.8471
Revised: January 15, 2014
Accepted: April 1, 2014
Published online: July 14, 2014
Processing time: 268 Days and 20.4 Hours
Pancreatic cancer carries a terrible prognosis, as the fourth most common cause of cancer death in the Western world. There is clearly a need for new therapies to treat this disease. One of the reasons no effective treatment has been developed in the past decade may in part, be explained by the diverse influences exerted by the tumour microenvironment. The tumour stroma cross-talk in pancreatic cancer can influence chemotherapy delivery and response rate. Thus, appropriate preclinical in vitro models which can bridge simple 2D in vitro cell based assays and complex in vivo models are required to understand the biology of pancreatic cancer. Here we discuss the evolution of 3D organotypic models, which recapitulare the morphological and functional features of pancreatic ductal adenocarcinoma (PDAC). Organotypic cultures are a valid high throughput preclinical in vitro model that maybe a useful tool to help establish new therapies for PDAC. A huge advantage of the organotypic model system is that any component of the model can be easily modulated in a short time-frame. This allows new therapies that can target the cancer, the stromal compartment or both to be tested in a model that mirrors the in vivo situation. A major challenge for the future is to expand the cellular composition of the organotypic model to further develop a system that mimics the PDAC environment more precisely. We discuss how this challenge is being met to increase our understanding of this terrible disease and develop novel therapies that can improve the prognosis for patients.
Core tip: Pancreatic cancer carries a terrible prognosis, as the fourth most common cause of cancer death in the Western world. One of the reasons no effective treatment has been developed in the past decade may in part, be explained by the influences exerted by the tumour microenvironment. The tumour stroma cross-talk in pancreatic cancer can influence chemotherapy delivery and response rate. Organotypic models of pancreatic cancer allow new therapies that can target the cancer, the stromal compartment or both to be tested in a model that mirrors the in vivo situation and can help improve patient prognosis.
- Citation: Coleman SJ, Watt J, Arumugam P, Solaini L, Carapuca E, Ghallab M, Grose RP, Kocher HM. Pancreatic cancer organotypics: High throughput, preclinical models for pharmacological agent evaluation. World J Gastroenterol 2014; 20(26): 8471-8481
- URL: https://www.wjgnet.com/1007-9327/full/v20/i26/8471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i26.8471
Pancreatic cancer has one of the highest mortality rates among malignancies, and is the fourth most common cause of cancer death in the Western world[1,2]. With an overall 5-year survival rate of 6% and median survival of less than six months, pancreatic ductal adenocarcinoma (PDAC) carries one of the bleakest prognoses in all of medicine. Surgery offers the only hope of a possible cure for patients; however even of those 10% of patients eligible for curative resection, only 21% will survive to five years[3]. This is due to the fact that, at diagnosis, distant metastases are common[4]. Clearly there is an urgent need for therapies for PDAC. One of the possible reasons that targeted therapies fail to improve the prognosis of patients with PDAC may, in part, be explained by the diverse influences exerted by the tumour microenvironment. Delineating the signalling networks within the tumour microenvironment, may help to explain the huge discrepancy between relative success and effectiveness of therapies in preclinical assay (predominately 2D cell based assays and xenograft mouse models) and their abject failure in human PDAC.
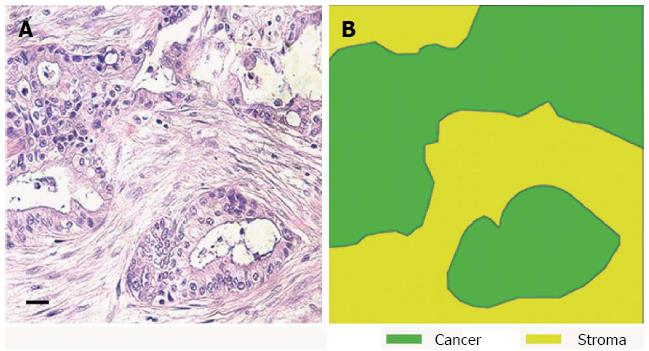
Many epithelial malignancies, including breast, prostate, skin and pancreatic cancers, often exhibit a significant stromal reaction around the tumour cells[5-9]. Once thought to be a bystander, it is becoming increasingly evident that the stroma not only functions as a mechanical barrier but also constitutes a dynamic compartment that is critically involved in the process of tumour formation, progression, invasion, and metastasis[10,11]. In particular, PDAC shows the most prominent stromal reaction or “desmoplasia” (defined as proliferation of fibrotic tissue with an altered ECM which contributes to tumour growth and metastasis) (Figure 1)[12]. This surrounding tumour environment is an highly heterogeneous and complex mixture of cells from different lineages; fibroblasts, pancreatic stellate cells, smooth muscle cells, immune, inflammatory, neural, adipose and endothelial cells[13-16].
The high proportion of stromal cells in pancreatic cancer (up to 80% of the tumour volume[17]) is associated with overexpression of a number of paracrine and autocrine signalling factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor β (TGFβ), insulin-like growth factor I (IGF-I), fibroblast growth factor (FGF) and their respective receptors as well as secretion of Matrix metalloproteinases (MMPS) and proteases which serve to fuel pancreatic cancer proliferation, metastasis and invasion[18-22]. In turn, pancreatic cancer cells secrete growth factors such as FGFs, TGFβ, IGF and platelet derived growth factor (PDGF)[23]. This interaction between cancer cells and stroma leads to altered transcription in stromal components, such as fibroblasts and inflammatory cells, promoting cancer cell motility and resistance to hypoxia. The net result is an unique tumour micro-environment, where tumour cells become inaccessible to chemotherapy and metastasise readily, leading to poor chemotherapy response rate[17].
These studies have highlighted the importance of stroma-cancer cross-talk. Thus, just studying pancreatic cancer cells without any stromal representation does not reflect accurately the in vivo situation. Cells grown on 2D tissue culture plates or in Transwell™ inserts differ in their morphology, differentiation and cell-cell and cell-matrix interactions compared to cells in vivo[24,25]. There is a need for physiologically relevant in vitro model systems that allow us to investigate and interrogate cancer and stromal cell behaviour and their interactions. Thus, 3D organotypic models are an invaluable research tool[26].
Improved understanding of the mechanisms that mediate epithelial-stromal interactions in PDAC is now possible due to the isolation, and in vitro culture, of pancreatic stellate cells (PSC), the key cells driving the desmoplastic reaction[21]. In the healthy pancreas, PSCs make up 4%-7% of all pancreatic cell types and exist in a quiescent state[27]. Quiescent PSCs are characterised by lipid droplets rich in vitamin A, resembling hepatic stellate cells (HSCs) first described by in the 19th century[28]. They express desmin and glial fibrillary acid protein (GFAP) marker which serve to distinguish them from pancreatic fibroblasts[29]. In acute and chronic inflammatory conditions, PSCs are activated. This is characterised a loss of fat droplets, expression of α-smooth muscle actin (αSMA), and an increased synthesis and secretion of several ECM proteins such as fibronectin, laminin and collagen type I and III[27,30,31].
The isolation and immortalisation of PSCs from human and rat pancreas has provided an additional tool for studying PSC activation and can overcome the limitations of culturing primary stellate cells. While immortalised stellate cells have provided a valuable tool in the study of PSC function, it is important to validate findings using primary PSCs[14]. PSCs have been immortalised using either SV40 large T antigen or human telomerase in human PSCs as we have previously successfully done in our laboratory[24,32-38]. Immortalised PSCs display an activated phenotype in 2D culture. Importantly PSC cell line is comparable to activated PSCs, which include expression of αSMA and ECM proteins. Importantly, expression profiling of primary and PSC cell lines have shown only a few differences, with differential differences expression of ECM proteins, cytokines and integrins[37]. In addition, both immortalised and primary PSCs respond to TGF-β or PDGF in a similar manner[33]. Thus primary and immortalised PSCs have facilitated for the dissection of important cross-talk between PSCs and pancreatic cancer cells and are an important source to explore the tumour promoting aspects of tumour myofibroblasts in PDAC[16].
The bidirectional interaction between PSCs and pancreatic cancer cells has been studied using co-culture or well-established 2D in vitro assays such as wound assays or Transwell™ inserts to study migration[39]. Co-culturing of PSCs and pancreatic cancer cells showed that PSCs can increase the proliferation and migration of pancreatic cancer cells, while inhibiting apoptosis by the release of several cytyokines and growth factors. Similarly, culturing PSCs in the conditioned medium of pancreatic cancer cells increases the proliferation, matrix synthesis and motility of PSCs, most likely via FGF-2, PDGF and TGF-β[22,40].
The desmoplasia in PDAC is believed to have a detrimental effect on the successful response to chemotherapy and radiotherapy[40,41]. In vitro experiments have shown that PSCs can increase the stem cell characteristic of pancreatic cancer cells, a possible mechanism of resistance to therapy[42]. Furthermore, in areas of the tumour that are hypoxic as a result of hypovascularity and profuse stroma provides a micro-environment in which pancreatic cancer cells thrive[43]. In vitro studies have shown that, co-culturing PSCs and pancreatic cancer cells under hypoxic conditions, PSCs are able to influence PCC invasion more strongly than in normoxic conditions[44]. Thus, pharmacological targeting of PSCs is an attractive option in treating PDAC.
Animal models, such as xenografts, orthotopic grafts or genetically engineered mice (GEM), have validated many in vitro findings. Early subcutaneous mouse models, in which PSCs and pancreatic cancer cells were injected into the flanks of immunocompromised mice, demonstratied that, in the presence of PSCs, pancreatic cancer cell proliferation increased and tumours formed more rapidly than when pancreatic cancer cells were injected alone[22]. Apte and colleagues showed that injection of pancreatic cancer cells (MiaPaCa-2 and AsPC-1 cell lines), together with primary human PSC into the mouse pancreas was able to stimulate fibrosis, tumour growth and metastasis[40]. More recently, sex mismatch studies (injection of male PSCs and female pancreatic cancer cells into the pancreas of female mice), have shown that Y chromosome positive PSCs are able to migrate through blood vessels, together with cancer cells, localising to distant sites, such as the liver and diaphragm, where they are able to facilitate seeding, survival and growth of pancreatic cancer cells[45].
The development of genetically engineered mouse (GEM) models of PDAC has provided the most physiologically relevant model that closely mimics the situation in human cancer. Most of the GEM models of PDAC are based on the conditional, pancreas-specific, expression of the Kras oncogene (KRASG12D), present in 90% of human PDAC cases[46], this is facilitated by expressing Cre recombinase under the control of the embryonic pancreas lineage determining transcription factor Pdx-1 or Ptf1/p48 (“KC” mice). KC mice develop pancreatic tumours ranging from precursor pancreatic intra-ductal neoplasms (PanINs) to fully invasive and metastatic disease[47,48], albeit with a long latency period of up to a year. These KC mice have been crossed with mice harbouring several additional mutations, to investigate their contribution to the rapid progression to PDAC. GEM models of PDAC have been developed with activating mutations in TGFβ receptor and/or inactivation of tumoral suppressors such as p53 (“KPC” mice), INK4A/ARF and Smad4, which are the most common PDAC drivers[49]. There are several excellent reviews on the various GEM models that have been developed for studying the development of PDAC[50-52]. The generation of complex allele combinations together with the latency period involved in the development of tumour makes these models inherently expensive. Further criticism against GEM models of PDAC has focused on the multi-focality of their PDAC, involvement of whole pancreas with tumours, histological variants commonly observed, presence of tumours in other organs as well as genetic homogeneity; features missing in the human PDAC[53]. Thus, 3D organotypic models may be an attractive option as a preclinical tool, bridging the gap between traditional 2D cell culture assays and the complex GEM models.
The idea of recapitulating the physiomimetic 3D environment started in the 1960’s growing 3D tissue explants in tissue culture media (organ cultures). Organ cultures of neural tissue explants are perhaps the best established model[54]. These models are still being used to study the basis of neurological diseases and injuries[55]. Organ cultures are also used in the study of cardiovascular function[56], angiogenesis[57], thymus[58], skin[59], bone[60], and urogenital tissues[61].
In cancer research, there has been an abundance of evidence suggesting that 3D models are superior to the conventional 2D culture in plastic flasks. However, current preclinical research still relies heavily on the latter[62]. From the simplest form: the “monotypic” cell model, comprising just one epithelial cell type, 3D co cultures have progressively evolved to contain multiple cell types, thus enabling study of their respective contributions[63]. An early example was the the “skin equivalent”, achieved by culturing keratinocytes either on de-epidermalised dermis or on collagen gels embedded with dermal fibroblasts[64,65].
The success of pioneering studies with breast epithelial cells cultured in, or on, a reconstituted basement membrane (e.g., Matrigel) undergoing glandular differentiation forming with apico-basal polarity and a central hollow lumen[66], have led to similar experiments for the liver, salivary gland, bone, lung, skin, intestine, kidney and thyroid glands[64,67-71]. The choice of cell source and ECM is critical in developing a representative model. For example, human luminal epithelial cells, grown in laminin rich basement membrane analogue (Matrigel) form acini[72]; however when grown in collagen I, these same cells show an altered integrin profile and abnormal polarity[73].
These 3D models have increased our understanding of how cells perceive biochemical and physical cues from the surrounding microenvironment[74]. For example, β1 integrin is expressed in normal breast epithelial cells but is lost when cells transform into a malignant phenotype. Re-expression of β1 integrin in 3D matrices induces the reversion of the tumor phenotype by allowing the malignant cells to differentiate into glands[75].
The incorporation of tissue specific stromal cells is critical for approximation to the in vivo condition. Thus, the isolation and availability of human PSCs hase been critical to the development of PDAC organotypic cultures[26].
Pancreatic cancer cell lines and normal pancreatic ductal epithelial cells (HPDE) previously have been cultured on type I glycosaminoglycan scaffolds and in collagen type I or Matrigel. Given only epithelial cells were in these models, the effect of the stroma on tumour cell behaviour was absent[76-78]. However, these studies were able to show that pancreatic cancer cells embedded into Matrigel formed spheroids with a distinct morphology and loss of apico-basal polarity as compared to culturing in 2D[76].
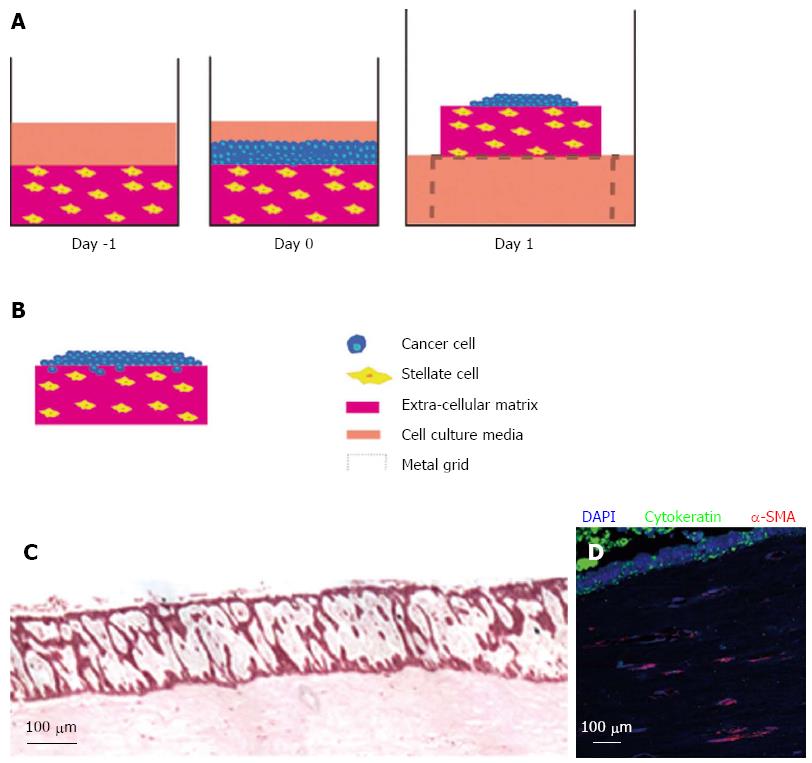
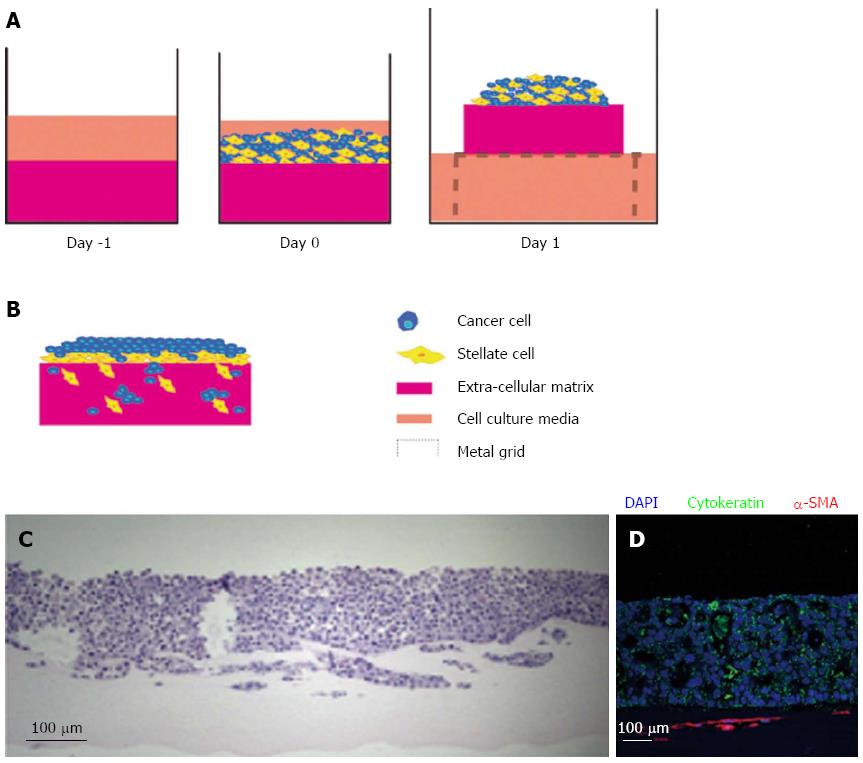
The introduction of stromal cells in PDAC 3D organotypic cultures was first demonstrated by our laboratory[24]. Depending on the hypothesis being explored, the flexible 3D models of PDAC can be set up distinctly. Pancreatic cancer cells can be embedded into the ECM gel consisting of collagen and Matrigel to simulate cells that have already invaded into the stroma However, in order to understand the influence of PSCs on the behaviour of invaded pancreatic cancer cells these cells can be embedded in an ECM gel together with cancer cells (Figure 2).
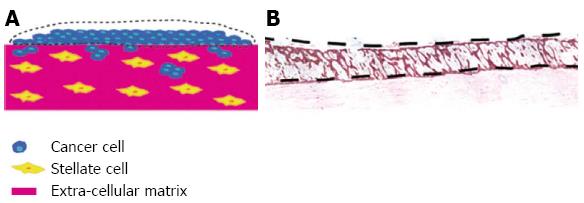
Submerged ECM gels (when pancreatic cancer cells are grown on top of the gel and PSCs are embedded) are designed to model the early events in tumour progression. When pancreatic cancer cells are cultured on top of this model, they form luminal structures that resemble ducts (Figure 3). Using this model, we have shown that PSCs induce Ezrin translocation from the apical to the basal compartment of the cells is an early event in pancreatic cancer cell invasion[24,79]. This phenomenon has been validated across a range of human gastro-intestinal tumours[80,81]. Finally, in order to study the invasion of pancreatic cancer cells in the 3D model, the submerged culture system can be raised upon a grid (‘air-liquid’ model) and fed from underneath, creating a gradient that stimulates pancreatic cancer cells to invade while at the same time recapitulates cancer-stellate cell interaction in vivo (Figure 4).
Using the air liquid 3D model we have shown that the presence of PSCs leads to a significant increase, and altered sub-cellular distribution, of β-catenin in pancreatic cancer cells. Treating these 3D co cultures with All Trans Retinoic Acid (ATRA, which renders PSC quiescent) dampens Wnt-β catenin signalling resulting in reduced pancreatic cancer invasion[16]. Importantly, these results were confirmed in vivo, whereby treating KPC mice with ATRA led to disruption lead to disruption of the activated stroma and increase in apoptosis of tumour cells. These sets of observations validate the use of the organotypic model as a tool to assess new therapies in PDAC.
3D organotypic models provide a perfect intermediate between 2D cultures and GEM. Use of distinct cell types in these co-culture allows assessment of changes in signalling cascades and molecular targets resulting from cancer-stroma cross-talk in the absence of noise from other stromal elements present in vivo. Thus the relative contribution of each cell type in the complex microinvrionment can be assessed. Using this approach Kadaba et al[14] isolated cancer cells from organotypic models of various organ including pancreas, skin and oesophagus after the cancer cells were exposed in 3D to their respective stromal cells (Figure 5). They demonstrated that cancer cell stromal interactions significantly alter proliferation, cell cycle, cell movement, cell signalling and inflammatory response in addition to changing stiffness in the ECM gel. Importantly, changes in stiffness of ECM gels was particularly prominent as the proportion of PSC in the ECM gel increased, a finding highly pertinent to drug delivery and perfusion in PDAC[41]. This study also highlighted the possible need for multidrug targeting or use of pleiotropic agents in PDAC therapy.
Despite the importance of multiple pathways in PDAC, the proto-oncogene Src has been heralded as a potential single molecular therapeutic target[82]. The conundrum of promise of Src inhibitors in combination with chemotherapy in vitro and the in vivo reduction of metastasis in KPC mice by 50%, was explored in organotypic cultures[82]. Using fluorescence lifetime imaging microscopy (FILM) to measure fluorescence resonance energy transfer (FRET) an ECFP-YFP Src reporter, in PDAC cells in organotypic cultures Anderson and colleagues investigated the influence of tumour microenvironment on Dasatinib delivery in PDAC[83]. In organotypic PDAC models with cancer cells expressing the Src biosensor cultured on top of an ECM gel with embedded primary human fibroblasts, they were able to show quantitatively that the microenvironment contribution to poor drug delivery to tumour cells is dependent on distance of cells from the invasive edge. This was validated in subcutaneous in vivo models due to the limitations of microscopy techniques precluding orthotopic or GEM models. This study demonstrated the adaptability of the organotypic model as powerful tool to address hypotheses at the molecular level in a complex microenvironment.
3D organotypic models that mimic the morphological and functional features of their in vivo parental tissues have potential for bridging the gap between cell-based discovery research and animal models[84,85]. A huge advantage of the organotypic system is that any component of the model can readily be modulated in a short time-frame. For example, the matrix composition can be altered to reflect the in vivo situation. The increase in ECM stiffness exerts elevated force on transformed cells increasing cellular response and resulting in increased tumour growth, survival and motility[14,86].
The relative paucity of primary stellate cells to conduct all the experiments in sufficient replicates lead us to generate a mini organotypic culture system (Figure 6) which give comparable results to the conventional “air liquid” co culture model[14]. Additional cell types can be titrated in such as stellate cells[14] or endothelial cells (Di Maggio, unpublished observations). For example, to assess the role of stroma on angiogenesis, in oesophageal cancer endothelial cells on a 2D monolayer have been cultured with fibroblast and cancer cells embedded in a collagen gel layered on top[87]. Elsewhere investigation of the role of macrophages in malignant growth of human squamous cell carcinoma has been investigated in organotypic cultures[88]. Immune response and inflammation play an important role in the desmoplastic reaction and inflammation is thought to activate pancreatic stellate cells[13,89].
Therapeutic agents such as chemotherapy (Gemenzitdis and Carapuca and Ghallab, unpublished observations), small molecules[90] or RNAi (Arumugam and Watt, unpublished observations) can be tested in these organotypic cultures. The best dosage and regimen can then be taken in small animals thus reducing animal usage[16,83]. Examples from other related fields include testing Met-kinase inhibitor or COX-2 inhibitor in skin cancer models[91], tyrosine kinase inhibitors for breast cancers[92] and Eps8 and HAX1 or β6 integrin[93] RNAi in cancer cells prior to their incorporation into organotypic cultures to assess the effects on cell invasion.
Finally, many PDAC patients present very late with their disease when metastasis have already occurred. Thus, treating PDAC cells immediately after seeding in a 3D environment does not reflect the true clinical setting as tumours are well established at the time of patient treatment. We currently are investigating the effect of treating organotypic models once they are established and invasion of PDAC and/or stromal cells has begun. It is likely this would give a better understanding of the treatment regimen that is required when novel therapies emerge into a preclinical setting.
Organotypic culture models are valuable tools for studying the mechanisms of pancreatic cancer, providing an easily manipulated system in which specific questions can be addressed, thus facilitating the translation of basic science to the clinic. Allowing manipulation of cell types, matrix composition, and exogenous therapies, these physiologically relevant model systems are reproducible, experimentally flexible and offer targeted high-throughput platforms. Although the organotypic model provides a physiologically relevant means to study the tumour stroma interactions and the use of new therapies to target the cross talk, it remains a simplified representation of the complex in vivo situation and it still remains critical to test new therapies in orthotopic or transgenic models of the disease. However, the use of the organotypic model as a preclinical tool is becoming increasingly important and our group, as well as others, are modulating the 3D cultures to recapture other important aspects of the tumour microenvironment that can influence cancer cell behaviour. Thus, 3D organotypic models have potential for bridging the gap between cell based discovery and complex animal models. By providing an environment in which cell behaviour and novel treatment options can be investigated in an easily reproducible and controlled manner, these models more precisely mimic pancreatic cancer, thus providing a major contribution to preclinical drug and therapeutic discovery.
P- Reviewers: Apte MV, Liu QD S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Tassi E, Henke RT, Bowden ET, Swift MR, Kodack DP, Kuo AH, Maitra A, Wellstein A. Expression of a fibroblast growth factor-binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006;66:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008;10:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Iqbal N, Lovegrove RE, Tilney HS, Abraham AT, Bhattacharya S, Tekkis PP, Kocher HM. A comparison of pancreaticoduodenectomy with extended pancreaticoduodenectomy: a meta-analysis of 1909 patients. Eur J Surg Oncol. 2009;35:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 5. | Moinfar F, Man YG, Bratthauer GL, Ratschek M, Tavassoli FA. Genetic abnormalities in mammary ductal intraepithelial neoplasia-flat type (“clinging ductal carcinoma in situ”): a simulator of normal mammary epithelium. Cancer. 2000;88:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320-1326. [PubMed] |
| 7. | Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002-5011. [PubMed] |
| 8. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 9. | Woenne EC, Lederle W, Zwick S, Palmowski M, Krell H, Semmler W, Mueller MM, Kiessling F. MMP inhibition blocks fibroblast-dependent skin cancer invasion, reduces vascularization and alters VEGF-A and PDGF-BB expression. Anticancer Res. 2010;30:703-711. [PubMed] |
| 10. | Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3231] [Cited by in RCA: 3322] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 11. | Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1315] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 12. | Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 13. | Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 14. | Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, Soylu E, Ghallab M, Bor D, Froeling FE. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486-1497, 1486-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 17. | Li D, Abbruzzese JL. New strategies in pancreatic cancer: emerging epidemiologic and therapeutic concepts. Clin Cancer Res. 2010;16:4313-4318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ozawa F, Friess H, Tempia-Caliera A, Kleeff J, Büchler MW. Growth factors and their receptors in pancreatic cancer. Teratog Carcinog Mutagen. 2001;21:27-44. [PubMed] |
| 19. | Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, Kobrin MS, Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993;53:5289-5296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Apte MV, Wilson JS. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 482] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 23. | Korc M. Growth factors and pancreatic cancer. Int J Pancreatol. 1991;9:87-91. [PubMed] |
| 24. | Froeling FE, Mirza TA, Feakins RM, Seedhar A, Elia G, Hart IR, Kocher HM. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 803] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 26. | Froeling FE, Marshall JF, Kocher HM. Pancreatic cancer organotypic cultures. J Biotechnol. 2010;148:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 715] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | Wake K. “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971;132:429-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 255] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 550] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 30. | Fujita H, Ohuchida K, Mizumoto K, Nakata K, Yu J, Kayashima T, Cui L, Manabe T, Ohtsuka T, Tanaka M. alpha-Smooth Muscle Actin Expressing Stroma Promotes an Aggressive Tumor Biology in Pancreatic Ductal Adenocarcinoma. Pancreas. 2010;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 797] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 32. | Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 934] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 33. | Jesnowski R, Fürst D, Ringel J, Chen Y, Schrödel A, Kleeff J, Kolb A, Schareck WD, Löhr M. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005;85:1276-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol. 2003;9:2751-2758. [PubMed] |
| 35. | Mathison A, Liebl A, Bharucha J, Mukhopadhyay D, Lomberk G, Shah V, Urrutia R. Pancreatic stellate cell models for transcriptional studies of desmoplasia-associated genes. Pancreatology. 2010;10:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Satoh M, Masamune A, Sakai Y, Kikuta K, Hamada H, Shimosegawa T. Establishment and characterization of a simian virus 40-immortalized rat pancreatic stellate cell line. Tohoku J Exp Med. 2002;198:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Sparmann G, Hohenadl C, Tornøe J, Jaster R, Fitzner B, Koczan D, Thiesen HJ, Glass A, Winder D, Liebe S. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G211-G219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Li NF, Kocher HM, Salako MA, Obermueller E, Sandle J, Balkwill F. A novel function of colony-stimulating factor 1 receptor in hTERT immortalization of human epithelial cells. Oncogene. 2009;28:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 40. | Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M. Pancreatic stellate cells--role in pancreas cancer. Langenbecks Arch Surg. 2008;393:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2535] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 42. | Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, Hamada H, Kobune M, Satoh K, Shimosegawa T. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 43. | Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 463] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 44. | Eguchi D, Ikenaga N, Ohuchida K, Kozono S, Cui L, Fujiwara K, Fujino M, Ohtsuka T, Mizumoto K, Tanaka M. Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor. J Surg Res. 2013;181:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 46. | Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1454] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 47. | Grippo PJ, Tuveson DA. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev Res (Phila). 2010;3:1382-1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277-5287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7:251-258. [PubMed] |
| 50. | Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L. Mouse models of pancreatic cancer. World J Gastroenterol. 2012;18:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 51. | Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249-4256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Pérez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 53. | Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2012;61:1488-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Ecob MS. The application of organotypic nerve cultures to problems in neurology with special reference to their potential use in research into neuromuscular diseases. J Neurol Sci. 1983;58:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Edelman DB, Keefer EW. A cultural renaissance: in vitro cell biology embraces three-dimensional context. Exp Neurol. 2005;192:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Partridge CR, Johnson CD, Ramos KS. In vitro models to evaluate acute and chronic injury to the heart and vascular systems. Toxicol In Vitro. 2005;19:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 246] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 58. | Owens BM, Hawley RG, Spain LM. Retroviral transduction in fetal thymic organ culture. Methods Mol Med. 2005;105:311-322. [PubMed] |
| 59. | Botham PA. The validation of in vitro methods for skin irritation. Toxicol Lett. 2004;149:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Orr FW, Lee J, Duivenvoorden WC, Singh G. Pathophysiologic interactions in skeletal metastasis. Cancer. 2000;88:2912-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Atala A. Engineering tissues and organs. Curr Opin Urol. 1999;9:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1902] [Cited by in RCA: 1959] [Article Influence: 108.8] [Reference Citation Analysis (4)] |
| 63. | Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 416] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 64. | Kopan R, Fuchs E. A new look into an old problem: keratins as tools to investigate determination, morphogenesis, and differentiation in skin. Genes Dev. 1989;3:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Fartasch M, Ponec M. Improved barrier structure formation in air-exposed human keratinocyte culture systems. J Invest Dermatol. 1994;102:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821-14826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 517] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 67. | Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG). J Cell Sci. 1996;109:2013-2021. [PubMed] |
| 68. | Sakamoto T, Hirano K, Morishima Y, Masuyama K, Ishii Y, Nomura A, Uchida Y, Ohtsuka M, Sekizawa K. Maintenance of the differentiated type II cell characteristics by culture on an acellular human amnion membrane. In Vitro Cell Dev Biol Anim. 2001;37:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990;63:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717-7722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Mauchamp J, Mirrione A, Alquier C, André F. Follicle-like structure and polarized monolayer: role of the extracellular matrix on thyroid cell organization in primary culture. Biol Cell. 1998;90:369-380. [PubMed] |
| 72. | Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol. 1998;10:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 242] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945-1957. [PubMed] |
| 74. | Radisky D, Muschler J, Bissell MJ. Order and disorder: the role of extracellular matrix in epithelial cancer. Cancer Invest. 2002;20:139-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA. 1995;92:7411-7415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 76. | Grzesiak JJ, Bouvet M. Determination of the ligand-binding specificities of the alpha2beta1 and alpha1beta1 integrins in a novel 3-dimensional in vitro model of pancreatic cancer. Pancreas. 2007;34:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA. Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun. 2007;358:698-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Deramaudt TB, Takaoka M, Upadhyay R, Bowser MJ, Porter J, Lee A, Rhoades B, Johnstone CN, Weissleder R, Hingorani SR. N-cadherin and keratinocyte growth factor receptor mediate the functional interplay between Ki-RASG12V and p53V143A in promoting pancreatic cell migration, invasion, and tissue architecture disruption. Mol Cell Biol. 2006;26:4185-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Kocher HM, Sandle J, Mirza TA, Li NF, Hart IR. Ezrin interacts with cortactin to form podosomal rosettes in pancreatic cancer cells. Gut. 2009;58:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Arumugam P, Partelli S, Coleman SJ, Cataldo I, Beghelli S, Bassi C, Wijesuriya N, Aleong JA, Froeling FE, Scarpa A. Ezrin expression is an independent prognostic factor in gastro-intestinal cancers. J Gastrointest Surg. 2013;17:2082-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Li NF, Gemenetzidis E, Marshall FJ, Davies D, Yu Y, Frese K, Froeling FE, Woolf AK, Feakins RM, Naito Y. RhoC interacts with integrin α5β1 and enhances its trafficking in migrating pancreatic carcinoma cells. PLoS One. 2013;8:e81575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, Doyle B, McKay C, Heung MY, Oien KA. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 83. | Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J, Edward M, Campbell AD, McGarry LC, Evans TR. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res. 2013;73:4674-4686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1282] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 85. | Barrila J, Radtke AL, Crabbé A, Sarker SF, Herbst-Kralovetz MM, Ott CM, Nickerson CA. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol. 2010;8:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 86. | Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 620] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 87. | Lioni M, Noma K, Snyder A, Klein-Szanto A, Diehl JA, Rustgi AK, Herlyn M, Smalley KS. Bortezomib induces apoptosis in esophageal squamous cell carcinoma cells through activation of the p38 mitogen-activated protein kinase pathway. Mol Cancer Ther. 2008;7:2866-2875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Linde N, Gutschalk CM, Hoffmann C, Yilmaz D, Mueller MM. Integrating macrophages into organotypic co-cultures: a 3D in vitro model to study tumor-associated macrophages. PLoS One. 2012;7:e40058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [PubMed] [DOI] [Full Text] |
| 90. | Coleman SJ, Chioni AM, Ghallab M, Anderson RK, Lemoine NR, Kocher HM, Grose RP. Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol Med. 2014;6:467-481. [PubMed] |
| 91. | Nystrom ML, McCulloch D, Weinreb PH, Violette SM, Speight PM, Marshall JF, Hart IR, Thomas GJ. Cyclooxygenase-2 inhibition suppresses alphavbeta6 integrin-dependent oral squamous carcinoma invasion. Cancer Res. 2006;66:10833-10842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Chioni AM, Grose R. FGFR1 cleavage and nuclear translocation regulates breast cancer cell behavior. J Cell Biol. 2012;197:801-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 93. | Ramsay AG, Keppler MD, Jazayeri M, Thomas GJ, Parsons M, Violette S, Weinreb P, Hart IR, Marshall JF. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res. 2007;67:5275-5284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |